Abstract
We compared the efficacy and safety between once-daily dosing and on-demand use of udenafil for type 2 diabetic patients with erectile dysfunction (ED). A multi-center, randomized, open-label, parallel-group, 12-week study was conducted. 161 patients who improved with on-demand 200 mg of udenafil according to Sexual Encounter Profile (SEP) diary Question 2 and 3 (Q2 and Q3) were randomized into 200 mg on-demand (n = 80) or 50 mg once-daily (n = 81) dosing groups for 8 weeks. The dosing period was followed by a 4-week treatment-free period. The primary efficacy endpoint was the change of the International Index of Erectile Function (IIEF) erectile function domain (EFD) score. The secondary efficacy endpoints included changes to the SEP diary Q2, Q3, IIEF Q3, Q4, other domains of IIEF, Global Assessment Question, and shift to the normal rate (EFD ≥ 26). Vascular endothelial markers were also assessed. The IIEF-EFD score of both groups improved after 8 weeks of treatment (P < 0.0001). There was no statistically significant difference between two groups. Improvement was not maintained after the treatment-free follow-up period. Similar results were observed in the secondary efficacy endpoints. There was also no significant difference in vascular endothelial markers. Daily udenafil was well-tolerated, and there was no significant difference in the adverse drug reactions and adverse events between the two groups. Flushing and headache were the most frequent adverse events. Both regimens improved ED in diabetic patients and were well-tolerated. Further studies are needed to assess the effect of daily udenafil treatment in diabetic patients.
Keywords: erectile dysfunction, International Index of Erectile Function, once-daily dosing, phosphodiesterase type 5 inhibitor, type 2 diabetes mellitus, udenafil
INTRODUCTION
Erectile dysfunction (ED) is defined as the consistent inability to attain or maintain a penile erection of sufficient quality to permit satisfactory sexual intercourse.1 ED is a common complication of diabetes, with an estimated prevalence of 20%–85% (ranging from mild to complete ED),2 which occurs at an earlier age than in nondiabetic men. In a cross-sectional survey of 541 diabetic male patients at a community-based clinic, the prevalence of ED increased progressively with age, from 6% in men aged 20–24 years to 52% in those aged 55–59 years.3 Diabetic men with ED tend to be less responsive to treatment because the pathogenesis of diabetes-associated ED may be multifactorial as indicated from data generated from experimental studies.4,5 Although treatment with phosphodiesterase type 5 (PDE5) inhibitor is less effective in diabetic patients than in nondiabetic ED patients, PDE5 inhibitor is well-tolerated and effective in improving ED in men with diabetes.6,7,8 In addition, PDE5 inhibitors are currently the first-line oral therapy for patients who experience ED of any etiology.9 Accumulating evidence indicates that daily administration of a PDE5 inhibitor is effective and well-tolerated treatment in improving erectile function10,11 and endothelial function12,13,14 in ED patients.
Udenafil is a selective PDE5 inhibitor. Based on clinical kinetics data of phase 1 trials involving healthy male subjects, udenafil is rapidly absorbed, reaching peak plasma concentrations at 0.8–1.3 h and then declines monoexponentially, with a terminal half-life (T1/2) between 7.3 and 12.1 h in the single-dose group.15,16,17 Udenafil demonstrated significant improvement in erectile function and was well-tolerated in diabetic patients.18 The objective of this study was to compare the efficacy and safety between once-daily dosing and on-demand use of udenafil for type 2 diabetic patients with ED and assess udenafil's effect on endothelial biomarkers.
MATERIALS AND METHODS
Study design
This randomized, open-label, parallel-group study was conducted in seven centers in Korea. There was a 4-week run-in period after screening. During the run-in period patients were administered four tablets of 200 mg of udenafil (ZydenaTM, Dong-A ST, Seoul, Korea) and asked to have four sexual intercourses 4 times each on separate days. Those patients who answered “yes” >50% to Sexual Encounter Profile (SEP) Question 2 and 3 (Q2 and Q3) were randomized to an 8-week treatment period with on-demand 200 mg of udenafil or 50 mg of udenafil daily. A 4-week treatment-free period followed the treatment period. The study design is illustrated in Figure 1. The participants visited the hospital every 4 weeks after screening. The study medicine, udenafil, was controlled to match the same total dose in both groups. For the on-demand regimen, the patients were educated take 200 mg of udenafil from 30 min to 12 h before sexual intercourse and the maximal dose was 200 mg once a day. For the daily dosing regimen, the patients were instructed to take one dose of 50 mg of udenafil at the same time of the day. Efficacy and safety data were collected at screening, baseline, after the treatment period and after the treatment-free follow-up period. The trial was approved by the “Ministry of Food and Drug Safety” of South Korea. Clinical trial plan number: DA8159_DDM_IV. KFDA IND approval number: pharmaceutical management division-462.
Figure 1.
