Abstract
The pedunculopontine tegmental nucleus (PPTg) targets nuclei in the basal ganglia, including the substantia nigra pars compacta (SNc), in which neuronal loss occurs in Parkinson’s disease, a condition in which patients show cognitive as well as motor disturbances. Partial loss and functional abnormalities of neurons in the PPTg are also associated with Parkinson’s disease. We hypothesized that the interaction of PPTg and SNc might be important for cognitive impairments and so investigated whether disrupting the connections between the PPTg and SNc impaired learning of a conditioned avoidance response (CAR) by male Wistar rats. The following groups were tested: PPTg unilateral; SNc unilateral; PPTg-SNc ipsilateral (ipsilateral lesions in PPTg and SNc); PPTg-SNc contralateral (contralateral lesions in PPTg and SNc); sham lesions (of each type). SNc lesions were made with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine HCl (MPTP, 0.6 μmoles); PPTg lesions with ibotenate (24 nmoles). After recovery, all rats underwent 50-trial sessions of 2-way active avoidance conditioning for 3 consecutive days. Rats with unilateral lesions in PPTg or SNc learnt this, however rats with contralateral (but not ipsilateral) combined lesions in both structures presented no sign of learning. This effect was not likely to be due to sensorimotor impairment because lesions did not affect reaction time to the tone or footshock during conditioning. However, an increased number of non-responses were observed in the rats with contralateral lesions. The results support the hypothesis that a functional interaction between PPTg and SNc is needed for CAR learning and performance.
Keywords: active avoidance, Parkinson, basal ganglia, dopamine
Introduction
The pedunculopontine tegmental nucleus (PPTg) is now accepted as one of the basal ganglia family of structures, being intimately connected with many parts of the basal ganglia, such as the globus pallidus, subthalamic nucleus and substantia nigra pars compacta (SNc). Understanding the connection between the PPTg and SNc is particularly important because they are implicated in the pathology of Parkinson’s disease. Parkinson’s disease patients present motor impairments, but also have cognitive deficits that appear in advance of the motor impairments (Carbon and Marie, 2003; Dubois and Pillon, 1997; Juri and Chana, 2006). This is not surprising, given that the basal ganglia play a role in action selection and in the learning of action-outcome associations and stimulus-response habits (Balleine, Liljeholm, and Ostlund, 2009; Cohen and Frank, 2009; Da Cunha, Wietzikoski, Dombrowski, Santos, Bortolanza, Boschen, and Miyoshi, 2009; Izquierdo, Bevilaqua, Rossato, Bonini, Da Silva, Medina, and Cammarota, 2006; Packard and McGaugh, 1992; 1996; White, 2009; Yin and Knowlton, 2006). Critical to such associations is the phasic release of dopamine (DA) in the striatum, which selectively reinforces corticostriatal synaptic activity. Striatal DA is provided by neurons in the SNc and adjacent ventral tegmental area (VTA) and, because the PPTg is a source of excitatory input to these, it is possible that the PPTg provides information that allows computation of a DA-mediated reward prediction error signal critical to the striatal operations (Mena-Segovia, Winn, and Bolam, 2008; Okada, Toyama, Inoue, Isa, and Kobayashi, 2009; Pan and Hyland, 2005). In this paper we attempt to determine whether the interaction between PPTg and SNc is important for the formation of associations – for learning.
The role of DA in stimulus-response-outcome learning was more extensively studied in tasks in which an action result in a reward. However, the literature also shows a devastating effect of the lesion of the SNc (Da Cunha, Gevaerd, Vital, Miyoshi, Andreatini, Silveira, Takahashi, and Canteras, 2001; Timar, Knoll, Jona, and Knoll, 1974) or the administration of DA receptor antagonists in conditioned avoidance learning (CAR) (Aguilar, Mari-Sanmillan, Morant-Deusa, and Minarro, 2000; Ogren and Archer, 1994). CAR learning can be modeled by the 2-way active avoidance task in which rats learn to anticipate an imminent footshock (unconditioned stimulus: US) and avoid it by an instrumental response after the presentation of a warning stimulus (conditioned stimulus: CS). In the present study we tested whether a functional interaction between the PPTg and SNc is necessary for learning this. The disconnection of these structures was achieved by combined lesions, based on the premise that if two structures are serially connected, their function can be impaired by combined hemilesions of these structures on opposite sides of the brain (when those hemi-lesions – in this case unilateral lesion of either SNc or PPTg – are independently ineffective) (Parkinson, Robbins, and Everitt, 2000). In the present study, impairment is expected after combined unilateral lesion of the PPTg and SNc in different hemispheres because they are mostly ipsilaterally connected (Beninato and Spencer, 1987; 1988; Clarke, Hommer, Pert, and Skirboll, 1987; Gould, Woolf, and Butcher, 1989; Mena-Segovia et al., 2008; Woolf and Butcher, 1986). In view of the reciprocal connections between the PPTg and the SNc (Beninato and Spencer, 1987; 1988; Clarke et al., 1987; Gould et al., 1989) and that they are needed for learning of stimulus-response-outcome associations (Da Cunha et al., 2009; Zokoll, Klump, and Langemann, 2008), we predicted that animals bearing combined unilateral lesion of both structures (in the different hemispheres) would be impaired to learn this CAR task.
Material and Methods
Subjects and housing
Adult male Wistar rats from the colony of the Universidade Federal do Parana, weighing 280-310 g at the beginning of the experiments were used. The animals were maintained in a temperature-controlled room (22 ± 2 o C) on a 12/12 h dark/light cycle (lights on at 7:00 a.m.) with food and water available ad libitum. All surgical interventions were done under appropriate anaesthesia and efforts were made to minimize the number of animals used and their suffering in the experimental procedures adopted for the in vivo studies. These procedures were previously approved by the Animal Care and Use Committee of the Universidade Federal do Parana (protocol 195) and were in compliance with the guidelines with the UK Animals (Scientific Procedures) Act 1986 and European Communities Council Directive of 24 November 1986 (86/609/EEC).
A total of 78 animals were used in these experiments. Data from 35 of these was included in this study; the others died (28 rats) before completing the experiments, or were excluded according to histological (12 rats) or turning behaviour criteria (3 rats). Surgical interventions using MPTP or excitotoxins in SNc and PPTg are known to be associated with relatively high mortality rates, both during and after surgery (Ferro et al, 2005; Wilson et al, 2009). The combination of lesions used here reflects this, with the combined PPTg and SNc lesions accounting for 68% of the fatalities; note that sham lesions were not associated with this high mortality rate.
Surgery
Surgery was conducted 21 days before the start of behavioural experiments. All the rats received atropine sulfate (0.4 mg/kg, i.p.) to suppress salivation, penicillin G-procaine (20,000U in 0.1 ml, i.m.), and were anesthetized with 3 ml/kg equithesin (1% sodium thiopental, 4.25% chloral hydrate, 2.13% magnesium sulfate, 42.8% propylene glycol, and 3.7% ethanol in water, i.p.).
The animals were randomly assigned to one of 5 groups: PPTg unilateral (unilaterally lesioned in the PPTg); SNc unilateral (unilaterally lesioned in the SNc); PPTg-SNc ipsilateral (ipsilaterally lesioned in the PPTg and SNc); PPTg-SNc contralateral (contralaterally lesioned in the PPTg and SNc). Sham lesions of each type were made. The rats were placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA) with the incisor bar set 3.3 mm below the interaural line and ibotenic acid (Tocris-Cookson Ltd, Bristol, UK; 24 nmoles in 0.2 μl 0.12 M phosphate buffer, pH 7.4) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine HCl (MPTP, Sigma Chemical Co., St. Louis, MO, USA; 0.6 μmoles in 1 μl saline) was infused into the PPTg or SNc, respectively. The following coordinates were used (adapted from the stereotaxic atlas of Paxinos and Watson (2005)): PPTg: anteroposterior (AP) +0.8 mm from the interaural line; mediolateral (ML) ±1.8 mm from midline; dorsoventral (DV) −6.4 mm from the skull surface (SK); MPTP: AP +3.9 mm from the interaural line; ML ±2.1 mm; DV −7.7 mm from SK. The sham rats received the vehicle of one of the neurotoxins, as appropriate to the structure concerned. Ibotenic acid was infused into the PPTg by manual pressure ejection from glass micropipettes (35 μm external tip diameter), left in situ for 5 min after infusion to allow for diffusion away from the tip. Rats with lesions in both hemispheres received 2 separate unilateral operations, 7 days apart. The MPTP was infused into the SNc at a rate of 0.25 μl/min by a 30-gauge needle connected to a microinfusion pump (Insight, Ribeirao Preto, Brazil). The needle remained in place for an additional 2 min to maximize diffusion of the solution. Rats also received 3 i.p. injections of 120 mg/kg acetaldehyde 15 min before, at the beginning and 15 min after surgery to increase the effectiveness of the neurotoxin (Zuddas, Corsini, Schinelli, Johannessen, Diporzio, and Kopin, 1989). After surgery, all rats were allowed to recover from anaesthesia in a temperature controlled chamber and then placed back into their home cages.
