Abstract
Erectile dysfunction (ED) in men under the age of 40 was once thought to be entirely psychogenic. Over the last few decades, advances in our understanding of erectile physiology and improvements in diagnostic testing have restructured our understanding of ED and its etiologies. Although psychogenic ED is more prevalent in the younger population, at least 15%–20% of these men have an organic etiology. Organic ED has been shown to be a predictor of increased future morbidity and mortality. As such, a thorough work-up should be employed for any man with complaints of sexual dysfunction. Oftentimes a treatment plan can be formulated after a focused history, physical exam and basic lab-work are conducted. However, in certain complex cases, more testing can be employed. The major organic etiologies can be subdivided into vascular, neurologic, and endocrine. Specific testing should be directed by clinical clues noted during the preliminary evaluation. These tests vary in degree of invasiveness, precision, and at times may not affect treatment. Results should be integrated into the overall clinical picture to assist in diagnosis and help guide therapy.
Keywords: dysfunction, endocrine, erectile, men, neurologic, organic, vascular, young
INTRODUCTION
Erectile dysfunction (ED) is defined as the inability to achieve or maintain a sufficient erection to engage in sexual intercourse. Descriptions of sexual dysfunction can be traced back to 2000 BC. However, our modern understanding of erectile physiology began relatively recently in the 1970s.1 Once thought to be a problem derived from one's psyche, an improved understanding of physiology and pathophysiology has led to the discovery of numerous organic etiologies. Although the prevalence of organic ED increases with age, a significant cohort of men under the age of 40 suffers from such causes. This review article will focus on the work-up of young men with organic ED, without cardiovascular disease.
EPIDEMIOLOGY
Prevalence and aging
It has been established that prevalence of ED increases with age. In cross-sectional analysis of the American populous, prevalence of ED is 4-fold higher for men in their 70s compared to their 20s. Ultimately, approximately 52% of men between the ages of 40 and 70 have some degree of sexual dysfunction.2 And yet, a number of younger men (i.e., under 40) suffer from ED. Until relatively recently (1970s), ED in young men was thought to be psychogenic. However, several studies have identified organic causes in 15%–72% of this population.3 The range in prevalence varies widely, depending on patient population and defining criteria. One study included 948 men with an International Index of Erectile Function 5 score of <21. They each underwent a thorough psychiatric evaluation, nocturnal penile tumescence (NPT), and penile Doppler ultrasound. The authors determined that 14.8% of the 526 men under the age of 40 had organic ED. The etiology was further subdivided into arteriogenic (32.1%), venogenic (16.7%), neurogenic (12.8%), endocrinologic (2.6%), drug-induced (7.7%), mixed (11.5%) and unknown (16.6%).4 The main risk factors were determined to be current smoking (41.4%), diabetes mellitus (27.1%), hypertension (17.3%), hyperlipidemia (18.5%), perineal trauma (5.1%), spinal cord injury (4.5%), and drug consumption (4.5%). Another study out of the University of California San Francisco reviewed 100 men under the age of 40, who had ED, and determined that only 13% had exclusively psychogenic ED.5 These findings have raised awareness within the urologic community and suggested that young men with sexual dysfunction should not be dismissed without proper evaluation.
Erectile dysfunction as a predictor of future morbidity and mortality
The presence of ED can be an indicator of future morbidity and mortality. The most widely studied association is that of increased future cardiovascular events. Thompson et al.6 evaluated over 9000 men who were randomized to the placebo group in the Prostate Cancer Prevention Trial. They were followed for 10 years, and were evaluated every 3 months for the development of cardiovascular disease and incident ED. Of the 4247 men without ED at the outset of this study, 2420 (57%) developed ED within 5 years. Incident development of ED was associated with a hazard ratio of 1.25 (P = 0.04) for future cardiovascular events. Similarly, Inman et al.7 followed men with ED and noted an 80% increased risk of developing coronary artery disease after 10 years, in those under 50 years old. Their data also suggested that when ED occurs in younger men it is associated with an increased risk of future cardiac event, while it carries little prognostic importance in older men. This raises the argument that ED and CAD may result from similar underlying vascular insults. Endothelial dysfunction, blood vessel size, and androgen levels have all been proposed as possible contributors to this phenomenon.8,9 The presence of ED may be the first manifestation of poor future health. For this reason, it is imperative to increase awareness within the younger population. Making an early diagnosis can allow clinicians to target at-risk men, and help them curb poor health habits or diagnose co-morbid conditions at a younger age.