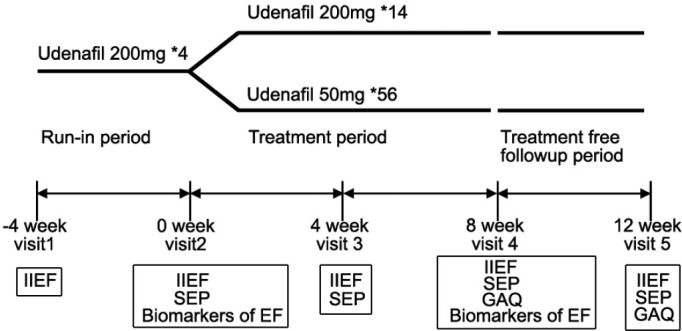
Study design. The efficacy variables listed in the box were assessed at each visit. IIEF: International Index of Erectile Function; SEP: Sexual Encounter Profile; GAQ: Global Assessment Question; EF: endothelial function. *Number of provided tablets.
Inclusion and exclusion criteria
Type 2 diabetic male patients aged 35–65 years who were in a stable heterosexual relationship and experienced ED for more than 6 months were included. The hemoglobin A1c level had to be ≤9% for at least 3 months, and the International Index of Erectile Function (IIEF) erectile function domain (EFD) score was required to be ≤25. All included patients understood and agreed to provide written informed consent form, and the trial was approved by Institutional Review Board of each institution and the Ministry of Food and Drug Safety of South Korea.
Patients who experienced stroke, myocardial infarction, coronary artery bypass graft surgery, heart failure, unstable angina, life-threatening arrhythmia or serious hypoglycemia during the previous 6 months were not allowed to participate. In addition, patients with the following conditions were excluded: diabetic ketoacidosis within the previous 3 years, proliferative diabetic retinopathy, other specific types of diabetes, spinal cord injury, prior radical prostatectomy or radical pelvic surgery, penile anatomic defect, uncontrolled hypotension or hypertension, hyperprolactinemia, low total testosterone level, aspartate transaminase/alanine transaminase level >3 times upper limit of normal, creatinine level >2.0 mg dl−1, use of nitrates or nitric oxide donor drugs or hypersensitivity or nonresponse to the PDE5 inhibitor. In addition, patients who were not interested in having sexual intercourse at least 4 times on different days during the run-in period of the study or who received any treatment for ED, including PDE5 inhibitor, in 2 weeks before the trial were excluded.
Efficacy variable
The primary efficacy measure was the change of the EFD score of the validated IIEF questionnaire19 before and after treatment in each group. The IIEF questionnaire was administered at every visit, including the screening visit. The secondary efficacy measures included the following: (1) IIEF Q3 (over the last month, when you attempted intercourse, how often were you able to penetrate your partner?) and Q4 (over the last month, during sexual intercourse, how often were you able to maintain your erection after you had penetrated your partner?), (2) the scores of the other four domains of IIEF, (3) SEP Q2 (“were you able to insert your penis into your partner's vagina?) and Q3 (did your erection last long enough for you to complete intercourse with ejaculation?), (4) the Global Assessment Question (GAQ, “has the treatment you have been taking over the last 4 weeks improved your erections?”), (5) the percentage of patients exhibiting a “shift to normal” (IIEF-EFD score ≥ 26), and (6) a change in endothelial biomarkers (endothelin-1, vascular cell adhesion molecule 1, intercellular adhesion molecule 1, high-sensitivity C-reactive protein, and tumor necrosis factor-alpha [TNF-α]).
Safety
All adverse events and adverse drug reactions were monitored and recorded. For each adverse event, the investigator assessed the severity (mild, moderate, or severe), and the relationship with the study drug (highly probable, probable, possible, unlikely, or unknown). Vital signs were evaluated at each visit, and clinical laboratory tests (complete blood count, chemistry, and urine analysis) were obtained at screening and after treatment. Twelve-lead electrocardiograms (ECG) parameter was evaluated at screening, after 8 weeks of treatment and after the treatment-free follow-up period.
Statistics
A sample size of 161 patients provided approximately 80% power to demonstrate the noninferiority of once-daily 50 mg of udenafil against on-demand 200 mg of udenafil. The noninferiority margin of 3.0 was derived from the statistical analyses of data from previous studies with udenafil and represents 40% of the difference in mean change from baseline in the IIEF-EFD score between patients who received 200 mg udenafil and placebo. This percentage is in accordance with the commonly accepted standard that the reference value for noninferiority be between one-third and one-half of the advantage value demonstrated by the reference group.20,21,22
Efficacy analyses included all randomized patients who took at least one dose of study medication, had a baseline measurement and had at least one postbaseline measurement. The last observation carried forward imputation methodology, in which a missing value was replaced by the most recent postbaseline measurement, was used. Safety analyses included all randomized patients who received at least one dose of the study drug and for whom any safety data were collected.
