Abstract
The aim of this study was to evaluate the semen quality of university students in Wuhan, the largest city in the world in terms of the number of university students. All student sperm donors recorded in the Hubei Province Human Sperm Bank from 1 March 2010 to 31 December 2013 were screened. At last, a total of 3616 semen samples from 1808 university student sperm donors were eligible and retrospectively analyzed. Each donor's semen parameters were averaged over two samples and compared with the World Health Organization criteria, and a generalized linear regression model was used to examine several determinants of semen quality. We found that the mean and median values were 3.0 ml and 2.8 ml for semen volume, 50.2 × 106 ml−1 and 50.0 × 106 ml−1 for sperm concentration, 148.1 × 106 and 142.1 × 106 for total sperm count, and 58.6% and 60.0% for total sperm motility. About 85.0% of donors had parameters that were all normal. Season and duration of abstinence were critical factors affecting semen quality. We also found a decrease in sperm concentration during the 4 years observation; however, this may not be a strong evidence to confirm the declining trend of semen quality. In conclusion, semen quality of university students in Wuhan was not optimal and should be paid high attention, long-term observation and further study should be carried out to confirm the present situation.
Keywords: semen analysis, semen quality, sperm donors, university students
INTRODUCTION
During the past several decades, many reports have suggested that the quality of semen in healthy men is declining,1,2,3 Swan et al.4 also corroborated a large annual decline in sperm concentration in European men (2.3%) and a smaller decline in US men (0.8%). However, this is a controversial issue, with several others reporting no significant change in human semen quality,5,6 these inconsistent conclusions may be due to geographic factors.
Young university students, who are at the highly reproductive period of life, may also exhibit a declining trend in semen quality. Mendiola et al.7 found that sperm concentration and total sperm count may have declined in young Spanish university students over the last decade. Li et al.8 also found that the sperm concentration and sperm viability rate of university students in the Chengdu area (South-West China) had a tendency to decrease. The factors leading to changes in human semen quality may be complex; environmental pollutants, seasonal variations and increased stress may be critical risk factors.9,10,11
Until date, large studies on the semen quality of university students in China have been rare. As the largest city in central China, Wuhan is an important part of the economic zone along the Yangtze River and an industrialized city with a high level of environment pollution.12 Because it has a hotter and longer summer than most other cities, Wuhan is called one of the “three furnaces” of China, where the temperature difference between summer and winter is large. Wuhan is also a major center of higher education in China; 85 colleges and universities are located there and more than 1.18 million students study there, making it the largest city in terms of university student numbers not only in China, but also in the world. As a result of the city's unique geographic and climate characteristics, university students in Wuhan may have semen characteristics which are distinct from those in other populations or other areas. Therefore, in this study we aimed to evaluate the semen quality of a large population of university students in Wuhan, China; to investigate whether the semen parameters in university students have declined in the past 4 years; and to determine whether age, season and abstinence were related to semen quality.
MATERIALS AND METHODS
This retrospective study was performed at the Hubei Province Human Sperm Bank of China. Screening data for all student sperm donors recorded in the Hubei Province Human Sperm Bank from 1 March 2010 to 31 December 2013 were reviewed, and relevant demographic and clinical information was collected and analyzed. Demographic information included age and university name. Clinical information included semen parameters, duration of abstinence and the date of semen analysis.
Criteria and screening of sperm donors in China
The screening of sperm donors are conducted strictly in accordance to the standard published by the Chinese Ministry of Health in 2003. The guidelines are the following:13 (1) donors must be between 22 and 44 years of age; (2) donors must be in good health, based on both physical examination and psychological evaluation by qualified doctors, and have no history of genetic disease in their family; (3) fresh semen is required to have a liquefaction time <60 min, sperm concentration ≥60 × 106 ml−1, progressive sperm motility ≥60% and percentage of normal morphology >30%; (4) postthaw semen is required to have a motility ≥40%, number of motile sperm per vial ≥12 × 106 and frozen-thaw survival rate ≥60%; (5) potential donors must undergo laboratory testing to exclude individuals at high risk for sexually transmitted infections and genetic diseases, including; HIV-1 and -2, hepatitis B and C, syphilis, gonorrhea, mycoplasma, chlamydia, cytomegalovirus, Toxoplasma gondii, Rubella virus, herpes simplex virus types 1 and 2, and karyotype analysis. If the results from all tests are negative, the donation process begins and semen samples are cryopreserved. The samples must be cryopreserved for a minimum 6 months quarantine period prior to allowing for HIV rescreening.