The neurotoxin ibotenate was used to lesion the PPTg because previous studies have shown that it cause loss of the neurons in the target structures, sparing axons of passage and nerve terminals (Coyle and Schwarcz, 1983; Fujimoto, Ikeguchi, and Yoshida, 1992) and does not cause aggressive behaviour, startle response, struggle reaction to capture and increased locomotor activity, as occurs after the lesion of the PPTg with kainate (Fujimoto, Yoshida, Ikeguchi, and Niijima, 1989). MPTP was used to lesion the SNc because it is most selective the neurotoxin to lesion dopaminergic neurons, sparing the other neurons of the SNc and causing depletion of striatal DA without alterations in other striatal monoamines (Harik, Schmidley, Iacofano, Blue, Arora, and Sayre, 1987). In addition, previous studies showed that MPTP-lesioned rats present memory deficits without other sensorimotor motor impairments (Da Cunha et al. 2001; Kumar, Kaundal, More, and Sharma, 2009; Wang, Liou, Pawlak, and Ho).
2-way active avoidance
The 2-way active avoidance apparatus was an automated 31 × 33 × 56 cm shuttle-box (Insight Instruments, Ribeirao Preto, Brazil) with the front made of Plexiglas and the floor made of parallel 5 mm caliber stainless-steel bars spaced 15 mm apart. The box was divided into 2 compartments of the same size by a wall, with a door that remained open during the tests. The animals were trained in 3 sessions, one on each of 3 consecutive days. In each session, after 10 min (Day 1) or 5 min (Days 2 and 3) of habituation, 50 sound cues (CS: 1.5 kHz, 60 dB, maximum duration of 10 s) were paired with a subsequent 0.5 mA footshock (US: maximum duration of 5 s, starting 5 s after the CS onset) until the rat crossed to the other compartment. Four measures of behaviour were taken: (i) avoidance: during presentation of the CS, the rat could turn off the sound and actively avoid the shock by crossing to the other chamber; (ii) escape: after the CS, when the US footshock was presented, the rat could escape by crossing to the other chamber; (iii) non-response: the trials in which the rat did not cross to the other chamber during either the CS or US presentation were counted as a non-response; (iv) inter-trial crossing (ITC): the time between each trial varied randomly, ranging from 10–50 s. During this, rats could spontaneously cross from one side of the apparatus to the other; the number of crossings was recorded as the ITC. The number of active avoidances, escapes, non-responses, and ITC crossings were recorded automatically by the apparatus and captured on computer.
Behavioural analysis of the lesion
Two days after the 2-way active avoidance sessions, the rats received 1 mg/kg apomorphine (Sigma-Aldrich, s.c.) and the number of 360° turns toward the lesioned side (ipsiversive) and toward the opposite side (contraversive) was scored over a period of 1 h in a Rota-Count apparatus(Columbus Instruments, Columbus, Ohio, USA). The SNc unilateral rats that made less than 80 ipsiversive turns / h were excluded from the analysis. This test was performed as a behavioural control for the lesion because it is known that MPTP rats bearing incomplete unilateral lesions of nigral cell loss present ipsiversive turning behaviour (Da Cunha, Wietzikoski, Ferro, Martinez, Vital, Hipolide, Tufik, and Canteras, 2008). The PPTg unilateral rats that made less than 80 contraversive turns / h were excluded from the analysis. Contraversive turning was expected from PPTg unilateral rats, as previously reported after inhibition of rat PPTg with GABA(A) agonists (Ikeda, Akiyama, Matsuzaki, Sato, Moribe, Koshikawa, and Cools, 2004) or blockade of muscarinic receptors in the SNc usually occupied by acetylcholine (ACh) released by PPTg neurons (Góngora-Alfaro, Hernandez-Lopez, Martinez-Fong, Flores, and Aceves, 1996).
Histological and neurochemical analysis of the lesions
Histological analysis was carried out on all rats in this study after behavioural procedures were complete. After a terminal anaesthetic dose of pentobarbitone, the brains were fixed in situ using transcardial perfusion at room temperature of heparinized 0.9% saline followed by 4% paraformaldehyde in phosphate buffer, pH 7.4. The brains were removed and post-fixed in 4% paraformaldehyde in phosphate buffer, pH 7.4. for 72 h at 4°C. The brains were then stored in 20% sucrose in 4% paraformaldehyde in phosphate buffer (pH 7.4) until they were cut. Eight series of sections 50 μ thick 200 μ apart were cut on a freezing microtome through the regions of interest. The sections were stored in cryoprotectant at –20°C until immunohistochemistry was carried out.
PPTg lesions and shams
Two series of sections 100 μ apart were processed free floating to demonstrate choline acetyltransferase (ChAT) and neuronal nuclear protein (NeuN) using immunohistochemical techniques. Primary antibodies were goat anti ChAT polyclonal antibody and mouse anti NeuN both from (Chemicon International Inc, Temecula, CA, USA), followed by appropriate Vector Elite Peroxidase ABC kits (Vector Labs, Peterborough, UK) and Sigma fast DAB substrate (Sigma Chemical Co., St Louis, MO, USA). After the NeuN stained sections were mounted, a light counterstain of cresyl fast violet was applied to allow non-neuronal structures (such as myelin, blood vessels and glial cells) to be identified using a light microscope to estimate the damage to PPTg. Remaining ChAT positive neurons were counted by an observer blind with respect to rats’ treatment group and the extent of the lesion was estimated by absence of neuronal structures and the presence of reactive gliosis and calcification seen in the NeuN / cresyl fast violet stain and scored on a linear scale of 0% (no lesion) to 100% (complete lesion).
SNc lesions and shams
One series of sections 200 μm apart were processed, free floating to demonstrate tyrosine hydroxylase (TH) using immunohistochemical techniques. Primary antibody was mouse anti-TH polyclonal antibody (Chemicon International Inc, Temecula, CA, USA) followed by appropriate Vector Elite Peroxidase ABC kits (Vector Labs, Peterborough, UK) and Sigma fast DAB (Sigma Chemical Co., St Louis, MO, USA). Using a light microscope, the extent of damage to midbrain DA neurons was estimated by an observer blind with respect to rats’ treatment group on a linear scale of 0% (no lesion) to 100% (complete lesion) from the remaining TH positive neurons in the SNc and VTA.
Neurochemical analysis of the effects of lesions was performed in rats that were not submitted to the behavioural tests. Twenty one days after surgery, rats were killed by rapid decapitation and their dorsal striata were dissected on ice and stored at −70° C. Endogenous levels of DA were assayed by reverse-phase HPLC with electrochemical detection (ED). The system consisted of a Synergi Fusion-RP C-18 reverse-phase column (150 × 4.6 mm i.d., 4-μm particle size, Phenomenex, Torrance, CA, USA), an dual coulometric electrochemical detector (Coulochem III, ESA, Chelmsford, USA), and an LC-20AT pump (Shimadzu, Kyoto, Japan). This detector consists of 2 cells successively connected, both containing a porous graphite working electrode together with associated reference and counter electrodes. The detector is equipped with a guard cell (ESA 5020) electrode set at +350 mV and the working electrodes (5011 analytical cell, ESA) set at E1=+100 and E2=+450 mV versus a solid state palladium reference electrode. The column was maintained inside a temperature-controlled oven (25°C). The tissue samples were homogenized with an ultrasonic cell disrupter (Sonics, Newtown, CT, USA) in 0.1 M perchloric acid. After centrifugation at 15,000 g for 30 min, 20 μl of the supernatant was injected into the chromatograph. The mobile phase, used at a flow rate of 1 ml/min, had the following composition: 15.7 g citric acid, 471.5 ml HPLC-grade water, 78 mg heptane sulfonic acid, 20 ml acetonitrile, and 10 ml tetrahydrofuran, pH 3.0. The peak areas of the external standards were used to quantify the sample peaks.
Data analysis
After examination of turning behaviour and histological analysis of the lesions, the numbers of rats included in the analysis of active avoidance were: PPTg unilateral, n = 6; SNc unilateral, n = 8; PPTg-SNc ipsilateral, n = 5; PPTg-SNc contralateral, n = 4. Data from sham rats of all types were pooled because no significant difference was found among the groups; n = 12. Another 9 sham, 9 PPTg unilateral, and 11 SNc unilateral, rats were used in the neurochemical analysis.
The 2-way active avoidance data (number of avoidances, escapes, ITC, and non-responses) were analyzed by two-way ANOVA, taking the trials blocks or training days as repeated measures. The avoidance data was also analyzed by three-way ANOVA, taking the number of non-response as covariate. Differences among groups were analyzed by the post hoc Newman-Keuls test. Correlations between two variables were analyzed by the Pearson test. Differences were considered to be statistically significant when p < 0.05.