The change from baseline in the IIEF-EFD score at 8 weeks was used to demonstrate the noninferiority of once-daily 50 mg of udenafil compared with on-demand 200 mg of udenafil. The once-daily dosing would be noninferior if the lower limit of the two-sided, 95% confidence interval (CI) was >3.0 in the analysis of change from baseline. For continuous secondary endpoints, treatment-group comparisons were performed using the two-sample t-test or Wilcoxon rank sum test. For dichotomous secondary endpoints, treatment-group comparisons were performed using the Chi-square test or Fisher's exact test.
For safety analysis, the number of adverse events that occurred and the number and percentage of patient who experienced the adverse events were collected.
RESULTS
Disposition and demographics
One hundred and sixty-one patients were randomized for treatment, and 141 patients completed the study. Eighty patients were randomized to take 200 mg of udenafil on-demand, and 81 patients were randomized to 50 mg of udenafil once-daily. Ten patients in each group prematurely discontinued the study, and the most common cause for the premature discontinuation was patient withdrawal of consent from the study, seven patients in each group. The flow of study participants is illustrated in Figure 2.
Figure 2.
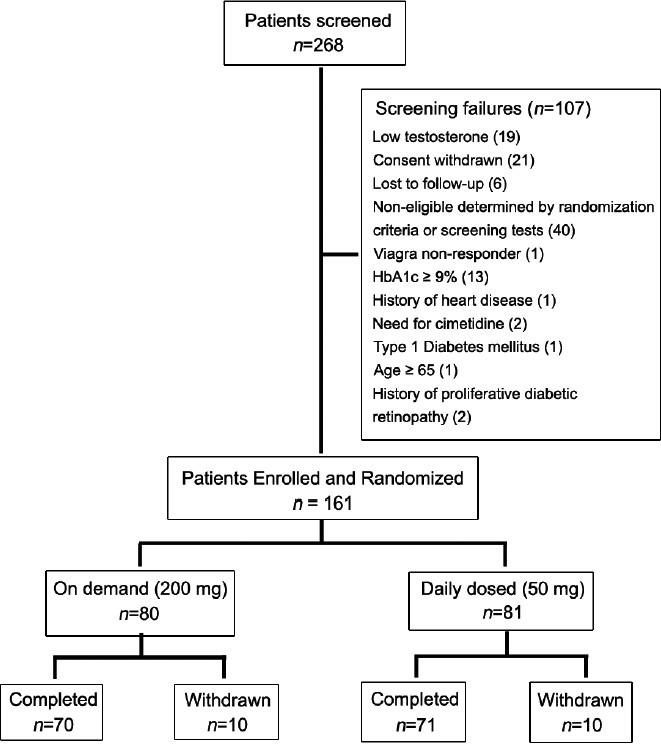
Flow of the participants through the study.
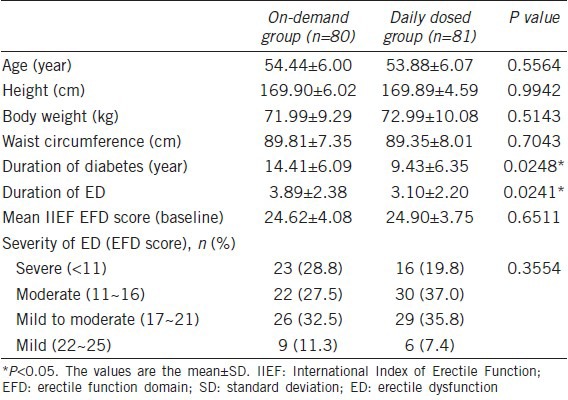
Table 1 shows the demographic data and baseline characteristics of each group. Age, height, body weight, waist circumference, and severity of ED showed no significant difference between the two groups, but the duration of diabetes and ED was longer in on-demand group.
Table 1.
Demographic data and baseline characteristics of each group
Primary efficacy variable
The EFD score of IIEF indicated significant improvement in both groups after 8 weeks of treatment compared with the screening period. After 8 weeks of treatment with on-demand 200 mg of udenafil and once-daily 50 mg of udenafil, the mean EFD score was 25.04 and 24.52, respectively (both P < 0.0001 vs screening), and the mean of change in the EFD score between screening and after treatment was 10.78 and 9.97, respectively. In the per-protocol (PP) analysis, the mean EFD score was 25.40 and 24.86, and the change in EFD score was 11.49 and 10.55, respectively. The change was also statistically significant in the PP analysis. After 4 weeks treatment-free follow-up period, the efficacy of treatment was not maintained in both groups, and the EFD score indicated a significant decrease in both groups (both P < 0.0001) (Figure 3). There was no significant difference in efficacy between the two groups. In addition, the efficacy of once-daily 50 mg of udenafil was not inferior to on-demand 200 mg of udenafil. The lower limit of the two-sided 95% CI for the difference in change from baseline and 8 weeks after treatment in the EFD of IIEF scores between once-daily 50 mg group and on-demand 200 mg group was −2.0. The lower limit was >3.0, and indicated noninferiority. The duration of diabetes and ED was longer in on-demand group. Therefore, primary efficacy analysis with covariates adjustment was conducted. The result of between group difference and 95% CI was −0.55 (−1.76, 0.67), and demonstrated noninferiority.