GENERAL DIAGNOSTIC PRINCIPLES
Historical perspective
Over the past 40–50 years, work-up and management of ED has changed immensely. Prior to the 1970s, diagnostic evaluation was limited to attaining a psychosexual history, and offered treatments were limited to psychosexual therapy and herbal supplementation. Since that time, many advances in work-up (i.e., NPT testing, penile duplex ultrasonography, intracavernous injections, and endocrine evaluation) and treatment (i.e., oral medications, intracavernosal pharmacotherapy, vacuum devices, the penile prosthesis) have greatly expanded detection and management of ED. The 21st century has looked to neuroimaging, biomarkers of vascular health to further refine and personalize work-up. There are on-going investigations into gene and stem cell therapies, as well as tissue engineering.10
Many clinicians recognize their responsibility in recognizing and treating ED, which has led to numerous consensus meetings in an attempt to formulate best-practice guidelines. The International Consultations on Sexual Medicine was one such series of meetings, first convened in 1999 with subsequent conferences in 2004 and 2009.1 Out of these meetings, a goal-directed approach was combined with evidence-based practice to create diagnostic and therapeutic algorithms and establish standard-of-care practices.
Initial evaluation
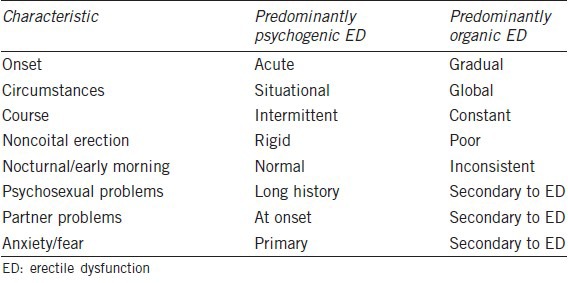
A detailed history is the first important step to the evaluation of any patient with ED. There are barriers to discussing sexual habits and problems. A clinician must approach the topic delicately and caringly in order to earn the patient's trust and be permitted to address his problem.1 An accurate sexual history is of central importance. Features such as onset, duration, severity, and etiology should be elucidated. A psychosocial history should be included as unstable interpersonal relationships, or emotional stressors can play a huge role in sexual health. A careful medical history can be used to pinpoint risk factors to a patient's ED. Determining underlying contributors can both help guide therapy and also determine potentially reversible problems. With a thorough history alone, ED can often be categorized into psychogenic, organic, or mixed (Table 1).11
Table 1.
Differential characteristics of psychogenic versus organic ED11
A physical exam can help further delineate possible etiologies and contributors to ED. At the very least, one's physical exam should include careful evaluation of the cardiovascular, neurologic, and genital systems. Physical signs of hypogonadism can point to an endocrine etiology. The presence of obesity (in particular visceral adiposity noted by waist circumference), high blood pressure, or abnormal pulses may require more extensive vascular work-up. Deficiencies in perineal sensation or evidence of peripheral neuropathy may be indicators of a diabetic or neurologic etiology. Penile deformity such as fibrous plaques, micropenis, chordee lends to the possibility of a physical impediment to sexual intercourse.10
For nearly all men with sexual dysfunction, serum chemistry (i.e., lytes, blood urea nitrogen, creatinine), fasting glucose, complete blood count, lipid profile, and serum testosterone should be attained. Then, depending on clues raised during one's history and physical exam, more directed lab-work could be conducted.
Distinguishing organic from psychogenic erectile dysfunction
The first distinction of ED that should be established is psychogenic from organic. Clues to suggest a psychogenic etiology include sudden onset, good quality spontaneous or self-stimulated erections, major life events, or previous psychological problems. Conversely, gradual onset, lack of tumescence, and normal libido are more suggestive of an organic etiology.12 One study classified men with ED into those that have difficulty achieving an erection from those who struggle with maintaining an erection. The authors noted that organic etiologies are significantly more prevalent within the group that cannot achieve tumescence.13 More research is required to explain this phenomenon, but the authors surmised that in order to obtain an erection, the vascular, neurologic, and endocrine systems must be working sufficiently well. There is data suggesting that certain organic etiologies, like venous leak, can also affect erection sustainability.14 However, the act of maintaining an erection appears to have a considerable psychologic component. In support of this fact, data suggests that men who struggle with maintaining an erection were younger, healthier, had a lower degree of penile insufficiency and a higher prevalence of normal nocturnal erections. Although the act of achieving and maintaining an erection are lumped under a common diagnosis, they may represent two distinct pathophysiologic disturbances.15 With a directed history, clinicians can distinguish between the two, which may alter work-up and ultimately treatment of the patient's dysfunction.
In more complex presentations, the utilization of NPT has proven to be beneficial. In this test, nocturnal monitoring devices measure the number of erections, duration of erections, and degree of rigidity while patients sleep. It is well-established that men experience nocturnal erections, particularly during the rapid eye movement phase. Normal NPT results include at least four erections, with a mean duration of 30 min, with a maximal rigidity above 70%.10 This test theoretically eliminates psychological interference. However, the test has drawbacks that limit its widespread use. It is an expensive test. In addition, false-negative results are documented, particularly in older or anxious patients. Finally, some questions have been raised as to whether the results can be extrapolated to the evaluation of sexually relevant erections. Nonetheless, NPT can be employed to help differentiate organic from psychologenic ED.