Results
Fig. 1 illustrates typical PPTg lesions. These extended into most parts of the PPTg with almost total destruction of Ch5 neurons (identified by ChAT immunostaining), in addition to the other NeuN-immunoreactive neurons of the PPTg. The neurons of the lateral dorsal tegmental nucleus (LTDg) and rostromedial tegmental nucleus (RMTg) were spared. Table 1 shows the loss of NeuN-immunostained neurons in the PPTg, which was of nearly 60-80% and significantly different from the sham group (F(3,20) = 24.52, p < 0.001 one-way ANOVA; p < 0.01, Newman-Keuls post hoc analysis). No significant difference was found among the PPTg unilateral, PPTg-SNc ipsilateral and PPTg-SNc contralateral groups (p > 0.1, Newman Keuls test). Table 1 also shows that, compared to the sham group, the SNc-lesioned rats presented a significant loss of nearly 50% of the TH-immunoreactive neurons, (F(3,19) = 3.99, p < 0.05, one-way ANOVA; p < 0.01, Newman-Keuls post hoc analysis). No significant difference was found among the SNc unilateral, PPTg-SNc ipsilateral and PPTg-SNc contralateral groups (p > 0.4, Newman Keuls test). This lesion was mostly restricted to the A9 neurons of the SNc, sparing most of the A10 neurons of the VTA (Fig. 2). This includes the dopaminergic neurons of the paranigral nucleus (PN) and parainterfascicular nucleus (PIF). The nondopaminergic neurons in the structures that are close to the SNc were also spared. The SNc-lesioned rats presented a gradient of loss of the TH-immunostained terminals, damage being more intense in the most caudal portions of the striatum. In the rostral striatum, TH loss was more apparent in the dorsolateral part, with the dorsomedial striatum and the nucleus accumbens partly spared (Fig. 3). Based on the histology, data of the following animals were excluded from the analysis: 10 rats with small lesions (1 PPTg unilateral; 3 SNc unilateral; 2 PPTg-SNc ipsilateral, and 4 PPTg-SNc contralateral) and 2 with that lost more than 80% of the neurons in the SNc (1 SNc unilateral e 1 PPTg-SNc ipsilateral).
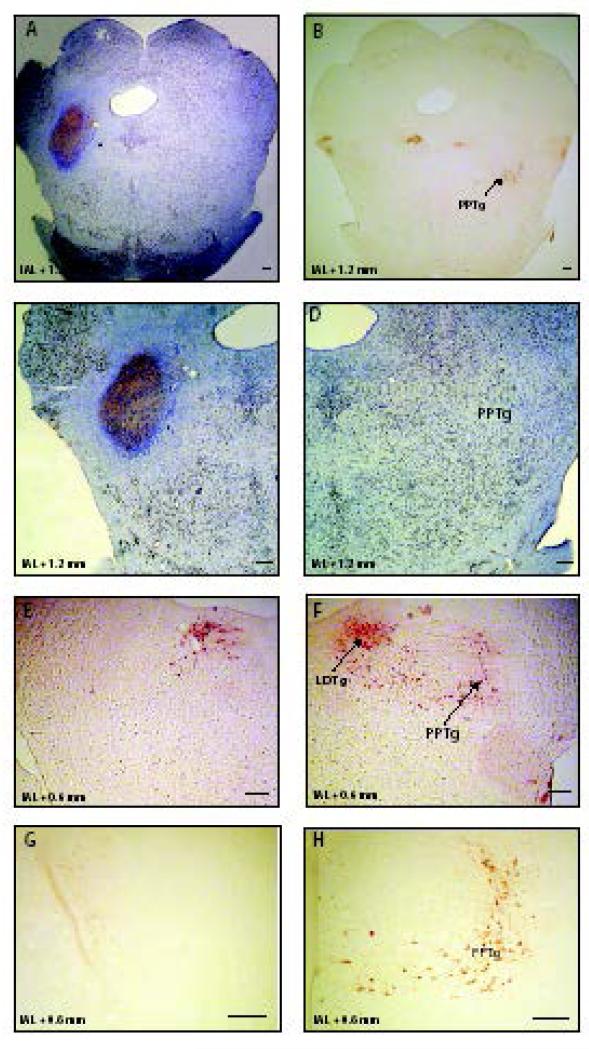
Fig. 1.
Representative left side PPTg ibotenic acid lesion. Panels A, C, D show cresyl violet and NeuN immunostained tissue and panels B, E, F, G and H show ChAT immunostained tissue. The sham-lesioned tissue is shown in the panels D, F, and H, and the lesioned tissue is shown in the panels C, E, and G. PPTg, pedunculopontine tegmental nucleus; LDTg, laterodorsal tegmental nucleus. Scale bar represents 200 μm.
Table 1.
Evaluation of the extent of the lesions in the PPTg and SNc
| % lesion | ||
|---|---|---|
| PPTg | SNc | |
|
|
||
| Sham | 0 ± 3 | 0 ± 20 |
| PPTg unilateral | 88 ± 6 (*) | - |
| SNc unilateral | - | 50 ± 10 (*) |
| PPTg-SNc ipsilateral | 63 ± 15 (*) | 45 ± 10 (*) |
| PPTg-SNc contralateral | 66 ± 14 (*) | 66 ± 9 (*) |
The effects of unilateral infusion of 24 nmoles ibotenic acid into the PPTg or 0.6 μmoles MPTP into the rat SNc on the extent of damage to the Neu-N positive neurons of the PPTg or to the TH-positive neurons of the SNc was estimated on a linear scale of 0% (no lesion) to 100% (complete lesion) expressed as the mean ± SEM.
P < 0.05 Newman-Keuls after two-way ANOVA.
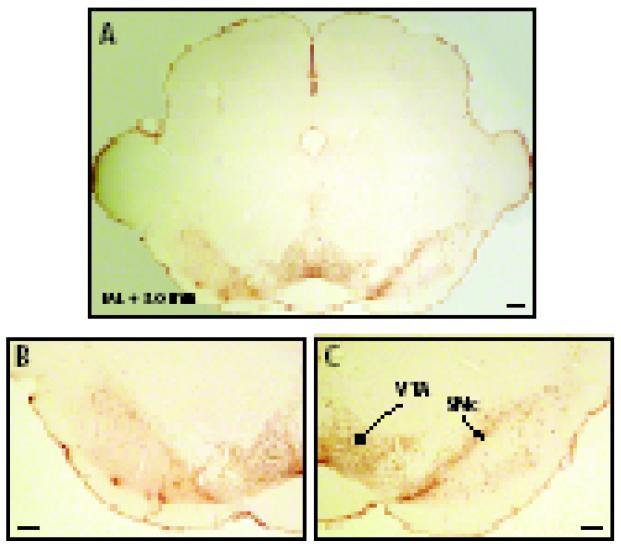
Fig. 2.
Representative TH-immunostained left side SNc MPTP lesion. Panel A shows the lesion on the left side and sham on the right side. Panel B shows detail of the VTA and SNc of lesioned side, panel C the SNc and VTA of the sham side. SNc, substantia nigra compacta; VTA, ventral tegmental area. Scale bar represents 200 μm.
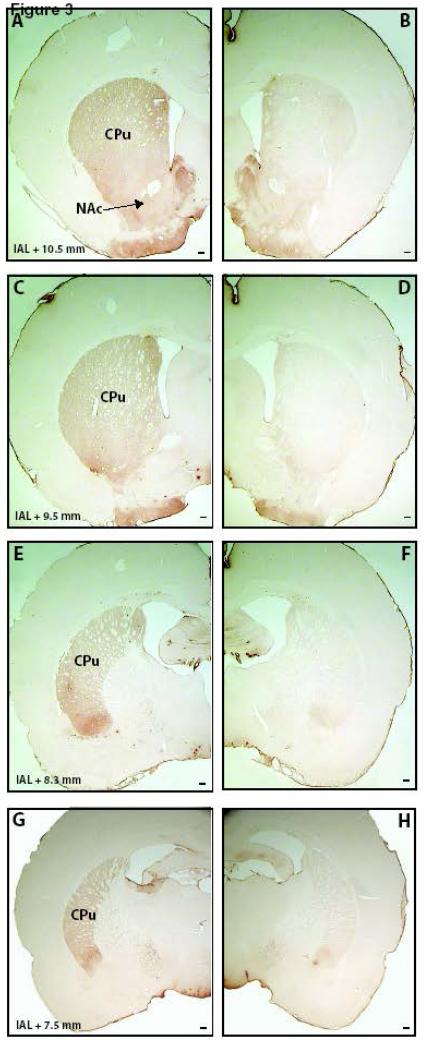
Fig. 3.
Representative TH-immunostained coronal slices of the striatum of a rat bearing an MPTP lesion of the right SNc. The slices are ordered in a rostral to caudal orientation. CPu, caudoputamen; NAc, nucleus accumbens. Scale bar represents 200 μm.