Figure 3.
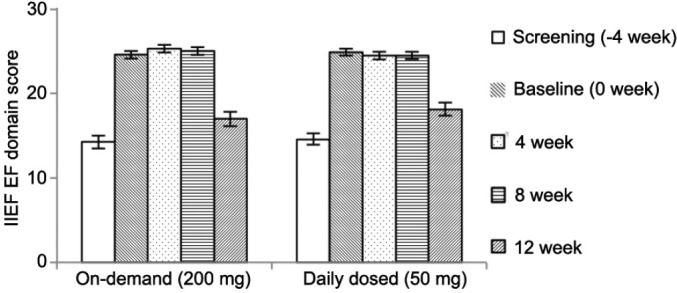
Primary efficacy variable. Erectile function domain score of International Index of Erectile Function in both treatment groups. After 8 weeks of treatment, a significant increase was observed compared with the screening period. After the 4 weeks treatment-free follow-up period, the erectile function domain score decreased significantly in both groups.
Secondary efficacy variable
The secondary efficacy parameters were Q3 and Q4 of the IIEF, the other four domains of the IIEF, Q2 and Q3 of SEP, GAQ, the percentage of patients returning to normal EF, and the change in biomarkers of endothelial function after treatment.
The IIEF Q3 (Q3, frequency of penetration) and Q4 (Q4, frequency of maintaining an erection) scores increased after 8 weeks of treatment compared with screening (P < 0.0001). The mean change in score was 1.91 ± 1.51 (Q3) and 1.82 ± 1.49 (Q4) in the on-demand group and 1.73 ± 1.30 (Q3) and 1.75 ± 1.26 (Q4) in the once-daily group. The scores after the treatment-free follow-up period was not maintained with the decrease of −1.44 ±1.55 (Q3) and −1.27 ± 1.68 (Q4) in the on-demand group and −0.96 ±1.53 (Q3) and −1.20 ± 1.44 (Q4) in the once-daily group. In addition, there was no significant difference in the change of IIEF Q3 and Q4 scores between the two groups.
All domains of IIEF, erectile function, intercourse satisfaction, orgasmic function, sexual desire, and overall satisfaction demonstrated improvement after treatment compared with screening (P < 0.0001) and the efficacy was not maintained after the 4 weeks treatment-free follow-up period. In addition, there was no significant difference between the two groups (Figure 4).
Figure 4.
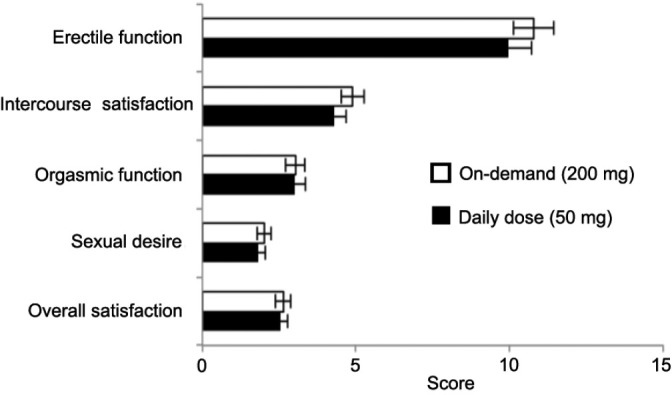
Five domains of International Index of Erectile Function (IIEF). The mean change from screening for each domain of IIEF. There were statistically significant changes for all of the domains of the IIEF in both treatment groups.
After 8 weeks treatment period, successful penetration (SEP Q2) was achieved in 95.05% of patients in the on-demand group and 94.56% in the daily-dosed group (96.27% vs 96.63% in PP analysis). Sexual intercourse was successfully completed (SEP Q3) in 84.82% of patients in the on-demand group and 83.95% in the daily-dosed group (85.90% vs 88.34% in PP analysis). SEP was assessed from baseline in the study, and there were no significant changes after treatment compared with baseline in SEP Q2 and SEP Q3. After the treatment-free follow-up period, the rate of successful penetration and successful intercourse attempts showed a significant decrease in both groups.
The proportion of yes responses to the GAQ was 89.7% for on-demand 200 mg of udenafil and 92.4% for daily 50 mg of udenafil. However, the proportion of yes responses decreased to 32.9% and 42.3% in the on-demand 200 mg of udenafil group and daily 50 mg of udenafil group, respectively, after the treatment-free follow-up period. There was no significant difference between two groups in GAQ after treatment (P = 0.5585) and after the treatment-free follow-up period (P = 0.2494).
