Abstract
Klinefelter syndrome (KS) is a hypergonadotropic hypogonadism characterized by a 47, XXY karyotype. The risk of testicular cancer in KS is of interest in relation to theories about testicular cancer etiology generally; nevertheless it seems to be low. We evaluated the need for imaging and serum tumor markers for testicular cancer screening in KS. Participants were 40 consecutive KS patients, enrolled from December 2009 to January 2013. Lactate dehydrogenase (LDH), alpha-fetoprotein (AFP), and beta-human chorionic gonadotrophin subunit (β-HCG) serum levels assays and testicular ultrasound (US) with color Doppler, were carried out at study entry, after 6 months and every year for 3 years. Abdominal magnetic resonance (MR) was performed in KS when testicular US showed micro-calcifications, testicular nodules and cysts. Nearly 62% of the KS had regular testicular echotexture, 37.5% showed an irregular echotexture and 17.5% had micro-calcifications and cysts. Eighty seven percent of KS had a regular vascular pattern, 12.5% varicocele, 12.5% nodules <1 cm, but none had nodules >1 cm. MR ruled out the diagnosis of cancer in all KS with testicular micro calcifications, nodules and cysts. No significant variations in LDH, AFP, and β-HCG levels and in US pattern have been detected during follow-up. We compared serum tumor markers and US pattern between KS with and without cryptorchidism and no statistical differences were found. We did not find testicular cancer in KS, and testicular US, tumor markers and MR were, in selected cases, useful tools for correctly discriminating benign from malignant lesions.
Keywords: abdomen magnetic resonance, alpha-fetoprotein, beta-human chorionic gonadotrohin subunit, klinefelter syndrome, lactate dehydrogenase, testicular ultrasound
INTRODUCTION
Klinefelter syndrome (KS) is a hypergonadotropic hypogonadism characterized by a 47, XXY or a mosaic karyotype, which affects approximately one in every 660 men, and <10% of the expected number is diagnosed before puberty.1 The “prototypic” KS man presents clinical findings of small testes, infertility, and tall stature. Biochemical findings include high levels of gonadotrophins (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) and low normal levels of testosterone. The testes in the adult KS man are characterized by diffuse hyalinization and fibrosis of the seminiferous tubules, hyperplasia of the interstitium, and the tubules may show residual foci of spermatogenesis. Cryptorchidism is present in 27%–37% of KS subjects, and is approximately 6 times more frequent than in the general male population.1 The phenotypic presentation can be quite variable and other conditions such as gynecomastia, metabolic syndrome, venous disease, leg ulcers, osteoporosis, and mild neurobehavioral alteration may be also present in these subjects.1,2,3,4,5,6,7,8,9 Subjects diagnosed with KS have raised mortality for several specific causes that comprise cardiovascular diseases and malignant tumors.10,11,12 Data from the literature show that these subjects have an elevated risk of developing malignancies of the lung, breast, non-Hodgkin lymphoma, testicular tumors, while their risk of prostate cancer appears to be reduced.11 The increased incidence of cryptorchidism and atrophy of the seminiferous tubules with relative hyperplasia of Leydig cells render KS a condition putting men at risk of developing testicular cancer,13 but the data on the incidence of testicular neoplasia in KS are still controversial.12,14,15 Cryptorchidism is an established risk factor for testicular germ cell tumors (TGCT) and about 10% of all cases of TGCT occurs in men with a history of cryptorchidism.16 Nevertheless, until now only few cases of TGCT have been reported in KS, one of them being an intrapelvic seminoma.17,18,19,20,21 Another case of a 35-year-old man with KS and a malignant Leydig cell tumor was described by Soria et al.22 Scrotal ultrasound (US) is the first-line diagnostic tool for testicular cancer diagnosis. Its sensitivity is almost 100%, and it is also recommended in the follow-up of patients at risk for microlithiasis, atrophy and irregular echotexture.23 Testicular sonography in men with KS reveals a heterogeneous irregular pattern with spread of hyper- and hypo-echoic foci, and blood flow of high resistance that could be suggestive of testicular cancer.24 In the case of equivocal US evaluations, magnetic resonance imaging (MRI) is an accurate and cost-effective diagnostic adjunct. Moreover, the serum tumor markers alpha-fetoprotein (AFP), lactate dehydrogenase (LDH), and beta-human chorionic gonadotrophin subunit (β-HCG) are critical for diagnosing germ cell tumors (GCTs), determining prognosis and assessing treatment outcome. Until now, no data are present in the literature on serum tumor marker evaluation together with diagnostic US of the testis and abdominal MR in men with KS. We have designed a prospective cohort study to evaluate KS subjects under testosterone replacement treatment, by using testicular ultrasonography, hormonal values, the tumor markers AFP, LDH, and β-HCG levels, and abdominal MR during 3 years of follow-up, for discriminating benign from malignant testicular lesions.