The major etiologies of organic ED in younger men include vascular, neurologic, endocrine disorders, and medications. Oftentimes the treatment plan for ED can be formulated with a focused history, physical exam and select lab-work. However, in certain complex presentations, or for the sake of diagnostic precision, further testing can be employed.
VASCULOGENIC ERECTILE DYSFUNCTION
Etiology
The most common vascular disorders that lead to ED in younger men include focal arterial occlusive disease, subclinical endothelial dysfunction, and Peyronie's disease. An example of focal arterial occlusive disease has been demonstrated in the bicycling community. A survey study of 160 cyclists following a 540-km Norweigan bicycle tour was conducted. Twenty one percentage of men reported penile numbness, with 13% admitting to symptoms of temporary new-onset impotence.16 Cross-sectional studies have established that men who ride > 3 h per week have an increased risk of developing ED. This is thought to be secondary to perineal pressure from a bicycle seat that temporarily occludes penile vessels. Arterial insufficiency causes an upregulation of connective tissue synthesis and inhibits vascular smooth muscle growth. This mechanism disrupts functional penile compliance, ultimately leading to ED.17 Subclinical endothelial dysfunction may also predispose young men to ED. In a study where young men with ED, but low risk for cardiovascular disease were compared to young men without ED, they carried more subclinical (albeit within normal range) cardiovascular risk factors: elevated systolic blood pressure, C-reactive protein levels, cholesterol, triglycerides, and carotid intimal-media thickness.18 This study suggested that although some patients may not have overt cardiovascular disease, they may still possess subclinical risk factors that predispose them to ED. Peyronie's disease is also strongly associated with sexual dysfunction. In fact, 21% of men under 40 years with Peyronie's disease experience ED. Larger plaque size, veno-occlusive dysfunction and impaired cavernosal arterial inflow may all contribute to ED in these men.19 There is also a significant degree of physical and psychological burden in Peyronie's disease, which makes for a multifactorial etiology.20 In a qualitative study of men with Peyronie's disease, four core domains were found to have an impact on sexual and psychosocial health in these patients: self-image, sexual function, pain and discomfort, and social isolation.21 These findings are suggestive of a mixed picture with psychogenic and organic contributors.
Vascular work-up
Intracavernosal injection and stimulation
A vascular work-up looks to evaluate one's ability to acquire and sustain an erection: arterial blood inflow, subsequent engorgement, and blood retention within corporeal bodies are all examined. Combined intracavernous injection and stimulation is a first-line option. Vasodilator drugs (e.g., papaverine, phentolamine, alprostadil) are injected at the lateral base of the penis using a small-gauge needle, and delivered directly into the corpus cavernosum. Assessment of penile rigidity and duration of response is then conducted. If a normal response is achieved, then work-up in other arenas (neurologic, psychogenic, and endocrine) may be pursued. However, it is important to recognize that false-positive results can occur in up to 20% of patients, particularly in men with penile arterial insufficiency,22 and false-negative results are also possible secondary to anxiety, needle phobia, or inadequate injection dosage.
Duplex ultrasonography
Drawbacks of intracavernosal injection include a degree of invasiveness and ultimately a subjective evaluation of penile rigidity by the assessor. Duplex ultrasound, on the other hand, is noninvasive and provides a quantitative component to the evaluation of blood-flow. In this test, a high-resolution ultrasonography and color-pulsed Doppler is used. Flow velocities are measured at the penis base, before and after vasodilator injections. In a normal Doppler study, the filling phase has characteristically high waveforms during systole and diastole.10 Peak systolic velocities (PSV) have been used to establish normal from abnormal erectile response. The mean PSV typically ranges from 35 to 47 cm s−1. If velocities dip below 25 cm s−1, abnormal pudendal arteriography is confirmed with 100% sensitivity, 95% specificity.23 With these values in mind, patients with a peak velocity of >35 cm s−1 are deemed to have normal cavernous arterial inflow, while those with values <25 cm s−1 are diagnosed with cavernous arterial insufficiency. In regards to veno-occlusive dysfunction, restrictive indices (RI) can be useful.
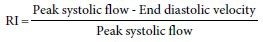
Normally, diastolic corporal flow should approach zero as rigidity is achieved. Hence, the RI calculation approaches a value of 1. Patients with an RI >0.9 have normal veno-occlusive function, and those with an RI <0.75 raise suspicion for veno-occlusive dysfunction, that is, dysfunctional corporal retention. One study used ultrasound to determine RI in men with negative responses to intracavernosal injection. They were stratified into three groups: RI <0.75, between 0.75 and 0.9, and >0.9. They all then underwent confirmatory penile cavernosography. In men with an RI >0.9, 90% had normal cavernosography. In those with a RI <0.75, 95.5% had corporal leakage. However, in the range of 0.75–0.9, 41% had normal caverosography while 59% demonstrated veno-occlusive dysfunction.24 This study illustrates the limitation of ultrasonography alone within the intermediate group. Ultrasonography has other limitations as well. Accurate testing may be affected by anatomic arterial variants. In addition, several researchers have suggested that ultrasonography, particularly in young men, can be confounded by false-positives. One study took a patient population of 71 men with suspected vascular ED, based on an initial poor response to pharmaco-injection. The patients were subdivided into four groups based on age: 20–29, 30–39, 40–49, and 50–59. Out of the 11 young men in the 20–29 age group, all 11 showed a poor response during the initial ultrasonography. However, all 11 showed an appropriate response to follow-up ultrasonography approximately 2 weeks later.25 This raised the issue of a psychogenic component to testing in this patient population.