The percentage of patients who achieved normal EFD scores (≥26) was 53.8% in the on-demand group and 43.0% in the once-daily group (58.1% vs 43.2% in PP analysis). There was no significant difference between the two groups (P = 0.1754). In addition, the percentage of patients with normal EFD scores decreased to 12.7% and 15.7% in the on-demand 200 mg of udenafil group and once-daily 50 mg of udenafil group, respectively (11.6% vs 18.2% in PP analysis) after the treatment-free follow-up period. There was also no significant difference between the two groups (P = 0.6051).
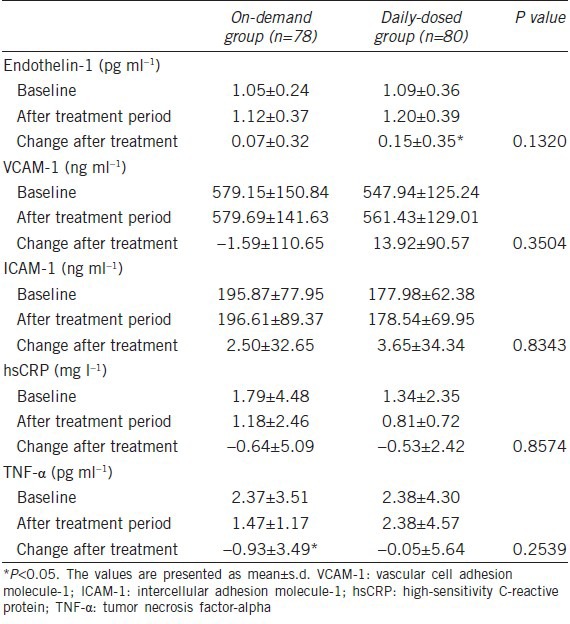
With regard to the change in biomarkers of endothelial function after treatment, endothelin-1 increased after treatment in the once-daily 50 mg of udenafil group (P < 0.01) and TNF-α decreased in the on-demand 200 mg of udenafil group (P < 0.05). However, the mean values for all biomarkers at baseline and after 8 weeks of treatment were within normal ranges in both treatment groups. In addition, there was no significant difference between the two groups in all biomarkers of endothelial function (Table 2).
Table 2.
Change in the biomarkers of endothelial function at baseline and after treatment
Safety
In this study, udenafil was well-tolerated in both groups, and most adverse events were mild to moderate in severity. The most commonly reported treatment-related adverse drug reaction was flushing in the on-demand group (8.9%, n = 7) and 3.8% (n = 3) in the daily-dosed group. Headache was reported in 2.5% (n = 2) of patients in the on-demand group and 1.3% (n = 1) in the daily-dosed group. There was no significant difference between the two groups in the incidence of treatment-related adverse drug reactions. One case in each group experienced an adverse event that led to discontinuation. Cellulitis occurred in one patient in the on-demand group, which was considered to be unlikely related to udenafil treatment; it was the only case of severe adverse event in the study.
There were no clinically significant changes in laboratory data. The slight decrease in the mean diastolic pressure (mean ± standard deviation: −3.19 ± 9.16 mmHg) in the on-demand group was noted. However, the change was within the normal reference range. An ECG abnormality was detected in one case in each group; however, it was temporary and not clinically significant.
DISCUSSION
This study demonstrated that daily 50 mg of udenafil is safe, well-tolerated, and has comparable efficacy to on-demand 200 mg of udenafil in patients with type 2 diabetes with ED. To our knowledge, this is the first study to compare the efficacy and biomarkers of endothelial function between once-daily and on-demand PDE5 inhibitor dosing in diabetic patients with ED. The primary and secondary efficacy parameters indicated significant improvement in erectile function in both groups after the treatment period when compared with screening. The mean IIEF-EFD score increased, and the efficacy of daily dosing was not inferior and was comparable to on-demand dosing. The mean IIEF-EFD score at baseline did not demonstrate the difference between the two groups. However, the duration of diabetes and ED was longer in the on-demand group. Therefore, primary efficacy analysis with covariates adjustment was conducted. The result of between group difference and 95% CI was −0.55 (−1.76, 0.67), demonstrated noninferiority for once-daily dosing.
Four other domain scores of IIEF and the mean score of Q3 of IIEF (successful penetration) and Q4 of IIEF (frequency of maintaining an erection) indicated a significant increase in both groups without between-group differences. Successful penetration and successful completion of sexual intercourse improved in both groups. In addition, improved erections were reported in 89.7% of patients in the on-demand group and 92.4% in the daily-dosed group after treatment. After the treatment-free follow-up period, the improvement in erectile function was not maintained in both treatment groups, indicating that once-daily 50 mg of udenafil treatment for 8 weeks did not provide a prolonged advantage over the on-demand udenafil regimen. In addition, there was no clinically significant change in biomarkers of endothelial function. Compliance with treatment was higher in the once-daily udenafil group compared with the on-demand group (97.10% ± 11.39% vs 91.06% ± 14.29%, P = 0.0039).
