Abstract
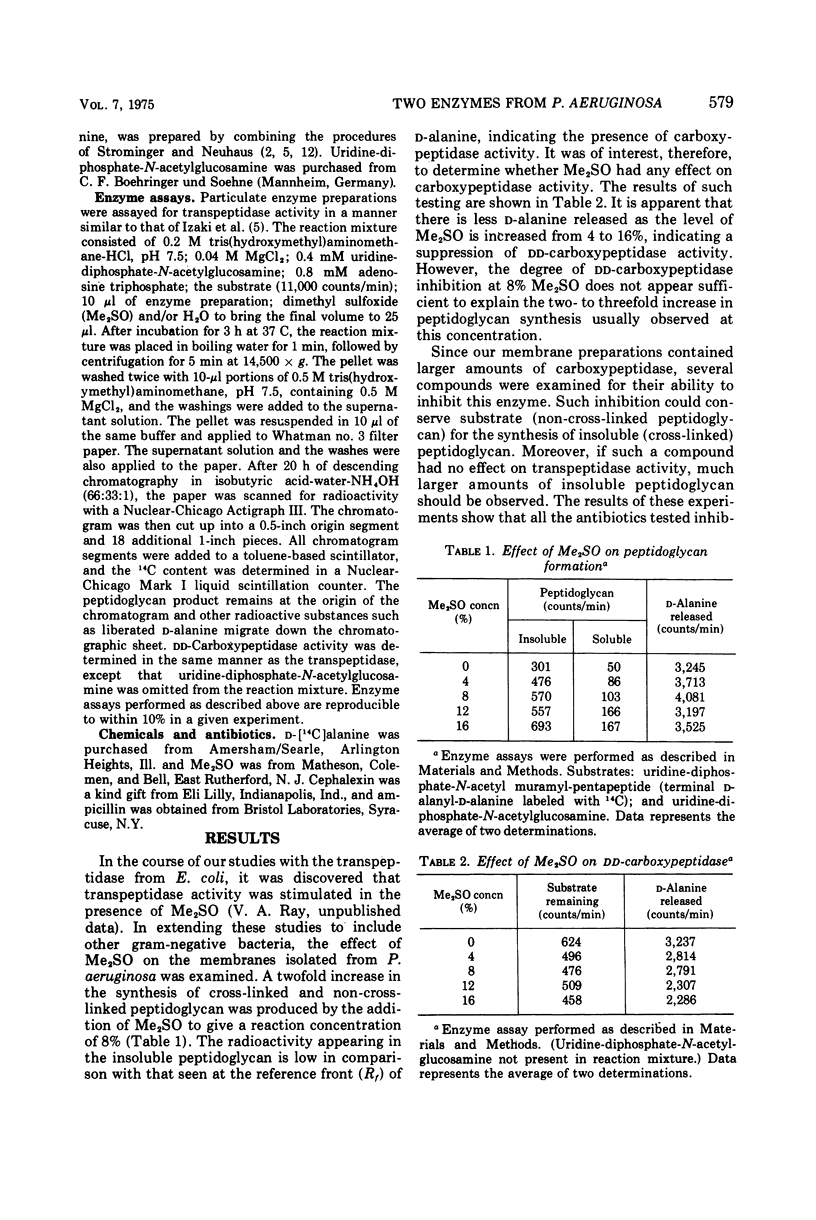
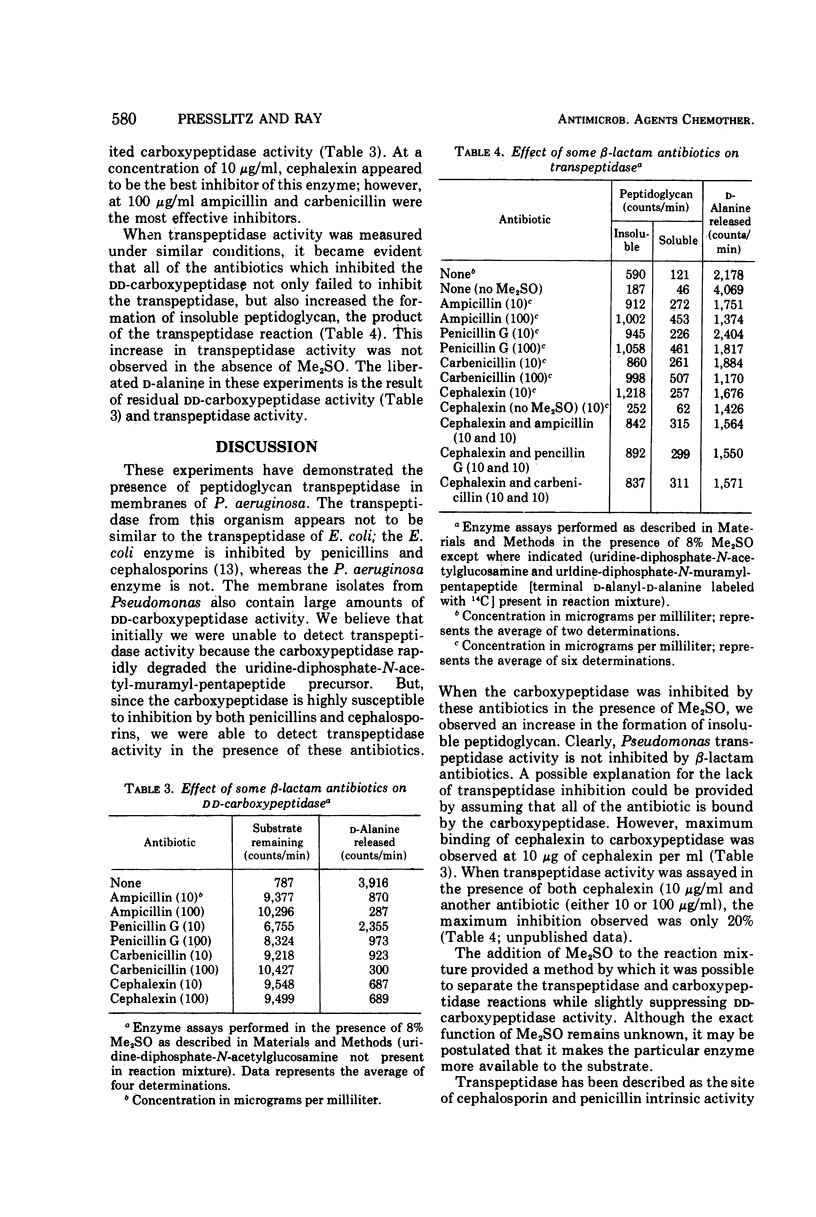
Peptidoglycan transpeptidase and dd-carboxypeptidase have been detected in isolated membranes of Pseudomonas aeruginosa. Cephalosporins and penicillins fail to inhibit the transpeptidase at concentrations as high as 100 μg/ml. dd-Carboxypeptidase, on the other hand, is sensitive to inhibition by β-lactam antibiotics. The presence of dimethyl sulfoxide in the reaction mixture results in a twofold stimulation of peptidoglycan formation, whereas dd-carboxypeptidase is inhibited approximately 30%. Maximum stimulation of transpeptidase occurs in the presence of both dimethyl sulfoxide and a β-lactum antibiotic. This is in sharp contrast to the transpeptidase from Escherichia coli, which is sensitive to inhibition by penicillins and cephalosporins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Shirai R., Shimada A., Ishimoto N., Ito E. Enzymatic synthesis of cell wall mucopeptide in a particulate preparation of Escherichia coli. Biochem Biophys Res Commun. 1966 May 25;23(4):466–472. doi: 10.1016/0006-291x(66)90751-0. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G. The enzymatic addition of D-alanyl-D-alanine to a uridine nucleotide-peptide. J Biol Chem. 1962 May;237:1601–1604. [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIV. Purification and properties of two D-alanine carboxypeptidases from Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3193–3201. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence P. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XVI. The reversible fixation of radioactive penicillin G to the D-alanine carboxypeptidase of Bacillus subtilis. J Biol Chem. 1970 Jul 25;245(14):3660–3666. [PubMed] [Google Scholar]
- Leyh-Bouille M., Coyette J., Ghuysen J. M., Idczak J., Perkins H. R., Nieto M. Penicillin-sensitive DD-carboxypeptidase from Streptomyces strain R 61. Biochemistry. 1971 May 25;10(11):2163–2170. doi: 10.1021/bi00787a032. [DOI] [PubMed] [Google Scholar]
- Leyh-Bouille M., Nakel M., Frère J. M., Johnson K., Ghuysen J. M., Nieto M., Perkins H. R. Penicillin-sensitive DD-carboxypeptidases from Streptomyces strains R39 and K11. Biochemistry. 1972 Mar 28;11(7):1290–1298. doi: 10.1021/bi00757a027. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C., LYNCH J. L. THE ENZYMATIC SYNTHESIS OF D-ALANYL-D-ALANINE. 3. ON THE INHIBITION OF D-ALANYL-D-ALANINE SYNTHETASE BY THE ANTIBIOTIC D-CYCLOSERINE. Biochemistry. 1964 Apr;3:471–480. doi: 10.1021/bi00892a001. [DOI] [PubMed] [Google Scholar]
- Strominger J. L. Penicillin-sensitive enzymatic reactions in bacterial cell wall synthesis. Harvey Lect. 1968 1969;64:179–213. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]