Penile angiography
Penile angiography is a third-line study used for evaluation of the penile vasculature. It is typically reserved for young patients with ED related to a traumatic arterial injury, or in patients with penile compression injury being considered for revascularization surgery.10 In this test, the internal pudendal artery is selectively cannulated, and then radiographic contrast is injected for visualization of the internal pudendal and penile arteries. Penile vascular anatomic variations exist, making it difficult for the angiographer to determine congenital from acquired abnormalities and limiting utility of this modality. However, there is promise in that penile angiography could potentially serve as a diagnostic and therapeutic option in select patients. Some argue that in men with evidence of penile arterial insufficiency who have failed pharmacotherapy, contemporary endovascular treatment options may have utility.26 At this time, however, supportive literature is limited to case reports or small nonrandomized clinical trials.
Penile magnetic resonance imaging
Several investigational vascular studies are underway. Penile magnetic resonance imaging (MRI) has promise in detailing penile anatomy and microcirculation. The use of MRI during the work-up of prostate cancer has recently become more popular. Given proximity of the genitals, penile anatomy and vasculature are often depicted on these imaging studies. Vargas et al.27 evaluated 50 prostate cancer patients who underwent an MRI pelvis for staging prior to prostatectomy. They found a correlation between a patient's self-reported sexual function and perfusion-related parameters noted on MRI. This data may help clinicians and patients navigate management options for prostate cancer in the future.
NEUROGENIC ERECTILE DYSFUNCTION
Etiologies
The neurologic system is intimately involved in proper erectile function. Peripheral, spinal, supraspinal, as well as somatic and autonomic pathways, are integrated with erectile physiology. In men under 40, common neurologic etiologies of ED include multiple sclerosis, epilepsy, intramedullary nailing of femoral fractures, and lumbar spine procedures.3 In a large population-based study using a national database, researchers found that patients with multiple sclerosis were 2.2 times more likely to have ED.28 The etiology of ED in this population is complex and likely involves an interplay between nerve dysfunction, cognitive decline, illness related stress, and perhaps inflammation-dependent endothelial dysfunction. ED is also seen in high prevalence amongst men with epilepsy. In a similar large population-based study, men with ED in their 30s, were 3.04 times more likely to have epilepsy.29 Men who suffer from femoral head fractures are at increased risk for ED following intramedullary nailing: up to 40.5%. This is thought to be related to counter-traction on the femoral head, leading to pudendal nerve damage.30 Similarly, in men under 50 who underwent lumbar spine surgical decompression, 34% experienced postprocedural ED. In addition, those who had persistent neurologic symptoms were at increased risk for persistent sexual dysfunction. It appears that permanent lumbar spine pathology places one at increased risk of ED.31 Men who sustain such injuries and undergo corrective surgical procedures should be counseled on the significant incidence of ED.
Neurologic work-up
Somatic nervous system
Numerous tests to evaluate the neurologic system as a cause of sexual dysfunction have been introduced. However, they carry limited utility to this point. Several barriers to mainstream use include their limited impact on management of ED, poor reproducibility, and sensitivity. In regards to work-up of somatic nervous system dysfunction, several tests exist. Penile glans biothesiometry involves placing an electromagnetic device onto the penile shaft and glans penis. Sensory perception is measured using various vibratory stimuli of varying amplitudes. However, questions regarding utility of this test have been raised as biothesiometry does not accurately mimic the neurophysiologic function of the glans penis. In addition, there is a marked intra-individual variation in vibration sensitivity, raising the issue of reproducibility and accuracy.32 The bulbocavernosus reflex latency test evaluates the somatosensory reflexogenic mechanism of erections. Two electrodes are placed on the penis, one at the corona and one approximately 3 cm proximal to the corona. A direct-current stimulator then delivers impulses via these electrodes, and a recorder measures response of the bulbocavernous muscles. Latency period is defined as the time from the electrical impulse delivery to the muscle response.10 The mean response time is approximately 30 ms, and an abnormal response is defined as > 3 standard deviations from the mean. Again, questions regarding the validity of this test have been raised as it is limited to evaluation of the pudendal nerve. Several studies contend that pathologic pudendal nerve conduction appears relatively late in various forms of neurogenic ED including diabetes, making it an unreliable diagnostic test.33 Dorsal nerve conduction velocity has also been studied in a similar fashion. Again two electrodes are placed on the penis, one at the glans and another at the base. A stimulus is delivered from each electrode. Conduction velocity is then calculated by dividing the distance between the electrodes by the difference in latency times recorded at each site. Slower conduction velocity has been associated with neurogenic ED. One study performed a series of perineal electrophysiological studies on 423 patients with ED. In men with neurologic ED, the most frequently observed finding was decreased conduction of the penile dorsal nerve (64.5%).34
Autonomic nervous system
Testing the autonomic system has proven to be even more challenging and limited than the somatic system. It remains less sensitive and reproducible. Measuring heart rate control and variation during quiet and deep breathing give insight into one's parasympathetic system. The sympathetic nervous system has been evaluated through the sympathetic skin response test, which involves delivering a shock stimulus at one location and recording the evoked potential elsewhere on the body.10 Penile thermal sensory testing assesses the thermal threshold of small sensory penile nerve fibers. Sensory nerve fibers that carry penile skin sensation travel with the cavernous nerves in the pelvis. With this in mind, Yiou et al.35 tested penile thermal sensation to determine the extent of cavernous nerve damage following radical prostatectomy. Forty-two patients underwent either nerve sparing or nonnerve sparing radical prostatectomy. Postoperatively, penile sensory thresholds for warm and cold sensations increased significantly after nonnerve sparing radical prostatectomy only. This paper argues in favor of testing penile sensation to evaluate the extent of cavernous nerve damage caused by prostatectomy.