The daily administration of PDE5 inhibitors produced favorable effects in previous clinical studies. McMahon compared the efficacy and safety of daily dosed tadalafil and on-demand tadalafil in the treatment of ED. Both on-demand and daily tadalafil enhanced all efficacy outcomes and were well-tolerated. The change in the IIEF domain score and the successful completion of sexual intercourse were higher with daily dosing. Seventy-two percent of patients preferred daily tadalafil and the main reasons for the preference were sexual spontaneity and efficacy.11 McMahon also reported that 10 mg and 20 mg of daily tadalafil were effective and well-tolerated in men previously unresponsive to on-demand tadalafil23 and proposed that the improvement in the erectile response is most likely related to improved endothelial function. Daily vardenafil24 and another study with tadalafil once-daily,25 also improved ED in patients who previously did not respond to on-demand PDE5 inhibitor treatment. However, there are some conflicting results regarding endothelial function improvement with PDE5 inhibitor treatment in other clinical studies. Zumbé et al. compared the efficacy of once-daily and on-demand vardenafil in the treatment of mild-to-moderate ED and evaluated the sustainability of improvement in erectile function after long-term treatment. Although both treatments improved erectile function, there was no significant difference between the groups in efficacy and treatment satisfaction. In addition, once-daily vardenafil treatment for 24 weeks did not provide prolonged advantages over on-demand administration.26 Another study with nightly dosing of sildenafil for 1 year demonstrated improved erectile function and endothelial function after a treatment-free follow-up period.27 Our study with once-daily and on-demand udenafil did not demonstrate a significant difference between the two groups and the treatment efficacy was not maintained after treatment. Diabetic patients constitute a difficult-to-treat subpopulation in the treatment of ED and have multiple risk factors for ED. Diabetic patients may need longer treatment periods or higher doses of PDE5 inhibitor for a prolonged effect after treatment or recovery from ED.
Erectile dysfunction is highly prevalent in patients with vascular diseases, such as coronary artery, cerebrovascular and peripheral arterial disease, whereas there is an increased risk of cardiovascular events (angina, myocardial infarction, stroke, transient ischemic attack, and intermittent claudication) in patients with preexisting ED. This observation implies an intimate nexus between the two conditions.28,29 The shared etiologic factor with vascular disease is endothelial dysfunction.30 Improvement in endothelial function in diabetes, which is associated with increased risk of cardiovascular disease, is particularly important. The effect of PDE5 inhibitors on endothelial function remains controversial. Some studies on sildenafil and tadalafil have demonstrated improvement in endothelial function assessed with peak systolic velocity, flow-mediated dilatation and biomarkers of endothelial function.13,14,27,31,32,33 On the other hand, in a study with coronary heart disease patients, sildenafil did not improve peripheral endothelium-dependent vasomotor or fibrinolytic function.34 Aversa et al. have reported improved endothelial function after chronic administration of sildenafil in men with type 2 diabetes without erectile dysfunction.12 However, another study with once-daily tadalafil in diabetes mellitus and ED patients demonstrated improvement in erectile function; however, there was no significant change in the endothelial biomarkers.10 Daily administration of PDE5 inhibitors may have benefits beyond treating ED. Although our study results did not indicate improvement in the biomarkers of endothelial function, recent data suggest that chronic dosing of PDE5 inhibitors may have prolonged beneficial effects on vascular endothelial function. Several factors may have contributed to the results regarding the biomarkers of endothelial function. These factors include diabetic patients being less responsive to treatment for ED when compared with nondiabetic patients as well as a short treatment period and low dosage of daily udenafil.
Both treatments with udenafil in diabetic patients were safe and well-tolerated. In addition, daily administration of udenafil was not associated with a higher incidence of treatment-related adverse events. The most commonly reported adverse events were flushing and headache, which are similar to previously reported adverse events in treatments with PDE5 inhibitors.11,18,23,35 All the adverse events associated with daily and on-demand udenafil treatment were mild-to-moderate in severity. The ECG and laboratory test results showed no evidence of abnormality related to udenafil treatment.
The unique feature of this study is that the dose of udenafil was controlled to match the same total dose in both groups. In addition, we compared the efficacy of daily dosing with the on-demand regimen with the integrated result of erectile function and endothelial biomarkers in diabetic patients with ED. The limitations of this study are that it was a two-arm, noninferiority trial that lacked a placebo group, and the number of included patients was relatively small. A larger controlled multi-center trial with longer treatment duration will be needed to assess the effect of daily PDE5 inhibitor administration on endothelial function and the prolonged effect after discontinuation. It would be interesting to determine the optimal treatment dose and duration according to the degree of ED, the number of risk factors, and whether there are special candidates who may benefit from chronic daily administration of PDE5 inhibitors.