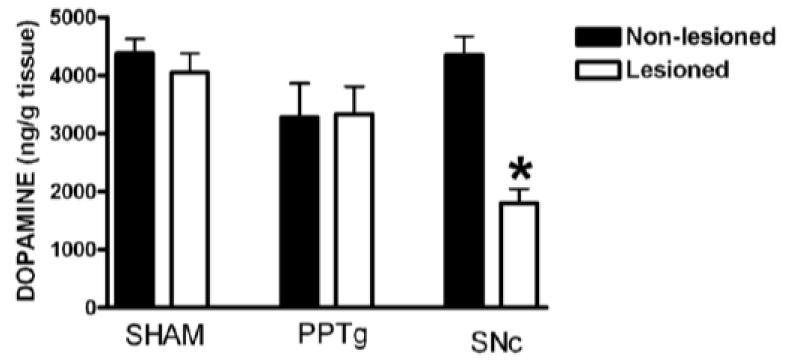
The effect of unilateral lesions of the PPTg or SNc on striatal DA is shown in Fig. 4. Two-way ANOVA showed significant effects of treatment (F(2,51) = 4.87, p < 0.05) and side (F(1,51) = 5.47, p < 0.05) and a significant interaction (F(2,51) = 9.40, p < 0.001). MPTP lesion of SNc caused a significant loss of nearly 60% of striatal DA on the lesioned side (p < 0.05, Newman-Keuls test) while PPTg excitotoxic lesions did not produce a significant reduction in striatal DA content (Fig. 4). These data clearly demonstrate different physical consequences of the SNc and PPTg lesions.
Fig. 4.
Effects of unilateral infusion of 0.6 μmol MPTP into the rat SNc or of 24 nmol ibotenic acid into the PPTg on striatal dopamine content. * P < 0.05 Newman-Keuls after two-way ANOVA.
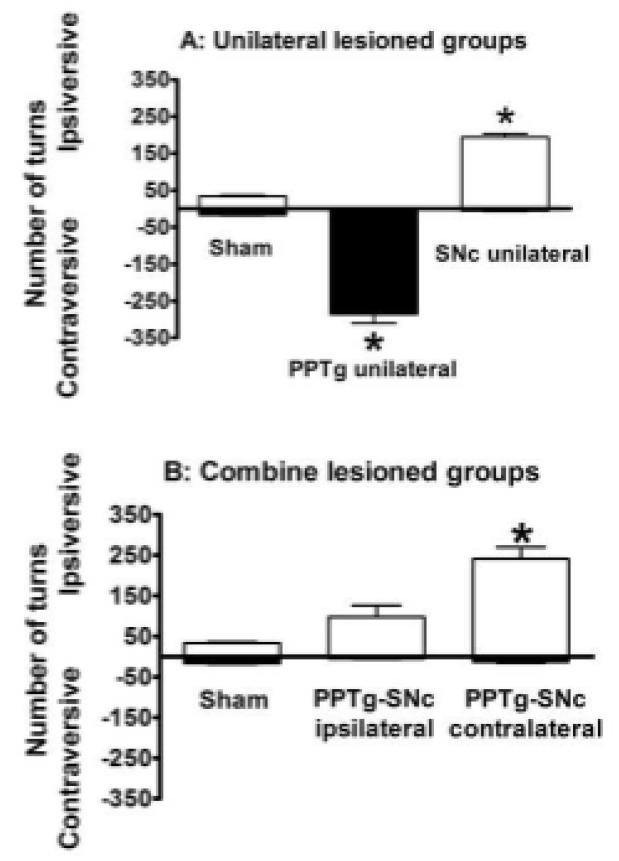
Two days after the 2-way active avoidance sessions, when challenged with 1 mg / kg s.c. apomorphine, PPTg unilateral rats made contraversive, and SNc unilateral rats ipsiversive turns (Fig. 5A). All PPTg unilateral rats included in the study made more than 80 contraversive turns / h and the SNc unilateral rats, more than 80 ipsiversive turns / h. The turning scores of both groups were significantly higher than the scores of the sham rats: PPTg unilateral, F(2,23) = 35.57; p < 0.001; p < 0.01 Newman Keuls test; SNc unilateral, F(2,23) = 36.85, p < 0.001; p < 0.01 Newman Keuls test. One PPTg unilateral and two SNc unilateral rats were excluded because of failure to meet this criterion.
Fig. 5.
Turning behaviour of unilaterally (A), ipsilaterally and contralaterally (B) PPTg and SNc lesioned rats challenged with 0.1 mg/kg apomorphine (i.p.). Data are expressed as the mean ±SEM. number of ipsiversive (positive scale) and contraversive turns (negative scale) counted over 1 h after drug challenge (n = 7 - 18 animals per group). * P ≤ 0.05 compared to the sham group (Newman–Keuls test after one-way ANOVA).
The turning scores of rats with combined lesions in PPTg and SNc were analyzed separately, because it was not possible to classify their turning behaviour as ipsiversive or contraversive in relation to the SNc and the PPTg at the same time. As can be seen in Fig. 5B, PPTg-SNc contralateral (F(2,18) = 8.62 p < 0.05; p < 0.05 Newman Keuls), but not the PPTg-SNc ipsilateral (p = 0.22, Newman Keuls), made significantly more turns towards the SNc lesioned side, compared to the sham lesioned rats. No significant difference in the number of contraversive turns was observed among the groups (F(2,18) = 1.13), p = 0.34). The PPTg-SNc combined lesioned rats showed ipsiversive rotation (as did the SNc unilaterally lesioned rats) but the presence of an ipsilateral PPTg lesion significantly attenuated ipsiversive rotation expected of rats with unilateral SNc lesions.
Three weeks after surgery, at the time of the 2-way active avoidance training, lesioned rats did not present turning behaviour or any other gross motor impairment when not drugged. They were not hypokinetic, aphagic or adipsic, and did not significantly differ from sham lesioned rats in relation to the reaction time in response to footshock (F(4,30) = 1.56, P = 0.20) or to the sound cue that signaled footstock (F(4,28) = 0.32, p = 0.85), as shown in Table 2. Reaction times to the footshock and sound cue were measured in the first trials, before they could be affected by learning.
Table 2.
Effect of PPTg and/or SNc lesion on reaction time to the footshock and sound cue
| Latency (s) | ||
|---|---|---|
| Footshock | Sound cue | |
|
|
||
| Sham | 1.4 ± 0.4 | 2.3 ± 0.3 |
| PPTg unilateral | 2.5 ± 0.6 | 2.7 ± 0.7 |
| SNc unilateral | 2.0 ± 0.4 | 2.9 ± 0.5 |
| PPTg-SNc ipsilateral | 1.7 ± 0.5 | 2.3 ± 0.7 |
| PPTg-SNc contralateral | 2.9 ± 0.7 | 3.0 ± 0.6 |
Data are expressed as the mean ± SEM. A one-way ANOVA showed no significant differences among the groups.
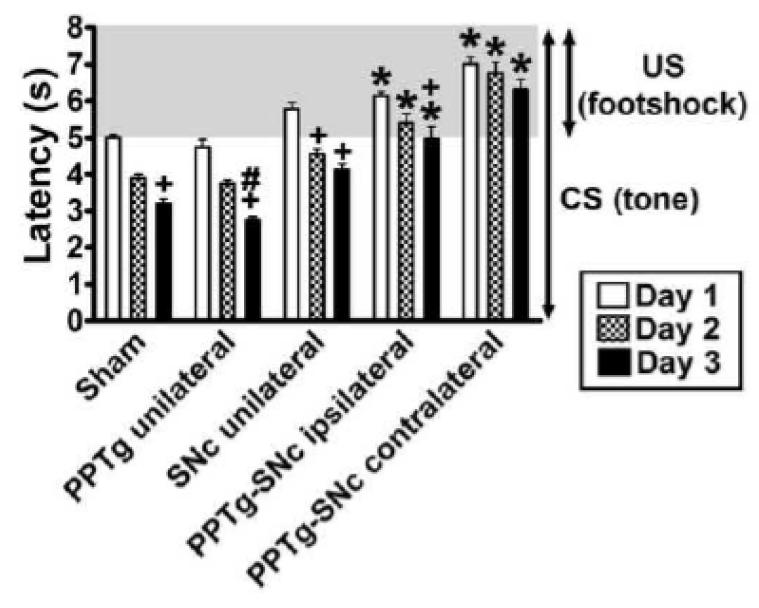
As can be seen in Fig. 6, different to the other groups, the PPTg-SNc ipsilateral rats, did not improve their latencies to respond to the CS (sound cue) across training days. In addition to this, the PPTg-SNc contralateral rats showed no sign of learning, even in the responding to the footshock (US). A two-way ANOVA of the mean latencies during the 3 days of training showed significant effects of treatment (F(4,30) = 7.00, p < 0.001), day of training (F(2,60) = 37.52, p < 0.001) and an interaction (F(8,60) = 0.97, p = 0.46). Post-hoc analysis is detailed in Fig. 6. Fig. S1 shows how the different groups improved their latency to respond to the US and CS in the first day of training.
Fig. 6.
Effect of the PPTg and/or SNc lesion on latency to respond to the CS and US during learning of a 2-way active avoidance response. Bars represent the mean ± SEM latencies to cross to the opposite side of the shuttle box after the CS-US onset averaged by day. * P < 0.05 compared to sham rats in the same day; + P < 0.05 compared to the same group on day 1; # P ≤ 0.05 compared to the same group on day 2; two-way ANOVA, followed by the Newman-Keuls test.