Screening criteria of sperm donors in our study
The screening criteria of sperm donors in our study were as follows: (1) male university students in Wuhan, 22–30 years old; (2) 2–7 days of abstinence before each ejaculation; (3) sperm donation of at least two samples (the first two eligible semen samples were screened); and (4) an interval between two semen samples of 2–28 days. A total of 1808 student donors with 3616 semen samples were eligible and screened for entry into the data analysis.
This study was approved by the Ethics Committee of the Reproductive Medicine Center, Tongji Medical College, Huazhong University of Science and Technology. All donors signed informed consent forms during their first visit to the human sperm bank, agreeing that their semen samples or data could be used by the human sperm bank for scientific research.
Semen analysis
All semen samples were obtained by masturbation into a wide-mouthed sterile plastic container in a separate room in the human sperm bank and were immediately delivered to the laboratory. Sexual abstinence for 2–7 days was requested, and the exact duration (in days) of abstinence was documented for each donor. The semen samples were marked with an anonymous serial number and were then incubated in a water-bath at 37°C pending analysis. All samples were analyzed within 60 min of collection.
Semen analysis was carried out following the recommendations of the World Health Organization (WHO) Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction.14 The semen quality parameters that were assessed included appearance, semen volume, viscosity, agglutination, liquefaction time, pH value, sperm concentration, sperm motility, and percentage of motile sperm. Semen volume was evaluated by semen weight, assuming a density of 1.0 g ml−1. The container was weighed before and after sample collection, and the difference between the weights was recorded as the volume. The pH value was measured using pH paper and compared with the calibration strip to determine the pH value. For the assessment of sperm concentration and motility, 10 μl of well-mixed semen was placed in a clean Makler chamber (which had been held at 37°C) and covered with the coverslip, then immediately examined at a total magnification of ×400. Ten of the 100 squares in the microscope field were randomly scanned and the sperm count was recorded by cytometer. With the help of an ocular grid, the proportion in each of four motility categories was assessed: fast progressive sperm (A), slow progressive sperm (B), nonprogressive sperm (C) and immotile sperm (D). To reduce variation in the assessment of sperm characteristics, all semen sample analyses were performed by three well-trained laboratory technologists using the same apparatus. During the research, internal quality control was performed to ensure that there was no significant difference between the results of the three technicians.
Statistical analysis
All the screened eligible donors provided at least two semen samples after 2–7 days of abstinence, of which the first two eligible samples were analyzed. To ensure more reliable results, we averaged the value of the seven semen parameters and the duration of abstinence over the two semen samples for statistical analysis. Because the distributions of these parameters were not normal, the percentiles, medians, and means were calculated. Percentages in accordance with the WHO criteria15 were also calculated. The data were summarized using medians, 25th and 75th percentiles, stratified by age, season of sperm donation (the date was determined according to the first donation time of the two screened donations), year of donation and duration of abstinence before ejaculation. We used the Kruskal–Wallis analysis of variance to compare medians between groups.
A generalized linear regression model was also used to examine the independent effects of risk factors on the semen parameters. All semen parameters were log-transformed (base e) to improve the normality of the dependent variables in the linear models. The possible risk factors, as independent variables, were re-evaluated with dummy variables representing different levels. The independent variables entered into the regression model were as follows: (1) age (years): 22–24, 25–27, 28–30, with 22–24 as the reference value; (2) year: 2010, 2011, 2012, 2013, with 2010 as the reference value; (3) season: spring (March-May), summer (June-August), autumn (September-November), winter (December-February the following year), with spring as the reference value; (4) duration of abstinence: 2–3.5 days, 4–5 days, 5.5–7 days, with 2–3.5 days as the reference value. Statistical analysis was performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA); P < 0.05 was considered as significant.