The efficacy of once-daily 50 mg of udenafil was not inferior and was comparable with on-demand 200 mg of udenafil. There was no significant between group differences observed in adverse drug reactions. Udenafil 50 mg once-daily may be an effective and safe treatment option for type 2 diabetic patients with ED. Further studies are required to establish the role and beneficial effects of the PDE5 inhibitor in diabetic patients.
AUTHOR CONTRIBUTIONS
KSP, KWM participated in designing the study, conducted the data acquisition, interpreted the data, drafted and revised the manuscript. SWP, BYC, IBP, YAS, THK conducted data acquisition, data interpretation, and revised the manuscript. SHP, JML involved in data interpretation, drafted and revised the manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was sponsored by Dong-A ST, Seoul, Korea.
REFERENCES
- 1.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 2.Romeo JH, Seftel AD, Madhun ZT, Aron DC. Sexual function in men with diabetes type 2: association with glycemic control. J Urol. 2000;163:788–91. [PubMed] [Google Scholar]
- 3.McCulloch DK, Campbell IW, Wu FC, Prescott RJ, Clarke BF. The prevalence of diabetic impotence. Diabetologia. 1980;18:279–83. doi: 10.1007/BF00251005. [DOI] [PubMed] [Google Scholar]
- 4.Ayan S, Yildirim S, Uçar C, Sarioglu Y, Gültekin Y, et al. Corporal reactivity to adenosine and prostaglandin E1 in alloxan-induced diabetic rabbit corpus cavernosum, and the effect of insulin therapy. BJU Int. 1999;83:108–12. doi: 10.1046/j.1464-410x.1999.00892.x. [DOI] [PubMed] [Google Scholar]
- 5.Azadzoi KM, Saenz de Tejada I. Diabetes mellitus impairs neurogenic and endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle. J Urol. 1992;148:1587–91. doi: 10.1016/s0022-5347(17)36975-6. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJ, Selam JL, Sweeney M, Ziegler D. Sildenafil citrate for the treatment of erectile dysfunction in men with type II diabetes mellitus. Diabetologia. 2001;44:1296–301. doi: 10.1007/s001250100656. [DOI] [PubMed] [Google Scholar]
- 7.Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421–6. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 8.Stuckey BG, Jadzinsky MN, Murphy LJ, Montorsi F, Kadioglu A, et al. Sildenafil citrate for treatment of erectile dysfunction in men with type 1 diabetes: results of a randomized controlled trial. Diabetes Care. 2003;26:279–84. doi: 10.2337/diacare.26.2.279. [DOI] [PubMed] [Google Scholar]
- 9.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Hatzichristou D, Gambla M, Rubio-Aurioles E, Buvat J, Brock GB, et al. Efficacy of tadalafil once daily in men with diabetes mellitus and erectile dysfunction. Diabet Med. 2008;25:138–46. doi: 10.1111/j.1464-5491.2007.02338.x. [DOI] [PubMed] [Google Scholar]
- 11.McMahon C. Comparison of efficacy, safety, and tolerability of on-demand tadalafil and daily dosed tadalafil for the treatment of erectile dysfunction. J Sex Med. 2005;2:415–25. doi: 10.1111/j.1743-6109.2005.20360.x. [DOI] [PubMed] [Google Scholar]
- 12.Aversa A, Vitale C, Volterrani M, Fabbri A, Spera G, et al. Chronic administration of Sildenafil improves markers of endothelial function in men with type 2 diabetes. Diabet Med. 2008;25:37–44. doi: 10.1111/j.1464-5491.2007.02298.x. [DOI] [PubMed] [Google Scholar]
- 13.Desouza C, Parulkar A, Lumpkin D, Akers D, Fonseca VA. Acute and prolonged effects of sildenafil on brachial artery flow-mediated dilatation in type 2 diabetes. Diabetes Care. 2002;25:1336–9. doi: 10.2337/diacare.25.8.1336. [DOI] [PubMed] [Google Scholar]
- 14.Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, et al. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol. 2005;47:214–20. doi: 10.1016/j.eururo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Salem EA, Kendirci M, Hellstrom WJ. Udenafil, a long-acting PDE5 inhibitor for erectile dysfunction. Curr Opin Investig Drugs. 2006;7:661–9. [PubMed] [Google Scholar]
- 16.Paick JS, Kim SW, Yang DY, Kim JJ, Lee SW, et al. The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. J Sex Med. 2008;5:946–53. doi: 10.1111/j.1743-6109.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 17.Park HJ, Park JK, Park K, Min K, Park NC. Efficacy of udenafil for the treatment of erectile dysfunction up to 12 hours after dosing: a randomized placebo-controlled trial. J Sex Med. 2010;7:2209–16. doi: 10.1111/j.1743-6109.2010.01817.x. [DOI] [PubMed] [Google Scholar]