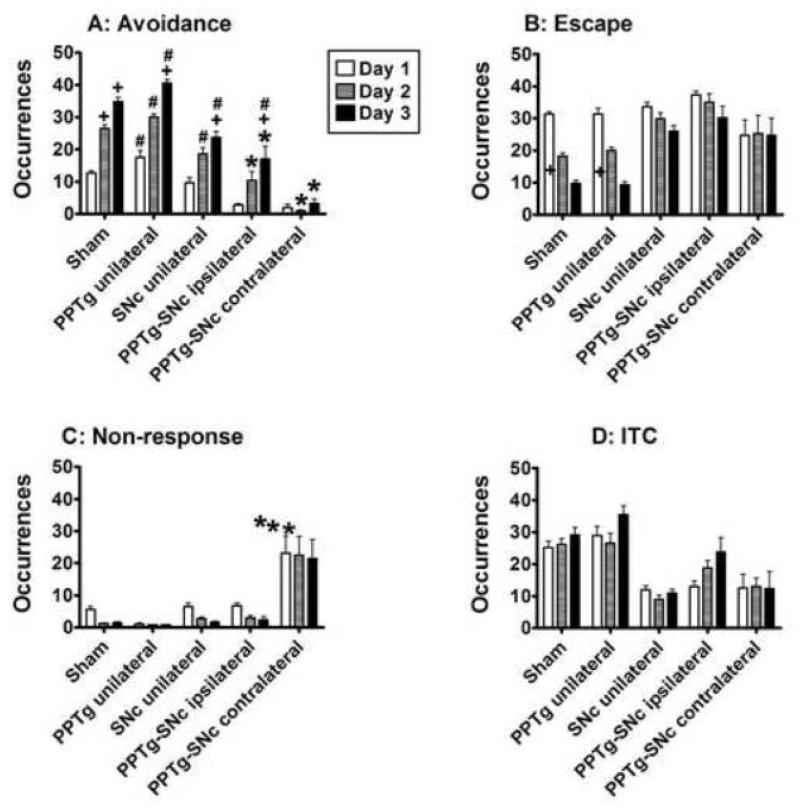
Similar conclusions were taken from analysis of the number of responses during the 2-way active avoidance training (Fig. 7 and Fig. S2). As can be seen in Table 3, 2-way ANOVA of the data averaged by day showed significant treatment effects on avoidance (PPTg-SNc ipsilateral and contralateral) and non-response scores (PPTg-SNc contralateral). These analyses also showed a significant session (training day) effects on avoidance, escape, and non-response scores but not a significant interaction between these 2 factors on all scores.
Fig. 7.
Effect of the PPTg and/or SNc lesion on learning of the 2-way active avoidance. The bars represent the mean ± SEM number of avoidance (A), escape (B), non-response (C) or inter-trial crossings (ITC, D) averaged by day; * P < 0.05 compared to sham in the same day; + P < 0.05 compared to the same group on day 1 ; # P ≤ 0.05 compared to the PPTg-SNc contralateral group in the same day; two-way ANOVA, followed by the Newman-Keuls test.
Table 3.
ANOVA statistics of the active avoidance scores
| Lesion | Session | Interaction | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| F (4,30) | P | F (2,60) | P | F (8,60) | P | |
| Avoidance | 5.57 | 0.05 | 28.27 | 0.001 | 1.79 | 0.09 |
| Escape | 2.31 | 0.08 | 12.63 | 0.001 | 1.91 | 0.07 |
| ITC | 1.94 | 0.12 | 1.16 | 0.31 | 0.35 | 0.93 |
| Non-response | 5.51 | 0.05 | 5.50 | 0.05 | 0.25 | 0.97 |
Rats bearing unilateral and combined (ipsilateral or contralateral) lesions in the PPTg and/or the SNc. Because no significant difference was found between rats bearing left or right lesions in one of the structures, data were combined into a single group. Avoidance, escape, ITC and non-response scores were analyzed by two-way ANOVA with repeated measures (training days).
Post-hoc analysis of the number of avoidances showed same pattern of improvement observed for the latencies to respond to the CS or US: the PPTg-SNc contralateral group was significantly worse than all the other groups and was the only one that did not improve this score. Although the PPTg-SNc ipsilateral rats improved their avoidances scores, they scored significantly worse than the sham rats. On the other hand, the PPTg and SNc unilateral rats were not significantly different of the sham rats (Fig. 7A). Analysis of the number of escapes also supports this conclusion because only the sham and PPTg unilateral rats significantly improved across training days (Fig. 7B). The ITC scores did not significantly differ among the groups (Fig. 7C) and the PPTg-SNc contralateral group was the only one to present a significantly higher number of non-responses (Fig. 7D). This might suggest that instead of a learning deficit, they were impaired in responding to the CS and US. However, correlation between the scores of avoidance and non-response for this group was weak (Table S1; see also Tables S2 and S3 for the correlations between the other variables). In addition, a three-way ANOVA of avoidance scores, taking the number of non-responses in the first day as a covariate showed that, independent of presenting a higher number of non-responses, the PPTg-SNc contralateral rats were impaired to learn this avoidance task (lesion factor: F(4,29) = 3.50, p < 0.05; session day factor: F(2,60) = 28.27, p < 0.001; interaction: F(8,60) = 1.79, p = 0.01; p < 0.01 Newman-Keuls pos hoc test).
Discussion
We have shown that combined unilateral lesions of the PPTg and SNc in opposite hemispheres impeded learning of the 2-way active avoidance task, but ipsilateral lesions in these two structures caused only a mild impairment, and unilateral lesion in just one of them caused no impairment at all. This strongly suggests that the interaction between these structures is necessary for CAR learning. This is not to argue that both PPTg and SNc have the same functional properties: striatal DA is depleted by the SNc lesions but not PPTg; and the rotational response to apomorphine differs after PPTg and SNc unilateral lesions. What the data do indicate though is that the two structures are operating within the same functional domain, and that combined contralateral lesions cause a profound impairment of learning without affecting reaction time measures of motor performance. This impairment of the PPTg-SNc contralateral lesioned rats shows that ipsilateral connections between the PPTg and SNc are critical for learning and that a contralateral connection is not sufficient to support learning.
These results are in agreement with previous studies showing interconnections between the PPTg and SNc (Beninato and Spencer, 1987; Clarke et al., 1987; Gould et al., 1989; Woolf and Butcher, 1986) are predominantly ipsilateral (Beninato and Spencer, 1987; Clarke et al., 1987; Gould et al., 1989; Mena-Segovia et al., 2008 for review). Other studies showed that the PPTg can modulate SNc activity by the release of ACh and glutamate (Di Giovanni and Shi, 2009; Futami, Takakusaki, and Kitai, 1995; Gould et al., 1989; Scarnati, Campana, and Pacitti, 1984; Scarnati, Proia, Campana, and Pacitti, 1986). Some authors hypothesize that, by this connection, the PPTg triggers the reward prediction error signal generated by midbrain DA neurons, a signal that is critical for learning of actions that result in a reward (Kobayashi and Okada, 2007; Mena-Segovia et al., 2008; Pan and Hyland, 2005). The mechanism for avoidance learning by a phasic release of DA is discussed below. There is also evidence of modulation of the PPTg by SNc neurons (Saavedra, Brownstein, Kizer, and Palkovits, 1976; Versteeg, Van Der Gugten, De Jong, and Palkovits, 1976). Support for a functional interaction between the PPTg and the SNc also comes from studies showing both hyperactivity (Carlson, Pearlstein, Buchholz, Iacono, and Maeda, 1999; Orieux, Francois, Feger, Yelnik, Vila, Ruberg, Agid, and Hirsch, 2000) and hypoactivity of PPTg neurons in animal models of Parkinson’s disease (Aravamuthan, Bergstrom, French, Taylor, Parr-Brownlie, and Walters, 2008; Gomez-Gallego, Fernandez-Villalba, Fernandez-Barreiro, and Herrero, 2007).
An ipsilateral projection from the PPTg to the SNc seems to be important also to the initiation of the avoidance response because the PPTg-SNc contralateral group was the only one to present a higher number of non-responses to the CS and US. This suggests that, much as with Parkinson’s disease patients (Grahn, Parkinson, and Owen, 2009a), they had difficulty in choosing and/or initiating proper actions. However, they were not impaired in performing all of the actions of their repertory: they were not impaired to move through the shuttle box in the inter-trials intervals, nor did they present slower responses to the CS or to the US. They simply failed to initiate such responses in the proper time as frequently as the others. Such an effect probably resulted from a deficient DA tone to stimulate D1 receptors in the striatal neurons of the direct pathway, responsible for disinhibition a specific action that was previously reinforced (Alexander, Delong, and Strick, 1986; Da Cunha et al., 2009).
Such avoidance failure might have affected learning, because they had fewer occasions to be reinforced, but the correlation between the number of avoidances and non-responses was weak and barely significant for the PPTg-SNc contralateral group. Therefore, it seems that, in addition to impairment in initiating the proper response, they were also impaired to learn this CAR task, since their avoidance score was significantly lower (even discounting the impact of the non-responses on it). In other words, PPTg-SNc contralateral rats did not learn from their experience, even in the few occasions when they were reinforced for presenting the avoidance response and in the many occasions when they were punished for not presenting the avoidance response. This probably resulted from a failure in presenting a phasic DA response in these occasions, as discussed below.