RESULTS
Subject characteristics
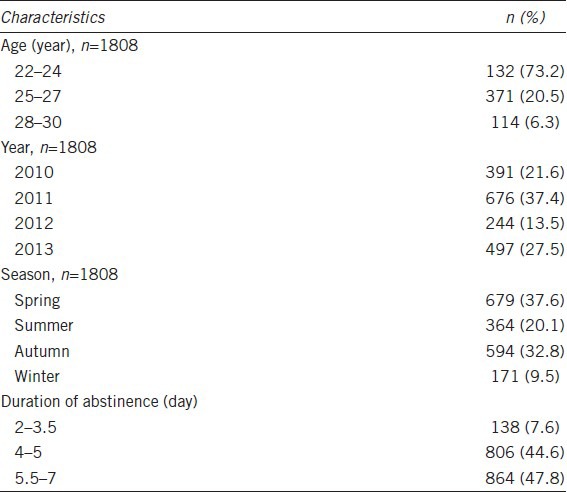
A total of 1808 eligible donors with 3616 semen samples were screened for entry into the data analysis. The general characteristics of the 1808 subjects were summarized in Table 1. The majority of the student donors (73.2%) were 22–24 years old, and the number of students donating sperm in 2011 (37.4%) and 2013 (27.5%) was more than that in 2010 (21.6%) and 2012 (13.5%). Students were more likely to donate sperm in spring and autumn than in summer and winter. The mean duration of abstinence was 5.1 days.
Table 1.
Characteristics of participants
Semen parameters
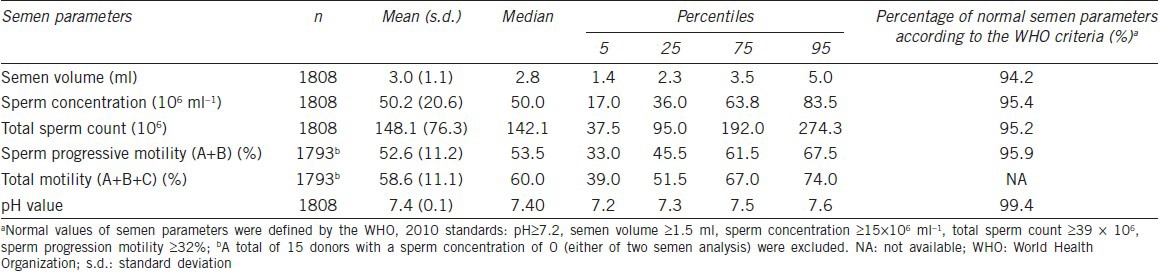
Table 2 shows the semen parameters of the study participants. All the referred semen parameters (semen volume, sperm concentration, total sperm count, progressive motility and pH value) were within high-normal values (94.2%, 95.4%, 95.2%, 95.9% and 99.4%, respectively) according to the WHO criteria (5th edition). Of all 1808 student donors, about 85.0% had parameters that were all normal, and 15.0% had at least one of the above semen parameters below normal threshold values. A total of 9 students (0.5%) were azoospermia (concentration of 0 was found in both the two semen analysis after centrifugation), 61 students (3.4%) were oligoozospermia (concentration <15 × 106 ml−1 was found in both the two semen analysis) and 15 students (0.8%) were severe oligoozospermia (concentration <5 × 106 ml−1 was found in both the two semen analysis).
Table 2.
Summary of semen parameters
Effect of various factors on semen parameters
Table 3 displays the effect of various factors on semen quality. The semen samples were grouped separately according to age, year, season and abstinence. The different semen parameters were examined and compared in relation to these variables. All semen parameters, except for sperm concentration (P = 0.004), were not significantly different between the different age groups. Regarding the year groups, all parameters were significantly different; semen volume showed a tendency to increase, but sperm concentration decreased, total sperm count showed erratic changes during the 4 years observation. Sperm concentration and total sperm count in spring were much higher than in other seasons. Duration of abstinence could obviously affect the semen parameters, it was positively correlated to semen volume (P < 0.001), sperm concentration (P = 0.049) and total sperm count (P < 0.001), but negatively related to progressive motility (P < 0.001) and total motility (P < 0.001).