ENDOCRINOGENIC ERECTILE DYSFUNCTION
Etiologies
Endocrinopathies can potentially affect erectile function, most notably hypogonadism, hyperthyroidism, hypothyroidism, and diabetes. The patient's history can raise suspicion for such diagnoses; however, there is significant variability in presentation. To aid in the diagnosis, several screening surveys have been suggested and tested, particularly in the case of hypogonadism. Yet, lack of sensitivity and specificity has led to limited utility. Ultimately, endocrinopathies are evaluated and diagnosed with the use of serum hormonal levels.
Androgen deficiency has been observed in as low as 2% and as high as 33% of all men with ED. In younger men, ED due to low testosterone is less common. One study found that only 4% of men under the age of 50 had low testosterone. In certain subgroups, however, hypogonadism can be more prevalent. In the HIV population, for instance, hypogonadism was observed in 16% of young and middle-aged men.36 Hypogonadism can be subdivided into primary (i.e., gonadal dysfunction) and secondary (i.e., central dysfunction) forms. Kleinfelter's syndrome, congenital hypogonadotropic hypogonadism and cryptorchidism fall into the primary division. Examples of secondary hypogonadism include head trauma, prolactinoma, pituitary surgery, alcohol or illicit drug abuse, and certain infiltrating disorders such as hemochromatosis.37
Erectile dysfunction is frequently found in men with thyroid dysfunction. Individuals with either hypo- or hyperthyroidism have, on average, less satisfactory erections.38 One trial of Greek men with thyroid dysfunction showed that 63% of men with hypothyroidism and 70% with hyperthyroidism had some form of ED, compared to 34% in the control group.39 The mechanism relating ED to thyroid dysfunction remains unknown to this point, although endothelial dysfunction has been suggested.
There is a well-established, strong association between diabetes and ED. The prevalence has been reported as high as 75% in some populations. In one prospective study, the incidence of ED in patients with diabetes was 68 cases per 1000 patients per year compared to 25.9 cases per 1000 patients per year in the general populous.40 In addition, presence of ED has been shown to be an independent risk factor for poor quality of life within the diabetic community.41 The prevalence of early-onset diabetes is becoming increasingly common and will likely contribute to the increasing prevalence of organic ED in young men.
Endocrine and hormonal work-up
Hypogonadism
Testosterone circulates in three forms: free (0.5%–3%), tightly bound to sex hormone binding globulin (SHBG) (30%), loosely bound to albumin and other similar serum (67%). Theoretically, the best measure of androgen status is determined by calculating bioavailable testosterone, that is, the summation of free and albumin-bound testosterone. However, for screening purposes total serum testosterone is thought to be sufficient. Blood draws should occur in the morning, when serum testosterone levels peak. The normal range is large, typically quoted between 280 and 1000 ng dl−1. There is such patient variability that the recommended level to treat remains controversial. However, if an initial testosterone level falls into the low-normal or below normal range, a repeat confirmatory test is recommended.42 If hypogonadism is suspected based on symptoms and serum testosterone, further work-up with serum luteinizing hormone (LH) and prolactin should be pursued. Because androgen levels are affected by the hypothalamic-pituitary-gonadal access, these additional tests can help pinpoint the dysfunctional organ.