In the present study we observed that rats bearing unilateral lesions in the PPTg or SNc could learn the 2-way active avoidance task as well as the controls. This does not mean that learning deficits can be caused only by a lesion in both structures. The rats used in the present study had only partial and unilateral lesions in the PPTg or SNc. Indeed, rats bearing almost total unilateral loss of SNc DA neurons presented a deficit to learn this task (data not shown), but they were excluded from this study because our purpose was not testing the effect of the lesion of the PPTg or SNc per se, but of the disconnection between them.
There is compelling evidence that the PPTg is important for procedural learning. This includes the studies showing that bilateral electrolytic (Satorra-Marin, Coll-Andreau, Portell-Cortes, Aldavert-Vera, and Morgado-Bernal, 2001) or excitotoxic lesions of the PPTg (Fujimoto et al., 1992; 1989; Kessler, Markowitsch, and Sigg, 1986) impair the ability of rats to learn active and inhibitory avoidance tasks, and that interference with the PPTg affects the ability to learn bar pressing or maze tasks motivated by either natural and drug rewards (Alderson, Brown, Latimer, Brasted, Robertson, and Winn, 2002; Alderson, Latimer, Blaha, Phillips, and Winn, 2004; Alderson and Winn, 2005; Corrigall, Coen, Zhang, and Adamson, 2001; Corrigall, Coen, Zhang, and Adamson, 2002; Inglis, Dunbar, and Winn, 1994; Inglis, Olmstead, and Robbins, 2000; Keating and Winn, 2002; Olmstead and Franklin, 1994; Olmstead, Robbins, and Everitt, 1998; Taylor, Kozak, Latimer, and Winn, 2004).
Likewise, there is extensive evidence that the SNc is critical for procedural learning. Rats bearing partial bilateral lesions of the SNc were impaired in learning 2-way active avoidance (Da Cunha, et al., 2001; Gevaerd et al., 2001; Gevaerd, Takahashi, Silveira, and Da Cunha, 2001), inhibitory avoidance (Del Guante, Rivas, Prado-Alcala, and Quirarte, 2004), the cued version of the Morris water maze (Da Cunha, Wietzikoski, Wietzikoski, Miyoshi, Ferro, Anselmo-Franci, and Canteras, 2003; Da Cunha, Wietzikoski, Wietzikoski, Silva, Chandler, Ferro, Andreatini, and Canteras, 2007; Ferro, Bellissimo, Anselmo-Franci, Angellucci, Canteras, and Da Cunha, 2005; Miyoshi, Wietzikoski, Camplessei, Silveira, Takahashi, and Da Cunha, 2002) and bar pressing for appetitive reward (Faure, Haberland, Conde, and El Massioui, 2005). Learning deficits have also been observed in mouse and monkey models of Parkinson’s disease, as well as in Parkinson’s disease patients (Fernandez-Ruiz, Wang, Aigner, and Mishkin, 2001; Grahn, Parkinson, and Owen, 2009b; Hood, Postle, and Corkin, 1999; Kimura, 1995; Knowlton, Squire, Paulsen, Swerdlow, Swenson, and Butters, 1996; Roncacci, Troisi, Carlesimo, Nocentini, and Caltagirone, 1996; Salmon and Butters, 1995). In addition, CAR learning is particularly sensitive to dopamine receptor antagonists (Aguilar et al., 2000; Ogren and Archer, 1994).
In line with the present study, there is strong support for the hypothesis that the PPTg and SNc are part of a brainstem system for coordinating responses to aversive unconditioned and conditioned stimuli. Both the PPTg and the SNc receive inputs from the RMTg, a GABAergic nucleus recently identified as a core structure in this system. The RMTg is activated by many aversive stimuli, including footshock (Jhou, Fields, Baxter, Saper, and Holland, 2009). This structure has been suggested to integrate inputs from many structures that process aversive stimuli, like the lateral habenula (LHb), central nucleus of the amygdala, bed nucleus of the stria terminalis, ventral periaqueductal gray matter (PAG), and lateral septum (Jhou et al., 2009; Sesack and Grace, 2010). The lesion of most of these structures also impairs active and passive fear responses (Schulz and Canbeyli, 1999; Sparks and Ledoux, 1995; Treit and Menard, 1997). When the RMTg and the LHb are activated by aversive stimuli they inhibit the release of DA by the midbrain neurons that are activated by rewarding conditioned and unconditioned stimuli and by the omission of an expected rewarding stimulus (Jhou et al., 2009). On the other hand, RMTg and LHb neurons are inhibited by rewarding CS or US (Jhou et al., 2009; Matsumoto and Hikosaka, 2009a). By this way these structures may lead the DA neurons to reduce their firing, providing a negative prediction error to the striatum. However, it is not clear how all these structures contributes to the generation of prediction error signals by DA neurons.
For many years the electrophysiological studies have only shown midbrain DA neurons that decreased their firing in response to mild aversive stimuli, in the manner described above (Morris, Schmidt, and Bergman, 2010; Redgrave, Gurney, and Reynolds, 2008; Schultz, 2007). However, recent studies reported that a subpopulation of midbrain DA neurons present a phasic activation in response to aversive conditioned and unconditioned stimuli (Brischoux et al., 2009; Joshua, Adler, Mitelman, Vaadia, and Bergman, 2008; Matsumoto and Hikosaka, 2009b). A recent review by Morris and co-workers (2010) argument that this finding poses a serious problem to explain how a punished action can be reduced, since phasic DA that follows the aversive stimulus would reinforce the punished action. This problem does not exist for learning of the 2-way active avoidance task if it is assumed the phasic DA reinforces an action that leads to halting or avoiding an aversive event. Therefore, we can say that the results of the present study are in agreement with the role suggested for the phasic release of DA in reinforcement learning.
The activation of DA receptors reinforces corticostriatal synapses between neurons that are active at the same time (Hebbian long-term potentiation) and weakens synapses between non-active corticostriatal neurons (Hebbian long-term depression, see Calabresi, Picconi, Tozzi, and Di Filippo, 2007; Calabresi, Pisani, Mercuri, and Bernardi, 1992; Wickens, 2009). This mechanism increases the likelihood that a stimulus (CS) paired with reward (US) can trigger the action performed to get it (Da Cunha et al., 2009). A similar mechanism may meditate avoidance learning: the likelihood of performing the action that halted an aversive US or halted the warning CS may increase with training because of phasic release of DA. The failure of the SNc-PPTg contralateral rats to learn this kind of conditional avoidance supports this explanation and suggests that the phasic response of midbrain DA neurons to aversive stimuli may be under the control of the PPTg, as proposed by Kobayashi & Okada (2007).
In conclusion, the findings of the present study give some clue about the architecture of PPTg-SNc connections and its functional relevance in the acquisition and expression of conditioned avoidance responses. The memory of how to avoid an aversive event is a kind of procedural memory – the memory of how to choose the proper action that results in getting a reward or avoiding an aversive outcome. Such knowledge is critical for improving treatments of diseases in which the basal ganglia fail to learn and/or initiate proper actions. This includes neurological diseases such as Parkinson’s and Huntington’s and psychiatric diseases such as schizophrenia and attention-deficit hyperactivity disorder. The first interventions in Parkinson’s disease patients with deep brain stimulation of the PPTg (Mazzone, Lozano, Stanzione, Galati, Scarnati, Peppe, and Stefani, 2005; Pierantozzi, Palmieri, Galati, Stanzione, Peppe, Tropepi, Brusa, Pisani, Moschella, Marciani, Mazzone, and Stefani, 2008; Stefani, Lozano, Peppe, Stanzione, Galati, Tropepi, Pierantozzi, Brusa, Scarnati, and Mazzone, 2007) stress the urgency of this knowledge in order to get the best from this kind of therapy and avoid possible cognitive side effects.
Supplementary Material
Acknowledgments
This study was supported by grants to CDC from the CNPq and CAPES, and by Wellcome Trust project grant 081128/Z/06/Z to PW, which supports Duncan A.A. MacLaren. The authors thank Edmar Miyoshi, Silvia N. Cordazzo, and Lindacir do Rocio Nascimento for their technical assistance.