Table 3.
Summary of semen parameters according to age, year, season, education and duration of abstinence
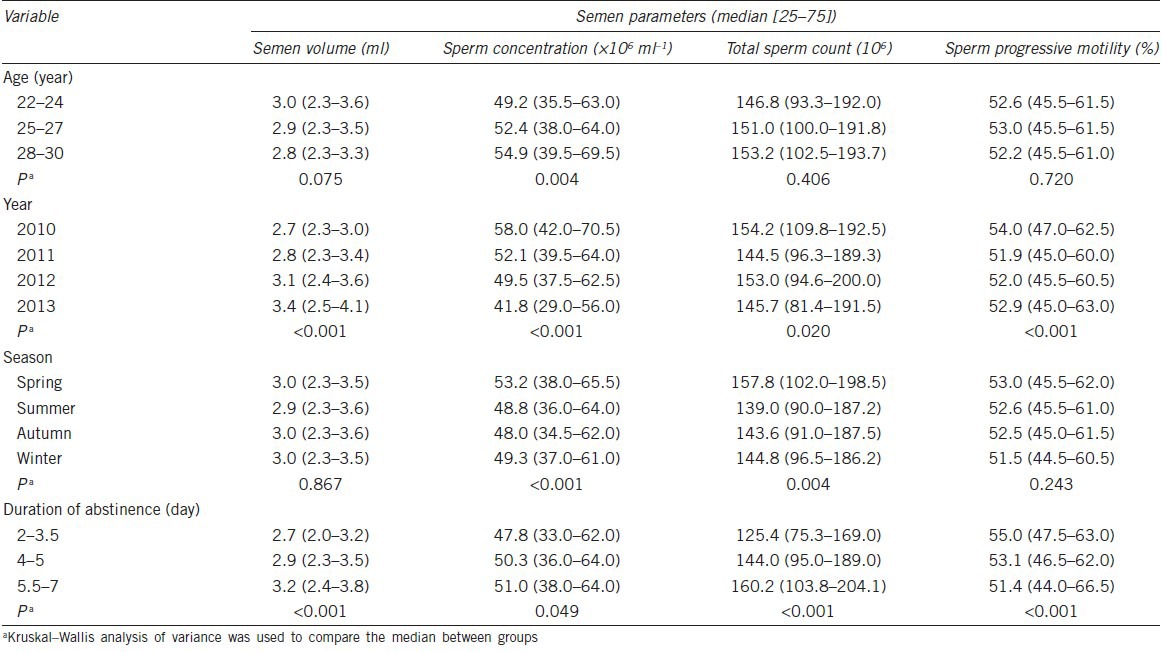
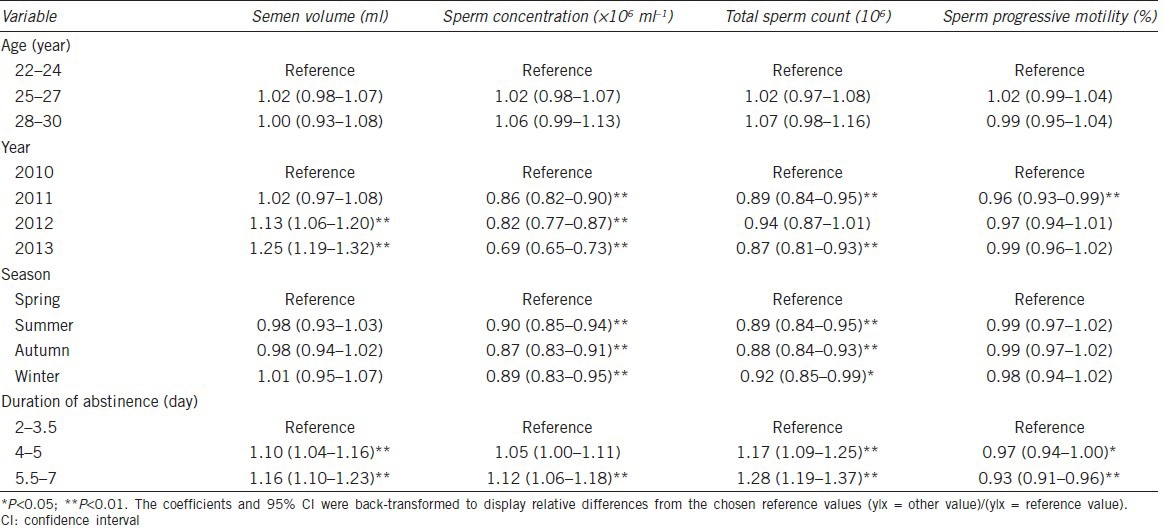
Table 4 shows the adjusted regression coefficients and P values for all considered factors in relation to the semen parameters. The coefficients listed were converted back to the original measure to reflect relative differences compared with the reference values. There was no significant difference in semen parameters among different age groups. Sperm concentration and total sperm count were significantly related to year, and semen volume was erratic in different years. Variation of seasons could obviously affect sperm concentration and total sperm count with the highest value in spring and lowest value in autumn. As for duration of abstinence, it was positively associated with semen volume, sperm concentration and total sperm count, but was negatively associated with progressive sperm motility.
Table 4.
Effects of various factors on semen parameters
DISCUSSION
Semen analysis is one of the most valuable methods for evaluating male reproductive health, and plays an important role in andrology. Nowadays, studies focusing on the semen quality of young university students are rare. In this study, 1808 eligible university students with 3616 semen samples were screened and analyzed. To our knowledge, this is the largest study focusing on the semen quality of university students in China. Sperm donors in China should be no <22 years old; thus we screened