In primary hypogonadism (i.e., gonadal dysfunction), LH and follicle-stimulating hormone (FSH) levels are appropriately elevated in response to low androgen level. Kleinfelter's syndrome falls into this category, with a prevalence of 1:500–1000. Many men do not present with the classic features of Kleinfelter's (i.e., micropenis, microorchidisism, eunuchoid body habitus) and appear in adulthood with complaints of ED or infertility. One 2010 studies evaluated 1386 male patients with ED and performed karyotype analysis on those with a testis volume < 6 ml. In total, 23/1386 men had Kleinfelter's syndrome, with a mean age of 40. Within this study, 22.7% of men with Kleinfelter's experienced severe ED, and 60.9% had hypoactive sexual desire.43
In secondary hypogonadism (i.e., central dysfunction), LH and FSH levels are inappropriately normal or low. Hyperprolactinemia can lead to secondary hypogonadism. Elevated prolactin levels suppress gonadotropin-releasing hormone secretion from the hypothalamus. This ultimately impairs the pulsatile secretion of LH, which is necessary for gonadal testosterone production.44 Causes include medications (antipsychotics, tricyclic antidepressants, opiates), prolactin-secreting tumors, hypothyroidism, cirrhosis and hypothalamic lesions. One study evaluated sexual functioning in men with heroin addiction. Results showed that 55% of these patients had elevated prolactin levels, along with an associated decline in erectile function (P = 0.011) and orgasm (P = 0.033).45 Regardless of etiology, elevated serum prolactin affects sexual functioning. In a series of 51 men with hyperprolactinemia with age-matched controls, 97% versus 14% of these men experienced a reduction in nocturnal erections. In addition, the number of nocturnal erections correlated with serum prolactin level.46 In the cases of hyperprolactinemia, MRI imaging of the pituitary should be considered, in particular when prolactin levels are very high (>200 ng ml−1). In these patients, the etiology is almost always due to prolactin secreting tumors. Of note, clinically nonfunctioning pituitary macroadenomas may cause secondary hypogonadism from mass effect on normal pituitary tissue. These patients may complain of headaches, have visual field defects, or seizures.47 These symptoms, paired with low testosterone warrant brain imaging regardless of prolactin level.
Thyroid dysfunction
Serum thyroid function tests should also be considered in endocrine work-up of ED. Hyperthyroidism can contribute to ED by increasing aromatization of testosterone into estrogen, ultimately raising levels of SHBG and decreasing percent of bioavailable testosterone. Diagnosis is often suspected from the symptomatology: fatigue, weight loss, hyperactivity, palpitations, and heat intolerance. It is confirmed when serum markers reveal high thyroid hormone concentrations (total or free T4) with low serum thyroid-stimulating hormone (TSH) levels.44 In the case of hypothyroidism, diagnosis is made when serum basal TSH is elevated, and thyroid hormone concentrations are low.
CONCLUSIONS
A significant number of young men suffer from ED. Although previously thought to be entirely psychogenic, approximately 15%–20% of cases are organic in origin. Young men with ED may be at higher risk for future morbidity and mortality, meaning that ED may be an early indicator of poor overall health. As such, increasing awareness, making the diagnosis, and targeting at-risk men is of critical importance. As previously discussed, ED may be the first indicator of cardiovascular disease. Perhaps select men with ED, in particular those with a vasculogenic etiology, should be referred to a cardiologist for further evaluation of overt or subclinical cardiovascular risk factors. Urologists and various other providers have an opportunity and responsibility to evaluate complaints of ED in young men, to work-up these complaints rather than dismiss them, and to understand how ED may be an indicator of a man's overall health.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Lue T. Ch. 23. Philadelphia: Elsevier Inc; 2011. Campbell-Walsh Urology. Physiology of Erection and Pathophysiology of ED. [Google Scholar]
- 2.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig W, Phillips M. Organic causes of erectile dysfunction in men under 40. Urol Int. 2014;92:1–6. doi: 10.1159/000354931. [DOI] [PubMed] [Google Scholar]
- 4.Caskurlu T, Tasci AI, Resim S, Sahinkanat T, Ergenekon E. The etiology of erectile dysfunction and contributing factors in different age groups in Turkey. Int J Urol. 2004;11:525–9. doi: 10.1111/j.1442-2042.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 5.Donatucci CF, Lue TF. Erectile dysfunction in men under 40: etiology and treatment choice. Int J Impot Res. 1993;5:97–103. [PubMed] [Google Scholar]