SUBJECTS AND METHODS
KS subjects
We designed a prospective cohort study, conducted from December 2009 to January 2013, in which consecutive KS subjects were recruited among those regularly attending the Endocrinology Unit at the Second University of Naples. The median follow-up duration of the study was 36 months (range 6–48 months). We obtained local Research Ethics Board approval for the study.
Forty consecutive men with KS recruited at the Endocrine Unit of the Second University of Naples entered the study. Inclusion criteria were a verified KS karyotype (39 subjects included were 47, XXY, 1 subject was 48, XXXY) and a signed informed consent form. All subjects included in this study, had been under replacement therapy with 1000 mg long-lasting intramuscular testosterone every 3 months (Nebido, Bayer HealthCare, Milan, Italy). All subjects had normal androgen levels on at least two occasions before entering the study. The mean duration of treatment at enrolment was 36 months. Hormonal replacement therapy was performed according to current guidelines.3 Patient data were collected at study entry, after 6 months, 1, 2 and 3 years follow-up. All 40 KS patients participated in the study.
KS men underwent work-up including a complete history and physical examination.25 Blood was obtained for a chemistry profile including LDH, complete blood count, and serum tumor markers including AFP and the β-HCG. Normal serum markers were identified according to the normal laboratory values.25
Imaging studies
Imaging studies were performed in all subjects. An expert radiologist performed testicular assessments with the patient in a horizontal position. The images were obtained with General Electric Logiq 9 US machine, using a linear broadband transducer operating at a range of 10–14 MHz. Each testis was measured in three projections by using frozen B-mode sonography, starting with the largest longitudinal diameter, followed by the largest testicular depth, and the width was measured by turning the transducer 90° to provide a transverse projection of the testis. The US computer calculated testicular volume. All the testicular parenchyma of each testis was scanned. Testicular sonography also included color Doppler imaging. All the images were recorded in the Digital Imaging and Communications in Medicine format.
MRI of the abdomen, pelvis and scrotum was also performed in selected cases. MRI was performed on a 1.5-Tesla Philips Achieva unit with a 14-cm circular surface coil. A T2-weighted fast spin-echo sequence with a 10 × 12 cm field of view and a 256 × 256 matrix in the three orthogonal planes were used for studying the scrotum.
Assays
Pituitary function was appropriately investigated in all subjects. In particular, basal anterior pituitary hormones were evaluated in duplicate: FSH, LH were determined by immunoradiometric assay with commercial kits. Moreover, target organ hormones (testosterone, estradiol, by radioimmunoassay) were also evaluated using commercial kits as previously reported.26 Biological and US imaging evaluation were performed at study entry, after 6 months, and every 12 months for 3 years.
Statistical analysis
Continuous variables were expressed as mean ± standard error of the mean, and categorical data was expressed as numbers and percentages. Statistical analysis was performed with the SPSS software (SPSS, Inc., Evanston, IL, USA). Student t-test for unpaired data, and the Mann-Whitney U test, for normally or non-parametrically-distributed variables were used for between groups comparisons, respectively. All statistical assessments were two-sided and were considered as significant at P < 0.05.
RESULTS
Forty KS subjects in management at the Complex Endocrinology Unit of the Second University of Naples entered the study. The anthropometric, clinical, and biochemical features of subjects are shown in Table 1. All KS men underwent a 3-year follow-up on their pathological condition, and all parameters were evaluated at study entry, after 6 months, and at 1, 2 and 3 years. The median follow-up duration was 36 months (range 6–48 months). Testicular cancer risk was assessed from the determination of blood levels of AFP, LDH, and β-HCG, and ultrasonography was used for morphological research according to testicular cancer workup guidelines.25 All subjects had normal serum tumor marker levels at the time of diagnosis (Figure 1). KS subjects showed no statistical differences between testosterone levels at the first control and in successive determinations. Figure 1 shows that no significant differences were found in the levels of AFP, β-HCG and LDH, which are predictive markers of cancer risk, at the first control and after 12, 24, and 36 months of follow-up. A significant increase, within the normal range values, was observed for LDH serum levels, when basal value was compared with serum levels detected at 12, 24, and 36 months of follow-up (Figure 1).
Table 1.