References
- Aguilar MA, Mari-Sanmillan MI, Morant-Deusa JJ, Minarro J. Different inhibition of conditioned avoidance response by clozapine and DA D-1 and D-2 antagonists in male mice. Behavioral Neuroscience. 2000;114:389–400. [PubMed] [Google Scholar]
- Alderson HL, Winn P. The pedunculopontine and reinforcement. Springer; New York: 2005. [Google Scholar]
- Alderson HL, Brown VJ, Latimer MP, Brasted PJ, Robertson AH, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus in rats: an examination of the perception of reward strength measured by responding on a progressive ratio schedule of reinforcement. Neuroscience. 2002;112:417–425. doi: 10.1016/s0306-4522(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P. An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience. 2004;125:349–358. doi: 10.1016/j.neuroscience.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aravamuthan BR, Bergstrom DA, French RA, Taylor JJ, Parr-Brownlie LC, Walters JR. Altered neuronal activity relationships between the pedunculopontine nucleus and motor cortex in a rodent model of Parkinson’s disease. Experimental Neurology. 2008;213:268–280. doi: 10.1016/j.expneurol.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behavioural Brain Research. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Beninato M, Spencer RF. A cholinergic projection to the rat substantianigra from the pedunculopontine tegmental nucleus. Brain Research. 1987;412:169–174. doi: 10.1016/0006-8993(87)91455-7. [DOI] [PubMed] [Google Scholar]
- Beninato M, Spencer RF. The Cholinergic Innervation of the Rat Substantia Nigra - a Light and Electron-Microscopic Immunohistochemical Study. Experimental Brain Research. 1988;72:178–184. doi: 10.1007/BF00248513. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4893–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends in Neurosciences. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. European Journal of Neuroscience. 1992;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Carbon M, Marie RM. Functional imaging of cognition in Parkinson’s disease. Current Opinion in Neurology. 2003;16:475–480. doi: 10.1097/01.wco.0000084225.82329.3c. [DOI] [PubMed] [Google Scholar]
- Carlson JD, Pearlstein RD, Buchholz J, Iacono RP, Maeda G. Regional metabolic changes in the pedunculopontine nucleus of unilateral 6-hydroxydopamine Parkinson’s model rats. Brain Research. 1999;828:12–19. doi: 10.1016/s0006-8993(99)01268-8. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Hommer DW, Pert A, Skirboll LR. Innervation of substantia nigra neurons by cholinergic afferents from pedunculopontine nucleus in the rat - neuroanatomical and electrophysiological evidence. Neuroscience. 1987;23:1011–1019. doi: 10.1016/0306-4522(87)90176-x. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behavioural Brain Research. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology. 2001;158:189–197. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology. 2002;160:198–205. doi: 10.1007/s00213-001-0965-2. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Gevaerd MS, Vital M, Miyoshi E, Andreatini R, Silveira R, Takahashi RN, Canteras NS. Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson’s disease amnesia. Behavioural Brain Research. 2001;124:9–18. doi: 10.1016/s0166-4328(01)00211-x. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Gevaerd MS, Vital M, Miyoshi E, Andreatini R, Silveira R, Takahashi RN, Canteras NS. Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson’s disease amnesia. Behavioural Brain Research. 2001;124:9–18. doi: 10.1016/s0166-4328(01)00211-x. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wietzikoski EC, Dombrowski P, Santos LM, Bortolanza M, Boschen SL, Miyoshi E. Learning processing in the basal ganglia: A mosaic of broken mirrors. Behavioural Brain Research. 2009;199:156–169. doi: 10.1016/j.bbr.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wietzikoski EC, Ferro MM, Martinez GR, Vital M, Hipolide D, Tufik S, Canteras NS. Hemiparkinsonian rats rotate toward the side with the weaker dopaminergic neurotransmission. Behavioural Brain Research. 2008;189:364–372. doi: 10.1016/j.bbr.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wietzikoski S, Wietzikoski EC, Miyoshi E, Ferro MM, Anselmo-Franci JA, Canteras NS. Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system. Neurobiology of Learning and Memory. 2003;79:236–242. doi: 10.1016/s1074-7427(03)00008-x. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wietzikoski S, Wietzikoski EC, Silva MHC, Chandler J, Ferro MM, Andreatini R, Canteras NS. Pre-training to find a hidden platform in the Morris water maze can compensate for a deficit to find a cued platform in a rat model of Parkinson’s disease. Neurobiology of Learning and Memory. 2007;87:451–463. doi: 10.1016/j.nlm.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Del Guante MAD, Rivas M, Prado-Alcala RA, Quirarte GL. Amnesia produced by pre-training infusion of serotonin into the substantia nigra. Neuroreport. 2004;15:2527–2529. doi: 10.1097/00001756-200411150-00019. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Shi WX. Effects of Scopolamine on Dopamine Neurons in the Substantia Nigra: Role of the Pedunculopontine Tegmental Nucleus. Synapse. 2009;63:673–680. doi: 10.1002/syn.20650. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. Journal of Neurology. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. Journal of Neuroscience. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wang J, Aigner TG, Mishkin M. Visual habit formation in monkeys with neurotoxic lesions of the ventrocaudal neostriatum. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4196–4201. doi: 10.1073/pnas.061022098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci MEM, Canteras NS, Da Cunha C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: Histological, neurochemical, motor and memory alterations. Journal of Neuroscience Methods. 2005;148:78–87. doi: 10.1016/j.jneumeth.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Ikeguchi K, Yoshida M. Impaired Acquisition, Preserved Retention and Retrieval of Avoidance-Behavior after Destruction of Pedunculopontine Nucleus Areas in the Rat. Neuroscience Research. 1992;13:43–51. doi: 10.1016/0168-0102(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Yoshida M, Ikeguchi K, Niijima K. Impairment of Active-Avoidance Produced after Destruction of Pedunculopontine Nucleus Areas in the Rat. Neuroscience Research. 1989;6:321–328. doi: 10.1016/0168-0102(89)90024-2. [DOI] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and Cholinergic Inputs from the Pedunculopontine Tegmental Nucleus to Dopamine Neurons in the Substantia-Nigra Pars Compacta. Neuroscience Research. 1995;21:331–342. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- Gevaerd MS, Miyoshi E, Silveira R, Canteras NS, Takahashi RN, Da Cunha C. L-dopa restores striatal dopamine level but fails m to reverse MPTP-induced memory deficits in rats. International Journal of Neuropsycopharmacology. 2001;4:361–370. doi: 10.1017/S1461145701002619. [DOI] [PubMed] [Google Scholar]
- Gevaerd MS, Takahashi RN, Silveira R, Da Cunha C. Caffeine reverses the memory disruption induced by intra-nigral MPTP-injection in rats. Brain Research Bulletin. 2001;55:101–106. doi: 10.1016/s0361-9230(01)00501-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Gallego M, Fernandez-Villalba E, Fernandez-Barreiro A, Herrero MT. Changes in the neuronal activity in the pedunculopontine nucleus in chronic MPTP-treated primates: an in situ hybridization study of cytochrome oxidase subunit I, choline acetyl transferase and substance P mRNA expression. Journal of Neural Transmission. 2007;114:319–326. doi: 10.1007/s00702-006-0547-x. [DOI] [PubMed] [Google Scholar]
- Góngora-Alfaro JL, Hernandez-Lopez S, Martinez-Fong D, Flores G, Aceves J. Circling behavior elicited by cholinergic transmission in the substantia nigra pars compacta: Involvement of nicotinic and muscarinic receptors. Neuroscience. 1996;71:729–734. doi: 10.1016/0306-4522(95)00485-8. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolf NJ, Butcher LL. Cholinergic Projections to the Substantia Nigra from the Pedunculopontine and Laterodorsal Tegmental Nuclei. Neuroscience. 1989;28:611–623. doi: 10.1016/0306-4522(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav Brain Res. 2009a;199:53–60. doi: 10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: neuropsychological studies. Behavioural Brain Research. 2009b;199:53–60. doi: 10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Harik SI, Schmidley JW, Iacofano LA, Blue P, Arora PK, Sayre LM. On the mechanisms underlying 1-methyl-4phenyl-1,2,3,6-tetrahydropiridine neurotoxicity: the effect of perinigral infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropiridine, its metabolite and their analogs in rat. Journal of Pharmacology and Experimental Therapeutics. 1987;241:669–676. [PubMed] [Google Scholar]
- Hood KL, Postle BR, Corkin S. An evaluation of the concurrent discrimination task as a measure of habit learning: performance of amnesic subjects. Neuropsychologia. 1999;37:1375–1386. doi: 10.1016/s0028-3932(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Stimulus-response and response-outcome learning mechanisms in the striatum. Behavioural Brain Research. 2009;199:129–140. doi: 10.1016/j.bbr.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Akiyama G, Matsuzaki S, Sato M, Moribe S, Koshikawa N, Cools AR. Role of GABA(A) receptors in the pedunculopontine tegmental nucleus in rat turning behavior. International Journal of Neuropsychopharmacology. 2004;7:S289–S289. [Google Scholar]