university student sperm donors aged from 22 to 30 years. To obtain more reliable results, the average value of the semen parameters and duration of abstinence taken over two instances of semen donation were used for statistical analysis.
Our results showed that the semen quality of the university student donors was not optimal. As shown in Table 5, semen volume as well as total sperm motility in our study was consistent with other reports from China and other areas in Europe in general. Sperm concentration in our study (mean value 50.2 × 106 ml−1) were lower than that in other studies in China16,17,18,19 and Sweden5 (mean value ranging from 55.5 × 106 ml−1 to 84.8 × 106 ml−1), but was similar to the result in Mendiola's study,7 which reported an obvious declining trend in sperm concentration and total sperm count among young Spanish university students. Total sperm count was lower than part of these studies, but was consistent with the result in Junqing's study,16 which also focused on the semen quality of young Chinese men with 22–30 years old. We also found a decrease in sperm concentration in our study during the 4 years observation (from 58.0 × 106 ml−1 in 2010 to 41.8 × 106 ml−1 in 2013, Table 3). After adjusting for potential confounders (age, year, season and duration of abstinence), sperm concentration and total sperm count also showed a tendency to decrease (Table 4), even the 4 years observation might not be a strong evidence to confirm the declining trend in semen quality; the phenomenon should be paid high attention.
Table 5.