- 18.Moon du G, Yang DY, Lee CH, Ahn TY, Min KS, et al. A therapeutic confirmatory study to assess the safety and efficacy of Zydena (udenafil) for the treatment of erectile dysfunction in male patients with diabetes mellitus. J Sex Med. 2011;8:2048–61. doi: 10.1111/j.1743-6109.2011.02268.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, et al. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 20.Gomery P, Bullock A, McGettigan J, Munarriz R, Natanegara F, et al. Tadalafil is efficacious in Black American and Hispanic men with erectile dysfunction: results from multiple observations in men with erectile dysfunction in national tadalafil study in the US (MOMENTUS) Int J Impot Res. 2007;19:76–83. doi: 10.1038/sj.ijir.3901484. [DOI] [PubMed] [Google Scholar]
- 21.D’Agostino RB Sr, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues – The encounters of academic consultants in statistics. Stat Med. 2003;22:169–86. doi: 10.1002/sim.1425. [DOI] [PubMed] [Google Scholar]
- 22.Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ. 1996;313:36–9. doi: 10.1136/bmj.313.7048.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon C. Efficacy and safety of daily tadalafil in men with erectile dysfunction previously unresponsive to on-demand tadalafil. J Sex Med. 2004;1:292–300. doi: 10.1111/j.1743-6109.04042.x. [DOI] [PubMed] [Google Scholar]
- 24.Javaroni V, Queiroz Miguez M, Burla A, Oigman W, Neves MF. Response to on-demand vardenafil was improved by its daily usage in hypertensive men. Urology. 2012;80:858–64. doi: 10.1016/j.urology.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 25.Kim ED, Seftel AD, Goldfischer ER, Ni X, Burns PR. A return to normal erectile function with tadalafil once daily after an incomplete response to as-needed PDE5 inhibitor therapy. J Sex Med. 2014;11:820–30. doi: 10.1111/jsm.12253. [DOI] [PubMed] [Google Scholar]
- 26.Zumbé J, Porst H, Sommer F, Grohmann W, Beneke M, et al. Comparable efficacy of once-daily versus on-demand vardenafil in men with mild-to-moderate erectile dysfunction: findings of the RESTORE study. Eur Urol. 2008;54:204–10. doi: 10.1016/j.eururo.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 27.Sommer F, Klotz T, Engelmann U. Improved spontaneous erectile function in men with mild-to-moderate arteriogenic erectile dysfunction treated with a nightly dose of sildenafil for one year: a randomized trial. Asian J Androl. 2007;9:134–41. doi: 10.1111/j.1745-7262.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 28.Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, Stefanadis C. The triad: erectile dysfunction - endothelial dysfunction - cardiovascular disease. Curr Pharm Des. 2008;14:3700–14. doi: 10.2174/138161208786898716. [DOI] [PubMed] [Google Scholar]
- 29.Chew KK, Bremner A, Jamrozik K, Earle C, Stuckey B. Male erectile dysfunction and cardiovascular disease: is there an intimate nexus? J Sex Med. 2008;5:928–34. doi: 10.1111/j.1743-6109.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 30.Kirby M, Jackson G, Simonsen U. Endothelial dysfunction links erectile dysfunction to heart disease. Int J Clin Pract. 2005;59:225–9. doi: 10.1111/j.1742-1241.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 31.Aversa A, Greco E, Bruzziches R, Pili M, Rosano G, et al. Relationship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: a pilot study. Int J Impot Res. 2007;19:200–7. doi: 10.1038/sj.ijir.3901513. [DOI] [PubMed] [Google Scholar]
- 32.Burnett AL, Strong TD, Trock BJ, Jin L, Bivalacqua TJ, et al. Serum biomarker measurements of endothelial function and oxidative stress after daily dosing of sildenafil in type 2 diabetic men with erectile dysfunction. J Urol. 2009;181:245–51. doi: 10.1016/j.juro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Deyoung L, Chung E, Kovac JR, Romano W, Brock GB. Daily use of sildenafil improves endothelial function in men with type 2 diabetes. J Androl. 2012;33:176–80. doi: 10.2164/jandrol.111.013367. [DOI] [PubMed] [Google Scholar]
- 34.Robinson SD, Ludlam CA, Boon NA, Newby DE. Phosphodiesterase type 5 inhibition does not reverse endothelial dysfunction in patients with coronary heart disease. Heart. 2006;92:170–6. doi: 10.1136/hrt.2004.059683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein I, Young JM, Fischer J, Bangerter K, Segerson T, et al. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care. 2003;26:777–83. doi: 10.2337/diacare.26.3.777. [DOI] [PubMed] [Google Scholar]