- Inglis WL, Dunbar JS, Winn P. Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and the acquisition of responding for conditioned reinforcement stimulated by amphetamine. Neuroscience. 1994;62:51–64. doi: 10.1016/0306-4522(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Olmstead MC, Robbins TW. Pedunculopontine tegmental nucleus lesions impair stimulus-reward learning in autoshaping and conditioned reinforcement paradigms. Behavioral Neuroscience. 2000;114:285–294. doi: 10.1037//0735-7044.114.2.285. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Da Silva WC, Medina JH, Cammarota M. The connection between the hippocampal and the striatal memory systems of the brain: a review of recent findings. Neurotoxicity Research. 2006;10:113–121. doi: 10.1007/BF03033240. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The Rostromedial Tegmental Nucleus (RMTg), a GABAergic Afferent to Midbrain Dopamine Neurons, Encodes Aversive Stimuli and Inhibits Motor Responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditionin trials. Journal of Neuroscience. 2008;28:11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juri C, Chana P. Levodopa for Parkinson’s disease. What have we learned?. Revista Medica De Chile. 2006;134:893–901. doi: 10.4067/s0034-98872006000700014. [DOI] [PubMed] [Google Scholar]
- Keating GL, Winn P. Examination of the role of the pedunculopontine tegmental nucleus in radial maze tasks with or without a delay. Neuroscience. 2002;112:687–696. doi: 10.1016/s0306-4522(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Kessler J, Markowitsch HJ, Sigg G. Memory related role of the posterior cholinergic system. International Journal of Neuroscience. 1986;30:101–119. doi: 10.3109/00207458608985660. [DOI] [PubMed] [Google Scholar]
- Kimura M. Role of basal ganglia in behavioral learning. Neuroscience Research. 1995;22:353–358. doi: 10.1016/0168-0102(95)00914-f. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Paulsen JS, Swerdlow NR, Swenson M, Butters N. Dissociations within nondeclarative memory in Huntington’s disease. Neuropsychology. 1996;10:538–548. [Google Scholar]
- Kobayashi Y, Okada KC. Reward prediction error computation in the pedunculopontine tegmental nucleus neurons. Reward and Decision Making in Corticobasal Ganglia Networks. 2007;1104:310–323. doi: 10.1196/annals.1390.003. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kaundal RK, More S, Sharma SS. Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behavioural Brain Research. 2009;197:398–403. doi: 10.1016/j.bbr.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:838–842. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nature Neuroscience. 2009a;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009b;459:838–842. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport. 2005;16:1877–1881. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia P, Winn P, Bolam JP. Cholinergic modulation of midbrain dopaminergic systems. Brain Research Reviews. 2008;58:265–271. doi: 10.1016/j.brainresrev.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da Cunha C. Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Research Bulletin. 2002;58:41–47. doi: 10.1016/s0361-9230(02)00754-2. [DOI] [PubMed] [Google Scholar]
- Morris G, Schmidt R, Bergman H. Striatal action-learning based on dopamine concentration. Experimental Brain Research. 2010;200:307–317. doi: 10.1007/s00221-009-2060-6. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Archer T. Effects of Typical and Atypical Antipsychotic-Drugs on 2-Way Active-Avoidance - Relationship to DA Receptor Blocking Profile. Psychopharmacology. 1994;114:383–391. doi: 10.1007/BF02249327. [DOI] [PubMed] [Google Scholar]
- Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y. Different Pedunculopontine Tegmental Neurons Signal Predicted and Actual Task Rewards. Journal of Neuroscience. 2009;29:4858–4870. doi: 10.1523/JNEUROSCI.4415-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin K,B,J. Lesions of the pedunculopontine tegmental nucleus block drug-induced reinforcement but not amphetamine-induced locomotion. Brain Research. 1994;638:29–35. doi: 10.1016/0006-8993(94)90629-7. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Robbins TW, Everitt BJ. Basal forebrain cholinergic lesions enhance conditioned approach responses to stimuli predictive of food. Behavioral Neuroscience. 1998;112:611–629. doi: 10.1037//0735-7044.112.3.611. [DOI] [PubMed] [Google Scholar]
- Orieux G, Francois C, Feger J, Yelnik J, Vila M, Ruberg M, Agid Y, Hirsch EC. Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience. 2000;97:79–88. doi: 10.1016/s0306-4522(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behavioural Neuroscience. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. Journal of Neuroscience. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. European Journal of Neuroscience. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press, Ltd; San Diego, CA, USA: 2005. [Google Scholar]
- Pierantozzi M, Palmieri MG, Galati S, Stanzione P, Peppe A, Tropepi D, Brusa L, Pisani A, Moschella V, Marciani MG, Mazzone P, Stefani A. Pedunculopontine nucleus deep brain stimulation changes spinal cord excitability in Parkinson’s disease patients. Journal of Neural Transmission. 2008;115:731–735. doi: 10.1007/s00702-007-0001-8. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Research Reviews. 2008;58:322–339. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Roncacci S, Troisi E, Carlesimo GA, Nocentini U, Caltagirone C. Implicit memory in Parkinsonian patients: Evidence for deficient skill learning. European Neurology. 1996;36:154–159. doi: 10.1159/000117234. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Brownstein MJ, Kizer JS, Palkovits M. Biogenic amines and related enzymes in the circumventricular organs of the rat. Brain Research. 1976;107:412–417. doi: 10.1016/0006-8993(76)90238-9. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N. Neurobiology of skill and habit learning. Current Opinion in Neurobiology. 1995;5:184–190. doi: 10.1016/0959-4388(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Satorra-Marin N, Coll-Andreau M, Portell-Cortes I, Aldavert-Vera L, Morgado-Bernal I. Impairment of two-way active avoidance after pedunculopontine tegmental nucleus lesions: effects of conditioned stimulus duration. Behavioural Brain Research. 2001;118:1–9. doi: 10.1016/s0166-4328(00)00306-5. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Campana E, Pacitti C. Pedunculopontine-Evoked Excitation of Substantia Nigra Neurons in the Rat. Brain Research. 1984;304:351–361. doi: 10.1016/0006-8993(84)90339-1. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Proia A, Campana E, Pacitti C. A Microiontophoretic Study on the Nature of the Putative Synaptic Neurotransmitter Involved in the Pedunculopontine-Substantia Nigra Pars Compacta Excitatory Pathway of the Rat. Experimental Brain Research. 1986;62:470–478. doi: 10.1007/BF00236025. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. (vol 80, pg 1, 1998) Journal of Neurophysiology. 1998;80:U32–U32. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annual Review of Neuroscience. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schulz D, Canbeyli R. Freezing behavior in BNST-lesioned Wistar rats. In: McGinty JF, editor. Advancing from the Ventral Striatum to the Extended Amygdala - Implications for Neuropsychiatry and Drug Abuse: in Honor of Lennart Heimer. 1999. pp. 728–731. [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks PD, Ledoux JE. Septal-lesions potentiate freezing behavior to contextual but not to phasic conditioned-stimuli in rats. Behavioral Neuroscience. 1995;109:184–188. doi: 10.1037//0735-7044.109.1.184. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Sy HN, Wu SL, Wang WF, Chen CH, Huang YT, Liou YM, Chiou CS, Pawlak CR, Ho YJ. MPTP-induced dopaminergic degeneration and deficits in object recognition in rats are accompanied by neuroinflammation in the hippocampus. Pharmacology Biochemistry and Behavior. 95:158–165. doi: 10.1016/j.pbb.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Taylor CL, Kozak R, Latimer MP, Winn P. Effects of changing reward on performance of the delayed spatial win-shift radial maze task in pedunculopontine tegmental nucleus lesioned rats. Behavioural Brain Research. 2004;153:431–438. doi: 10.1016/j.bbr.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Timar J, Knoll B, Jona G, Knoll J. Inhibition of acquisition of conditioned avoidance responses by substantia nigra lesion. Naunyn-Schmiedebergs Archives of Pharmacology. 1974;284:R83–R83. [Google Scholar]
- Treit D, Menard J. Dissociations among the anxiolytic effects of septal, hippocampal, and amygdaloid lesions. Behavioral Neuroscience. 1997;111:653–658. doi: 10.1037//0735-7044.111.3.653. [DOI] [PubMed] [Google Scholar]
- Versteeg DH, Van Der Gugten J, De Jong W, Palkovits M. Regional concentrations of noradrenaline and dopamine in rat brain. Brain Research. 1976;113:563–574. doi: 10.1016/0006-8993(76)90057-3. [DOI] [PubMed] [Google Scholar]
- Wang AL, Liou YM, Pawlak CR, Ho YJ. Involvement of NMDA receptors in both MPTP-induced neuroinflammation and deficits in episodic-like memory in Wistar rats. Behavioural Brain Research. 208:38–46. doi: 10.1016/j.bbr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- White NM. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behavioural Brain Research. 2009;199:3–23. doi: 10.1016/j.bbr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Wickens JR. Synaptic plasticity in the basal ganglia. Behavioural Brain Research. 2009;199:119–128. doi: 10.1016/j.bbr.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic Systems in the Rat-Brain .3. Projections from the Pontomesencephalic Tegmentum to the Thalamus, Tectum, Basal Ganglia, and Basal Forebrain. Brain Research Bulletin. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Zokoll MA, Klump GM, Langemann U. Auditory memory for temporal characteristics of sound. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2008;194:457–467. doi: 10.1007/s00359-008-0318-2. [DOI] [PubMed] [Google Scholar]
- Zuddas A, Corsini GU, Schinelli S, Johannessen JN, Diporzio U, Kopin IJ. MPTP treatment combined with ethanol or acetaldehyde selectively destroys dopaminergic-neurons in mouse substantia nigra. Brain Research. 1989;501:1–10. doi: 10.1016/0006-8993(89)91020-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.