ABSTRACT
Introduction:
Measurements of extracellular pH show that the micro environment of malignant tumors is more acidic than that of normal cells, whereas pH does not differ appreciable in normal and malignant cells. The acid micro environment of tumors is created by the secretion of tumor factors and ATP hydrolysis in hypoxic tumor tissue. In order to survive in a low pH-environment tumor cells develop regulatory mechanisms which keep their intracellular pH stable. Two of the most important systems are the Na+/H+ ion pump and the Na-dependent HCO3-/Cl- pump of stilbenian derivatives.
Material and methods:
Experiments were carried out on DBA mice of both sexes at the age of 4 month. Laboratory animals were grown in our institute and supplied with food and aqua ad libitum.
Results:
After termination of the experiments the mean tumor diameter in the control group was 12.4±0.8mm, in group A it was 6.9±0.6mm, and in group B we measured 6.6±3.1mm. At the final day the tumor size in treated animals was twice as small as in the control group. In addition we observed the rate of survival. In the control group only 18% of the animals were still alive at day 18. Considering the rate of survival a statistically significant difference between treated and untreated animals was observed. The survival of tumor cells is dependent on the function of these ion pumps which keep their intracellular pH values constant in the setting of an acid extracellular environment.
Conclusion:
The activity of the ion pump is especially important at the beginning of cell division and in cell proliferation. Our in vivo experiments demonstrate that prolonged administration of intratumoral ion pump inhibitors suppresses tumor growth as well as enhances survival of tumor-bearing animals. Research of inhibitors of ion pumps and their action in tumor growth opens new perspectives into pathophysiology of malignant tumors and may create new therapeutic options.
Keywords: Tumor, pH-value, Inhibitors for ion pumps, EIPA, DIDS
1. INTRODUCTION
There is an eminent difference between the micro environment of tumor tissues and normal tissue. Measurements of the extracellular pH showed that the mean pH derived from tumors is o.5 units lower compared to normal tissues. These lower pH-values in tumors are caused by an increase of lactate and the hydrolysis of ATP under hypoxic conditions (1, 2, 3). Interestingly, measurements of intracellular pH (pHi) show no significant difference between tumor cells and normal cells (4).
This indicates that tumor cells establish mechanisms which allow them to keep their pHi constant in a physiologic range, as alterations within intracellular hydrogen concentration would lead to biochemical perturbations and finally to cell death. Recent investigations show that acidification of cells is one of the major events in induction of apoptosis (5, 6). The maintenance of pHi is regulated by the Na+/H+ pump under hypoxic conditions and the Na-dependent HCO3/Cl- pump (6). The Na+/H+ exchanger regulates the concentration of sodium and hydrogen within the cell and contributes actively in cell volume control (7, 8). It is assumed that this ion pump takes part in the initialization of cell growth and proliferation in various cell species. The activation of the Na+/H+ pumps by different mitogens and growth factors, leads to alkalization which is a prerequisite for cell growth and proliferation.
This fact is confirmed by findings of decreased tumor growth in cells with non-functional Na+/H+ pumps (5, 6, 9, 10). Inhibition of the Na+/H+ pump could be achieved by amiloride and its analogues like e.g. 5-N-ethyl-N-isopropyl amiloride (EIPA). The Na-dependent HCO3/Cl- pump could be blocked by stilbestric derivatives as e.g. 4,4’diisathiocyanostiben-2,2’disulphonoc acid (DIDS). Recent in vitro studies feature a significant inhibition of tumor growth after blocking ion pumps (11, 12). The present work shows that inhibition of ion pumps under in vivo conditions leads to a drastic decrease of tumor growth combined with a prolonged rate of survival of laboratory animals.
2. MATERIAL AND METHODS
Laboratory animals
Experiments were carried out on DBA mice of both sexes at the age of 4 month. Laboratory animals were grown in our institute and supplied with food and aqua ad libitum.
Tumor
P815 cells derived from transformed mouse mastocitoma were cultured in modified Eagel’s medium (Gibco Laboratories) supplemented with !=% fetal calf serum (FCS, 50U/ml, polymixin, 5x10-5 mol/L 2-mercaptoethanol, 2.2 g/L sodium bicarbonate, and 15 mmol/L HEPES at 37 °C in a 5% CO2 atmosphere. Suspensions of P815 cells in physiological solutions (6x106 cells/0.3 ml) were intraperitoneally applied to DBA mice. After a follow-up of two weeks the occurrence of ascites was detected in animals (n=5). These animals became sacrificed and tumor cells were isolated by aspiration of the peritoneum (total cell count: 2.5x108). These cells were subcutaneously injected into the tights of new DBA mice (2.5x106 cells/0.1ml physiologic solution).