- 6.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 7.Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84:108–13. doi: 10.4065/84.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elesber AA, Solomon H, Lennon RJ, Mathew V, Prasad A, et al. Coronary endothelial dysfunction is associated with erectile dysfunction and elevated asymmetric dimethylarginine in patients with early atherosclerosis. Eur Heart J. 2006;27:824–31. doi: 10.1093/eurheartj/ehi749. [DOI] [PubMed] [Google Scholar]
- 9.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 10.Burnett A. Ch. 24. Philadelphia: Elsevier Inc; 2011. Campbell-Walsh Urology. Evaluation and Management of Erectile Dysfunction. [Google Scholar]
- 11.Miner M, Nehra A, Jackson G, Bhasin S, Billups K, et al. All men with vasculogenic erectile dysfunction require a cardiovascular workup. Am J Med. 2014;127:174–82. doi: 10.1016/j.amjmed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Ralph D, McNicholas T. UK management guidelines for erectile dysfunction. Br Med J. 2000;321:499–503. doi: 10.1136/bmj.321.7259.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corona G, Petrone L, Mannucci E, Mansani R, Balercia G, et al. Difficulties in achieving vs maintaining erection: organic, psychogenic and relational determinants. Int J Impot Res. 2005;17:252–8. doi: 10.1038/sj.ijir.3901298. [DOI] [PubMed] [Google Scholar]
- 14.Paul JF, Virag R. Does anatomy of the pubic arch interfere with the maintaining of erection? J Sex Med. 2013;10:777–81. doi: 10.1111/jsm.12026. [DOI] [PubMed] [Google Scholar]
- 15.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24:S17–37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 16.Andersen KV, Bovim G. Impotence and nerve entrapment in long distance amateur cyclists. Acta Neurol Scand. 1997;95:233–40. doi: 10.1111/j.1600-0404.1997.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 17.Sommer F, Goldstein I, Korda JB. Bicycle riding and erectile dysfunction: a review. J Sex Med. 2010;7:2346–58. doi: 10.1111/j.1743-6109.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- 18.Yao F, Huang Y, Zhang Y, Dong Y, Ma H, et al. Subclinical endothelial dysfunction and low-grade inflammation play roles in the development of erectile dysfunction in young men with low risk of coronary heart disease. Int J Androl. 2012;35:653–9. doi: 10.1111/j.1365-2605.2012.01273.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung E, De Young L, Brock GB. Penile duplex ultrasonography in men with Peyronie's disease: is it veno-occlusive dysfunction or poor cavernosal arterial inflow that contributes to erectile dysfunction? J Sex Med. 2011;8:3446–51. doi: 10.1111/j.1743-6109.2011.02501.x. [DOI] [PubMed] [Google Scholar]
- 20.Miner MM, Seftel AD. Peyronie's disease: epidemiology, diagnosis, and management. Curr Med Res Opin. 2014;30:113–20. doi: 10.1185/03007995.2013.842544. [DOI] [PubMed] [Google Scholar]
- 21.Rosen R, Catania J, Lue T, Althof S, Henne J, et al. Impact of Peyronie's disease on sexual and psychosocial functioning: qualitative findings in patients and controls. J Sex Med. 2008;5:1977–84. doi: 10.1111/j.1743-6109.2008.00883.x. [DOI] [PubMed] [Google Scholar]
- 22.Erdogru T, Kadioglu A, Cayan S, Tellaloglu S. Does the positive intracavernous papaverine test always indicate a normal penile vascular system? Eur Urol. 1997;31:323–8. doi: 10.1159/000474476. [DOI] [PubMed] [Google Scholar]
- 23.Quam JP, King BF, James EM, Lewis RW, Brakke DM, et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. AJR Am J Roentgenol. 1989;153:1141–7. doi: 10.2214/ajr.153.6.1141. [DOI] [PubMed] [Google Scholar]
- 24.Naroda T, Yamanaka M, Matsushita K, Kimura K, Kawanishi Y, et al. Clinical studies for venogenic impotence with color Doppler ultrasonography - Evaluation of resistance index of the cavernous artery. Nihon Hinyokika Gakkai Zasshi. 1996;87:1231–5. doi: 10.5980/jpnjurol1989.87.1231. [DOI] [PubMed] [Google Scholar]
- 25.Shamloul R. Peak systolic velocities may be falsely low in young patients with erectile dysfunction. J Sex Med. 2006;3:138–43. doi: 10.1111/j.1743-6109.2005.00136.x. [DOI] [PubMed] [Google Scholar]
- 26.Philip F, Shishehbor M. Current state of endovascular treatment for vasculogenic erectile dysfunction. Peripher Vasc Dis. 2013:15–360. doi: 10.1007/s11886-013-0360-8. [DOI] [PubMed] [Google Scholar]
- 27.Vargas HA, Donati OF, Wibmer A, Goldman DA, Mulhall JP, et al. Association between penile dynamic contrast-enhanced mri-derived quantitative parameters and self-reported sexual function in patients with newly diagnosed prostate cancer. J Sex Med. 2014 doi: 10.1111/jsm.12555. [DOI] [PubMed] [Google Scholar]