Summary of semen parameters of studies in different areas
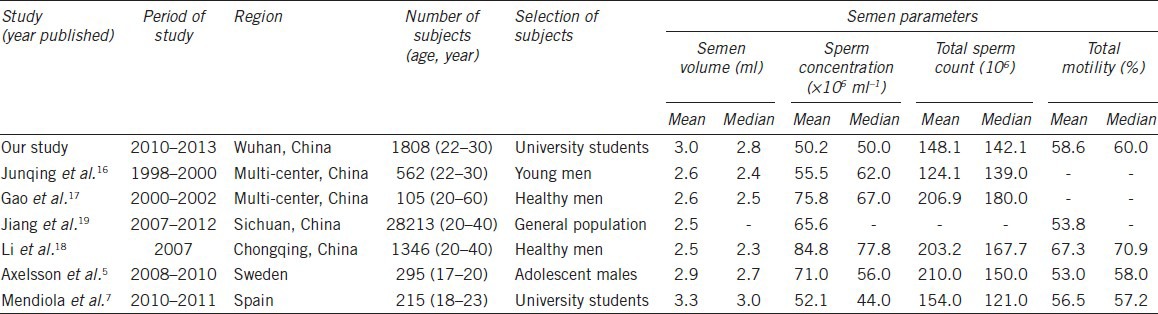
Our results showed that sperm concentration and total sperm count was lower than most of other referred studies (Table 5), and sperm concentration in 2013 was only 41.8 × 106 ml−1 (Table 3). The reasons leading to the serious situation should be deeply investigated. We speculate the reasons may be complex, such as environmental pollution, increased stress, climate, or a combination of these factors. Even though regional difference was reported to affect semen quality,20,21 university students in Wuhan came from different areas of China, so we think environmental pollution, stress as well as climate might be the key factors. Wuhan is an industrialized city with high levels of air pollution,12 these pollutants may originate from industrial emissions, vehicle exhausts, and burning of agricultural waste. Water pollutants such as polychlorinated biphenyls and heavy metal were found in high concentrations at several sites along the Wuhan section of the Yangtze River,22,23 which provided drinking water source for the citizen. Both the referred ambient air pollutants and water pollutants were reported to have a range of adverse effects on reproductive health,9,22,24 thus we speculate that pollutants may play a role in damaging human spermatogenesis. As Wilcox and Bonde25 stated, future fertility studies should consider the wide spectrum of environmental exposures that plausibly affect reproduction. Hence, we emphasize that semen quality of university students exposed to detrimental environmental factors should be monitored in the coming years. In addition, nowadays university students face greater psychological stress from study, emotions, and looking for work, which also have adverse effects on semen quality.11,26
Climate is another key factor that should not be ignored. The weather in Wuhan is characterized by a long and hot summer, cold winter, and short spring and autumn. Previous studies have indicated that semen quality was significantly correlated with temperature. Levitas et al.27 showed that sperm concentration and rapid progressive sperm motility decreased significantly from spring through summer and autumn, with recovery observed during winter; there was no change in semen volume. However, Zhang et al.10 indicated that semen volume in spring and autumn was significantly higher than that in other seasons, sperm concentration in summer was significantly lower than that in other seasons, and no difference was observed in spring and winter. Our results were not absolutely coincident with these; in our study, semen volume did not show obvious seasonal variation whereas sperm concentration and total sperm count decreased significantly from spring to summer and autumn, with no obvious recovery in winter, which may be because we divided the year into the traditional 3 months for each season. The weather may become hot in May and continue so even until October; as high temperature has an adverse effect on spermatogenesis, this may continue for a long time even after the temperature has dropped. In addition, seasonal variation may be a co-effect with air pollution in damaging human health, the acute effects of particulate air pollution may vary by season, with the largest effect in China occurring in winter and summer.28 Another phenomenon, that of people continually wearing thermal underwear in the cold of winter, should be considered, as it is known that thermal underwear can elevate scrotal temperature and affect semen parameters.29 In summary, we speculate that in our study the lower values for sperm concentration and total sperm count in winter may be due to seasonal particularity, heavier pollution, and the clothes-wearing habits of students.
The relationship between age and semen parameters has been investigated for some time and the conclusions are controversial.30,31 In our study, age seemed to have no obvious effect on semen parameters after adjustment for other potential confounders, namely that semen quality may not change with the age ranged from 22 to 30 years.
It has been well-demonstrated that duration of abstinence may influence semen quality. Li et al.18 reported that variation in abstinence (2–7 days) could affect semen volume, sperm concentration and total sperm count but had no effect on sperm motility. Gao et al.30 also indicated that progressive motility and total motility of sperm were not significantly different between a short period of abstinence (2–3 days) and a long period of abstinence (4–7 days). However, another study showed that semen volume was similar in 2–5 days abstinence group and 6–7 days abstinence group, but sperm motility was significantly different.32 Our results were partially inconsistent with them. We found that semen volume, sperm concentration and total sperm count gradually increased with increasing extent of abstinence. However, increasing duration of abstinence was negatively and significantly related to the progressive motility and total sperm motility (Tables 3 and 4). The inconsistent conclusions may be due to different study populations and this issue needs further study. According to our results, we suggest that accurate abstinence time should be considered in semen analysis even when it is in the recommended range.