Chemicals
EIPA (5-N-ethyl-N-isopropyl amiloride), DIDS (4,4’diisathiocyanostiben-2,2’disulphonoc acid) purchased by Sigma. RPMI culture medium was a kind gift from the Institute of Immunology, Zagreb.
Procedure of experiments
Research is realized from 01. 01. 2012 to 31. 12. 2012 at University hospital J.E. Goethe in Frankfurt. For all experiments DBA mice with P815-induced subcutaneous tumors were used. 20 days post tumor initialization animals were randomly divided in one control group and three groups which underwent different treatments. Group A consists of 16 mice which became treated with DIDS. Group B were 17 mice treated with EIPA, and group C were 15 mice treated with combination of both, EIPA and DIDS. The control group consists of 15 mice which became injected with 0.1ml physiological solution (0.9% NaCl). All animals were not anesthetized during injection. DIDS and/or EIPA were solved in physiologic solutions at concentrations derived from preliminary experiments (results not shown). 0.1ml DIDS (10-2mol/L) and/or EIPA (4.3x10-3mol/L) were injected into the tumor center. Tumor burden became estimated at day 1, 3, 4, 6, 7, 10, 12, 13, 15, 16 and 18 after treatment.
Assessment of Effects
Tumor sizes were estimated using a qualimeter, immediately before treatment with ion-pump inhibitors. The tumor size was given as the mean of the previous measurement. Moreover, we followed the rate of survival rate of the animals.
3. RESULTS
At the beginning of the series of experiments the mean tumor diameter in the control group was 9.0±0.9mm. Tumor diameter in group A was 8.4±0.6mm, in group B it was 8.6±0.8mm, and in group C it was 8.2±1.9mm. Between the different groups were no statistically relevant alterations concerning tumor size. According to the protocol we injected during 18 days inhibitors of ion pumps and physiologic solutions, respectively, into the tumors. The mean tumor diameter was in group A, B and C significantly lowered after day 6, respectively after the third injections of inhibitors (p<0.005) (Figure 1).
Figure 1.
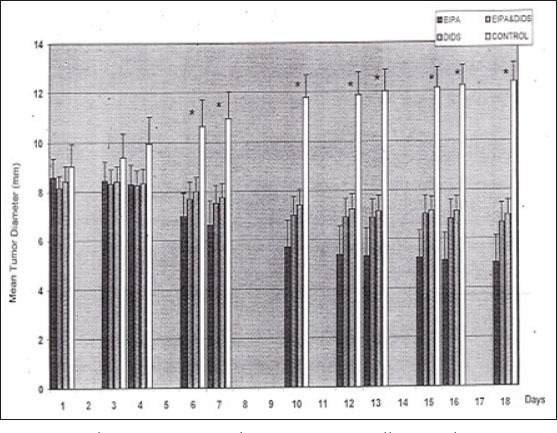
The mean tumor diameter was in all treated groups significantly lowered after day 6, respectively after the third injection of inhibitors (p<0.0005).
After termination of the experiments the mean tumor diameter in the control group was 12.4±0.8mm, in group A it was 6.9±0.6mm, and in group B we measured 6.6±3.1mm. At the final day the tumor size in treated animals was twice as small as in the control group (Figure 2). In addition we observed the rate of survival (Figure 3). In the control group only 18% of the animals were still alive at day 18. Considering the rate of survival a statistically significant difference between treated and untreated animals was observed.
Figure 2.
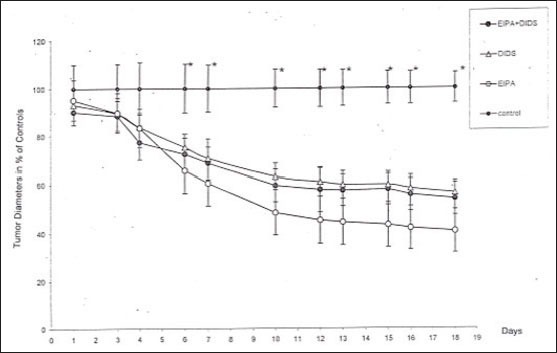
At the final day of series of experiments the tumor diameter in the treated groups was twice as small as than in the control group.
Figure 3.
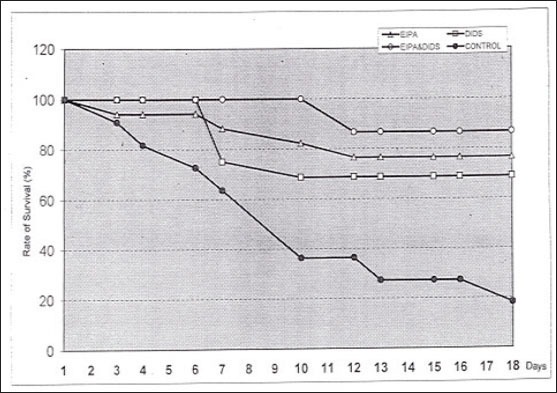
Representation of the rate of survival at the end of series of experiment.