- 28.Keller JJ, Liang YC, Lin HC. Association between multiple sclerosis and erectile dysfunction: a nationwide case-control study. J Sex Med. 2012;9:1753–9. doi: 10.1111/j.1743-6109.2012.02746.x. [DOI] [PubMed] [Google Scholar]
- 29.Keller J, Chen YK, Lin HC. Association between epilepsy and erectile dysfunction: evidence from a population-based study. J Sex Med. 2012;9:2248–55. doi: 10.1111/j.1743-6109.2012.02670.x. [DOI] [PubMed] [Google Scholar]
- 30.Mallet R, Tricoire JL, Rischmann P, Sarramon JP, Puget J, et al. High prevalence of erectile dysfunction in young male patients after intramedullary femoral nailing. Urology. 2005;65:559–63. doi: 10.1016/j.urology.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui MA, Peng B, Shanmugam N, Yeo W, Fook-Chong S, et al. Erectile dysfunction in young surgically treated patients with lumbar spine disease: a prospective follow-up study. Spine (Phila Pa 1976) 2012;37:797–801. doi: 10.1097/BRS.0b013e318232601c. [DOI] [PubMed] [Google Scholar]
- 32.Bemelmans B, Hendrixkx L, Koldewijn E, Lemmens W. Comparision of biothesiometry and neurophysiological investigations for the clinical evaluation of patients with erectile dysfunction. 1995;153:1483–6. [PubMed] [Google Scholar]
- 33.Kaneko S, Bradley WE. Penile electrodiagnosis. Value of bulbocavernosus reflex latency versus nerve conduction velocity of the dorsal nerve of the penis in diagnosis of diabetic impotence. J Urol. 1987;137:933–5. doi: 10.1016/s0022-5347(17)44298-4. [DOI] [PubMed] [Google Scholar]
- 34.Amarenco G, Kerdraon J. Perineal electrophysiological studies in erectile dysfunctions. Study of 423 cases. Ann Med Internet. 1993;144:383–8. [PubMed] [Google Scholar]
- 35.Yiou R, De Laet K, Hisano M, Salomon L, Abbou CC, et al. Neurophysiological testing to assess penile sensory nerve damage after radical prostatectomy. J Sex Med. 2012;9:2457–66. doi: 10.1111/j.1743-6109.2012.02793.x. [DOI] [PubMed] [Google Scholar]
- 36.Rochira V, Zirilli L, Orlando G, Santi D, Brigante G, et al. Premature decline of serum total testosterone in HIV-infected men in the HAART-era. PLoS One. 2011;6:e28512. doi: 10.1371/journal.pone.0028512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young J. Approach to the male patient with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2012;97:707–18. doi: 10.1210/jc.2011-1664. [DOI] [PubMed] [Google Scholar]
- 38.Veronelli A, Masu A, Ranieri R, Rognoni C, Laneri M, et al. Prevalence of erectile dysfunction in thyroid disorders: comparison with control subjects and with obese and diabetic patients. Int J Impot Res. 2006;18:111–4. doi: 10.1038/sj.ijir.3901364. [DOI] [PubMed] [Google Scholar]
- 39.Krassas GE, Tziomalos K, Papadopoulou F, Pontikides N, Perros P. Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat? J Clin Endocrinol Metab. 2008;93:1815–9. doi: 10.1210/jc.2007-2259. [DOI] [PubMed] [Google Scholar]
- 40.Bortolotti F, Coscelli A, Santeusanio F, Chatenoud L, Collie E. Erectile dysfunction in type 1 and type 2 diabetics in Italy. J Epidemiol. 2000;29:524–31. [PubMed] [Google Scholar]
- 41.Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Ranasinghe P, et al. Erectile dysfunction is a strong predictor of poor quality of life in men with type 2 diabetes mellitus. Diabet Med. 2014;31:699–706. doi: 10.1111/dme.12412. [DOI] [PubMed] [Google Scholar]
- 42.Morgentaler A, Khera M, Maggi M, Zitzmann M. Commentary: who is a candidate for testosterone therapy. A synthesis of international expert opinions? J Sex Med. 2014;11:1636–45. doi: 10.1111/jsm.12546. [DOI] [PubMed] [Google Scholar]
- 43.Corona G, Petrone L, Paggi F, Lotti F, Boddi V, et al. Sexual dysfunction in subjects with Klinefelter's syndrome. Int J Androl. 2010;33:574–80. doi: 10.1111/j.1365-2605.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 44.Maggi M, Buvat J, Corona G, Guay A. Hormonal causes of male sexual dysfunctions and their management (Hyperprolactinemia, thyroid disorders, GH Disorders, and DHEA) J Sex Med. 2012;10:661–77. doi: 10.1111/j.1743-6109.2012.02735.x. [DOI] [PubMed] [Google Scholar]
- 45.Trajanovska A, Vujovic V, Ignjatova L. Sexual dysfunction as a side effect of hyperprolactinemia in methadone maintenance therapy. Med Arh. 2013;67:48–50. doi: 10.5455/medarh.2013.67.48-50. [DOI] [PubMed] [Google Scholar]
- 46.De Rosa M, Zarrilli S, Vitale G, Di Somma C, Orio F, et al. Six months of treatment with cabergoline restores sexual potency in hyperprolactinemic males: an open longitudinal study monitoring nocturnal penile tumescence. J Clin Endocrinol Metab. 2004;89:621–5. doi: 10.1210/jc.2003-030852. [DOI] [PubMed] [Google Scholar]
- 47.Jaffe CA. Clinically non-functioning pituitary adenoma. Pituitary. 2006;9:317–21. doi: 10.1007/s11102-006-0412-9. [DOI] [PubMed] [Google Scholar]