The sample size is the most important strength of our study; the inclusion of 3616 semen samples from 1808 healthy university students make this study one of the largest ever of university students. Furthermore, the screened participants in our study had to provide at least two semen samples and the semen parameters were averaged over these two samples to reduce variability in our estimates of the quality and instability of participants’ semen; in most studies, only one semen sample was evaluated for each participant. We realize that our study was not without limitations. First, it was not based on a community population. Second, we did not collect questionnaire data from the sperm donors so we cannot provide strong evidence on semen quality affected by other risk factors (such as body mass index, tobacco use, alcohol use, dietary patterns and occupational exposure). However, the participants were recruited through posters, newspapers and other media and eligible students came from 80 universities, which represent almost 90% of all universities in Wuhan. Therefore, the participants can to a large degree represent the university student population in Wuhan.
CONCLUSIONS
Our results showed that the semen quality of university students in Wuhan was not optimal, we speculated that the environment pollution, increased stress as well as climate factors may be the main reasons that should be monitored in the coming years. We also found a decrease in sperm concentration during the 4 years observation even though this may not be a strong evidence to confirm the declining trend of semen quality. Long-term observation and further study should be carried out to confirm the present situation.
AUTHOR CONTRIBUTIONS
TQM, SHH and HTG carried out the semen analysis. MR and WX collected and collated the data, conceived the study and drafted the manuscript. QYW performed the statistical analysis. CHZ and CLX supervised the project and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Science and Technology Support Program of the Ministry of Science and Technology (Grant No. 2012BAI32B03).
REFERENCES
- 1.James WH. Secular trend in reported sperm counts. Andrologia. 1980;12:381–8. doi: 10.1111/j.1439-0272.1980.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 2.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–5. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 3.Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–6. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axelsson J, Rylander L, Rignell-Hydbom A, Giwercman A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod. 2011;26:1012–6. doi: 10.1093/humrep/der045. [DOI] [PubMed] [Google Scholar]
- 6.Pacey AA. Are sperm counts declining? Or did we just change our spectacles? Asian J Androl. 2013;15:187–90. doi: 10.1038/aja.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendiola J, Jørgensen N, Mínguez-Alarcón L, Sarabia-Cos L, López-Espín JJ, et al. Sperm counts may have declined in young university students in Southern Spain. Andrology. 2013;1:408–13. doi: 10.1111/j.2047-2927.2012.00058.x. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Huang P, Ma HZ, Ge L, Xie Y, Wan QZ. Investigation on the sperm quality of 549 college students in Chengdu area. Zhonghua Nan Ke Xue. 2003;9:673–5, 678. [PubMed] [Google Scholar]
- 9.Pant N, Pant A, Shukla M, Mathur N, Gupta Y, Saxena D. Environmental and experimental exposure of phthalate esters: the toxicological consequence on human sperm. Hum Exp Toxicol. 2011;30:507–14. doi: 10.1177/0960327110374205. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XZ, Liu JH, Sheng HQ, Wu HJ, Wu Y, et al. Seasonal variation in semen quality in China. Andrology. 2013;1:639–43. doi: 10.1111/j.2047-2927.2013.00092.x. [DOI] [PubMed] [Google Scholar]