4. DISCUSSION
Previous studies demonstrated the existence of Na+/H+ ion pumps and Na+-dependent HCO3/Cl- ion pumps in P815 cells (13). These ion pumps can be inhibited by the use of specific inhibitors like DIDS and EIPA. It was shown that DIDS and/or EIPA are able to decrease growth and proliferation of P815 cells and L292 cells under in vitro conditions (13). In the present work we could demonstrate a similar effect of inhibitors of ion pumps under in vivo conditions. We have compared animals treated with DIDS and/or EIPA and the referring controls regarding tumor growth and rate of survival. With means of statistics we could discover a distinct difference in tumor size in all treated animals compared to the control group (p<0.05). In all groups with treated animals (n=48) 37 animals featured reduced tumor sizes after repeated application of ion pump inhibitors. In the control group tumors grew and metastasized causing death of animals (confirmed by autopsy). Moreover, the rate of survival in treated animals was distinctly increased compared to untreated controls. It could be assumed that this effect was due to the application of DIDS and EIPA. We could not evaluate significant difference between both types of ion pump inhibitors. Results from the other investigator confirm the inhibitory effect of EIPA on tumor growth (14). It was shown that growth of GM-CSF-stimulated monocytes was inhibited by treatment with EIPA (15). Boyer and Tannock could demonstrate that the Na+/H+ ion pump is the major regulator for the intracellular pH in hypoxic areas of the tumor (16). They show an anti-tumoral effect in a mouse model by treatment with amiloride, an inhibitor of Na+/H+ ion pumps, in combination with hydrolasin and nigericin (16). Analogues of amiloride as e.g. 5-(N,N-hexamethylen) amiloride (HMA) in combination with hydrolasin and nigericin are also capable to inhibit growth of solid tumors (17). EIPA in combination with nigericin shows, among all amiloride analogues, the strongest effect in vivo. EIPA and nigericin together with hydrolasin are able to cause death of tumor cells in vivo (18). Regarding the body weight and the levels of serum creatinine, no toxic effect of EIPA and nigericin on normal tissues were detected (19). Tumor cells acidificated by nigericin show a dose-dependent susceptibility towards DIDS. The combination of DIDS and nigericin enhances the anti-tumoral effect of hyperthermia in vivo (20). The acidification of tumor cells can also be favored by treatment with some cytostatics (21, 22). An improved anti-tumoral effect of inhibitors of ion pumps was shown by Yamagata and Tannock (23) in a long-term application (72h) of DIDS and EIPA in vivo using C3H/HeJ and Balb/c mice carrying KHT or EMT-6 tumors.
5. CONCLUSION
Our results confirm that the repeated application (18d) of inhibitors of ion pumps in vivo reduce tumor growth. This study shows the effectiveness of drugs which regulate intracellular pH in tumor treatment and opens perspectives for new therapeutic options.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005 Dec;1(6):779–786. doi: 10.2217/14796694.1.6.779. [DOI] [PubMed] [Google Scholar]
- 2.Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y. Acid extracellular micro environment and cancer. Cancer Cell Int. 2013 Sep 3;13(1):89. doi: 10.1186/1475-2867-13-89. doi: 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barar J, Omidi Y. Dysregulated pH in Tumor Micro environment Checkmates Cancer Therapy. Bioimpacts. 2013;3(4):149–162. doi: 10.5681/bi.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol. 2013 Dec 17;4:370. doi: 10.3389/fphys.2013.00370. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harguindey S, Reshkin SJ, Orive G, Arranz JL, Anitua E. Growth and trophic factors, pH and the Na+/H+exchanger in Alzheimer's disease, other neurodegenerative diseases and cancer: new therapeutic possibilities and potential dangers. Curr Alzheimer Res. 2007 Feb;4(1):53–65. doi: 10.2174/156720507779939841. [DOI] [PubMed] [Google Scholar]
- 6.Reshkin SJ, Cardone RA, Harguindey S. Na+-H+exchanger, pH regulation and cancer. Recent Pat Anticancer Drug Discov. 2013 Jan 1;8(1):85–99. doi: 10.2174/15748928130108. [DOI] [PubMed] [Google Scholar]
- 7.Paulino C, Kühlbrandt W. pH- and sodium-induced changes in a sodium/proton antiporter. Elife. 2013 Jan 1;3:e01412. doi: 10.7554/eLife.01412. doi: 10.7554/eLife.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinothkumar KR, Smits SH, Kühlbrandt W. pH-induced structural change in a sodium/proton antiporter from Methanococcus jannaschii. EMBO J. 2005 Aug 3;24(15):2720–9. doi: 10.1038/sj.emboj.7600727. Epub 2005 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harguindey S, Arranz JL, Wahl ML, Orive G, Reshkin SJ. Proton transport inhibitors as potentially selective anticancer drugs. Anticancer Res. 2009 Jun;29(6):2127–2136. [PubMed] [Google Scholar]
- 10.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na+/H+antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—one single nature. Biochim Biophys Acta. 2005 Sep 25;1756(1):1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Xiong ZG. Ion channels as targets for cancer therapy. Int J Physiol Pathophysiol Pharmacol. 2011 Jun 30;3(2):156–166. [PMC free article] [PubMed] [Google Scholar]
- 12.Le Guennec JY, Ouadid-Ahidouch H, Soriani O, Besson P, Ahidouch A, Vandier C. Voltage-gated ion channels, new targets in anti-cancer research. Recent Pat Anticancer Drug Discov. 2007 Nov;2(3):189–202. doi: 10.2174/157489207782497244. [DOI] [PubMed] [Google Scholar]
- 13.Horvat B, Taheri S, Salihagić A. Tumour cell proliferation is abolished by inhibitors of Na+/H+and HCO3-/Cl-exchange. Eur J Cancer. 1992;29A(1):132–137. doi: 10.1016/0959-8049(93)90591-3. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Wang D, Dong W, Song Z, Dou K. Inhibition of Na(+)/H(+) exchanger 1 by 5-(N-ethyl-N-isopropyl) amiloride reduces hypoxia-induced hepatocellular carcinoma invasion and motility. Cancer Lett. 2010 Sep 28;295(2):198–204. doi: 10.1016/j.canlet.2010.03.001. doi: 10.1016/j.canlet.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Caracciolo D, Pannocchia A, Treves S, Ghigo D, Gallo E, Tarella C, Bussolino F, Turrini F, Tamponi G, Bosia A. Role of Na+/H+exchange in the granulocyte-macrophage colony-stimulating factor-dependent growth of a leukemic cell line. J Cell Physiol. 1990 Apr;143(1):133–139. doi: 10.1002/jcp.1041430118. [DOI] [PubMed] [Google Scholar]
- 16.Boyer MJ, Tannock IF. Regulation of intracellular pH in tumor cell lines: influence of microenvironmental conditions. Cancer Res. 1992 Aug 15;52(16):4441–4447. [PubMed] [Google Scholar]
- 17.Luo J, Tannock IF. Inhibition of the regulation of intracellular pH: potential of 5-(N,N-hexamethylene) amiloride in tumour-selective therapy. Br J Cancer. 1994 Oct;70(4):617–624. doi: 10.1038/bjc.1994.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maidorn RP, Cragoe EJ, Jr, Tannock IF. Therapeutic potential of analogues of amiloride: inhibition of the regulation of intracellular pH as a possible mechanism of tumour selective therapy. Br J Cancer. 1993;67:297–303. doi: 10.1038/bjc.1993.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasuda K, Lee C, Tannock IF. Antitumor activity of nigericin and 5-(N-ethyl-N-isopropyl)amiloride: an approach to therapy based on cellular acidification and the inhibition of regulation of intracellular pH. Oncol Res. 1994;6(6):259–268. [PubMed] [Google Scholar]
- 20.Lyons JC, Ross BD, Song CW. Enhancement of hyperthermia effect in vivo by amiloride and DIDS. Int J Radiat Oncol Biol Phys. 1993 Jan;25(1):95–103. doi: 10.1016/0360-3016(93)90150-t. [DOI] [PubMed] [Google Scholar]
- 21.Vukovic V, Tannock IF. Influence of low pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J Cancer. 1997;75(8):1167–1172. doi: 10.1038/bjc.1997.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood PJ, Sansom JM, Newell K, Tannock IF, Stratford IJ. Reduction of tumour intracellular pH and enhancement of melphalan cytotoxicity by the ionophore Nigericin. Int J Cancer. 1995 Jan 17;60(2):264–268. doi: 10.1002/ijc.2910600222. [DOI] [PubMed] [Google Scholar]
- 23.Yamagata M, Tannock IF. The chronic administration of drugs that inhibit the regulation of intracellular pH: in vitro and anti-tumour effects. Br J Cancer. 1996 Jun;73(11):1328–1334. doi: 10.1038/bjc.1996.254. [DOI] [PMC free article] [PubMed] [Google Scholar]