- 11.Gollenberg AL, Liu F, Brazil C, Drobnis EZ, Guzick D, et al. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril. 2010;93:1104–11. doi: 10.1016/j.fertnstert.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Xiang H, Mertz KJ, Arena VC, Brink LL, Xu X, et al. Estimation of short-term effects of air pollution on stroke hospital admissions in Wuhan, China. PLoS One. 2013;8:e61168. doi: 10.1371/journal.pone.0061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ping P, Zhu WB, Zhang XZ, Li YS, Wang QX, et al. Sperm donation and its application in China: a 7-year multicenter retrospective study. Asian J Androl. 2011;13:644–8. doi: 10.1038/aja.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. 4th ed. New York: Cambridge University Press; 1999. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 15.WHO. 5th ed. Geneva: World Health Organization; 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 16.Junqing W, Qiuying Y, Jianguo T, Wei Y, Liwei B, et al. Reference value of semen quality in Chinese young men. Contraception. 2002;65:365–8. doi: 10.1016/s0010-7824(02)00281-0. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Gao ES, Walker M, Yang Q, Wu JQ, et al. Reference values of semen parameters for healthy Chinese men. Urol Int. 2008;81:256–62. doi: 10.1159/000151400. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Lin H, Ma M, Li L, Cai M, et al. Semen quality of 1346 healthy men, results from the Chongqing area of South-West China. Hum Reprod. 2009;24:459–69. doi: 10.1093/humrep/den399. [DOI] [PubMed] [Google Scholar]
- 19.Jiang M, Chen X, Yue H, Xu W, Lin L, et al. Semen quality evaluation in a cohort of 28213 adult males from Sichuan area of South-West China. Andrologia 2013. 2013 Sep 30; doi: 10.1111/and.12168. doi: 10.1111/and.12168. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, et al. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–20. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu JQ, Yang QY, Tao JG, Li WY, Gao ES, et al. Epidemiological study on semen quality of 562 volunteers aged 22-30. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:44–8. Article in Chinese. [PubMed] [Google Scholar]
- 22.Yang Z, Shen Z, Gao F, Tang Z, Niu J. Occurrence and possible sources of polychlorinated biphenyls in surface sediments from the Wuhan reach of the Yangtze River, China. Chemosphere. 2009;74:1522–30. doi: 10.1016/j.chemosphere.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, Wang Y, Shen Z, Niu J, Tang Z. Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater. 2009;166:1186–94. doi: 10.1016/j.jhazmat.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Giaccio L, Cicchella D, Vivo BD, Lombardi G, Rosa MD. Does heavy metals pollution affects semen quality in men? A case of study in the metropolitan area of Naples (Italy) J Geochem Explor. 2012;112:218–25. [Google Scholar]
- 25.Wilcox AJ, Bonde JP. On environmental threats to male infertility. Asian J Androl. 2013;15:199–200. doi: 10.1038/aja.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lampiao F. Variation of semen parameters in healthy medical students due to exam stress. Malawi Med J. 2009;21:166–7. doi: 10.4314/mmj.v21i4.49635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitas E, Lunenfeld E, Weisz N, Friger M, Har-Vardi I. Seasonal variations of human sperm cells among 6455 semen samples: a plausible explanation of a seasonal birthpattern. (e1–6).Am J Obstet Gynecol. 2013;208:406. doi: 10.1016/j.ajog.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Peng RD, Meng X, Zhou Z, Chen B, et al. Seasonal variation in the acute effect of particulate air pollution on mortality in the China Air Pollution and Health Effects Study (CAPES) (259-65).Sci Total Environ. 2013:450–451. doi: 10.1016/j.scitotenv.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanger WG, Friman PC. Fit of underwear and male spermatogenesis: a pilot investigation. Reprod Toxicol. 1990;4:229–32. doi: 10.1016/0890-6238(90)90063-2. [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Gao ES, Yang Q, Walker M, Wu JQ, et al. Semen quality in a residential, geographic and age representative sample of healthy Chinese men. Hum Reprod. 2007;22:477–84. doi: 10.1093/humrep/del383. [DOI] [PubMed] [Google Scholar]
- 31.Levitas E, Lunenfeld E, Weisz N, Friger M, Potashnik G. Relationship between age and semen parameters in men with normal sperm concentration: analysis of 6022 semen samples. Andrologia. 2007;39:45–50. doi: 10.1111/j.1439-0272.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 32.Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, et al. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–54. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]