Demographic, anthropological, and biochemical data of the Klinefelter syndrome men studied (n=40)
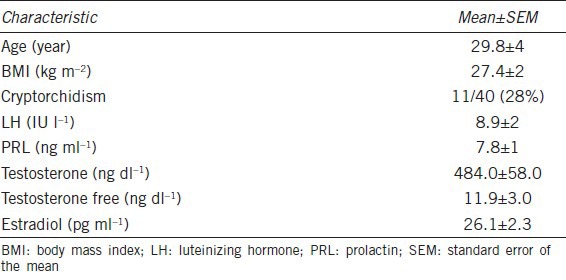
Figure 1.
Time course of serum alpha-fetoprotein, β-human chorionic gonadotropin, lactate dehydrogenase and testosterone before and after 3 years of follow-up in Klinefelter syndrome. *P < 0.05, **P < 0.01 compared with starting value. Values are mean ± standard error of the mean.
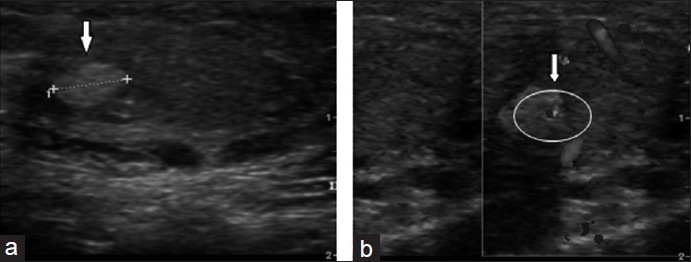
We studied several ultra-sonographic parameters, such as testicular size, echotexture, vascular pattern and the presence of micro-calcifications or other neoformations, such as testicular nodules and testicular cysts. The ultrasonography data are summarized in Table 2. In all men, testicular size was reduced according to the phenotype of the KS subject. The mean testicular volume was 2.1 ± 0.6 cm3 on the right and 2.3 ± 1.0 cm3 on the left. Twenty-seven of these (62.5%) showed regular echotexture, while in 15 subjects (37.5%), there was an irregular echotexture. Eight subjects (20%) had micro-calcifications. Of the vascular patterns, 35 patients (87.5%) had a regular vascular pattern after analysis with color Doppler, while in five subjects (2.1%) a varicocoele was found. Varicocele grade I was found in three men, and grade II in two. All clinically palpable varicocoeles were grade II. Three subjects (11.1%) showed testicular nodules < 1 cm, but none had nodules > 1 cm. Seven of the men (17.5%) showed the presence of testicular cysts. All the subjects with nodules were studied with MR, which ruled out the presence of cancer. Ultrasonography was repeated at 1, 2, and 3 years of follow-up, and no variations in ultrasonographic pattern were found in this period; the size of the nodules and the varicocoele stage were also not different from that at first detection. Figure 2a shows in panel A the longitudinal scrotal US scan of a man with KS revealing a hyper-echoic nodulation (arrow) with a diameter of 4.8 mm in the right testis. The testes present an inhomogeneous structure and volume reduction. As shown in Figure 2b, a small spot in color Doppler of the same nodulation is shown. Longitudinal scrotal ultrasonography of two representative KS subjects is depicted in Figure 3. Figure 3a shows a widespread inhomogeneous testicular structure with micro-calcifications (arrows) and small hypoecoic areola, and Figure 3b shows various testicular micro-calcifications (arrows) in an inhomogeneous testicular echo structure. We have compared biological marker levels and ultrasonographic features in KS subjects with and without a history of cryptorchidism. Eleven out of 40 KS subjects had a history of undescended testicle (28%). All subjects with undescended testicles underwent orchidopexy. The mean age at orchidopexy was 11 ± 6 years. No significant statistical differences were found between KS patents with and without cryptorchidism in any parameters evaluated (Table 1 and Figure 4).
Table 2.
Ultrasound characteristics of the testis in Klinefelter syndrome subjects
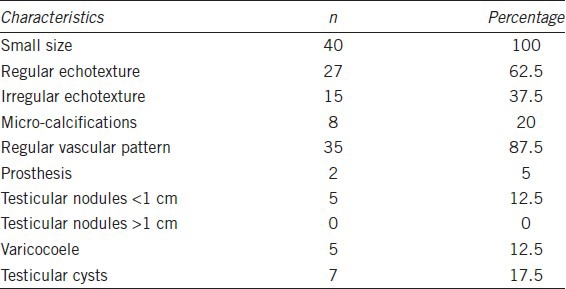
Figure 2.
Longitudinal scrotal ultrasonography of a man with Klinefelter syndrome. (a) Hyper-echoic nodulation (arrow) with a diameter of 4.8 mm in the right testis which shows inhomogeneous structure and volume reduction. (b) Small spot of color Doppler in the context of the same nodulation.
Figure 3.
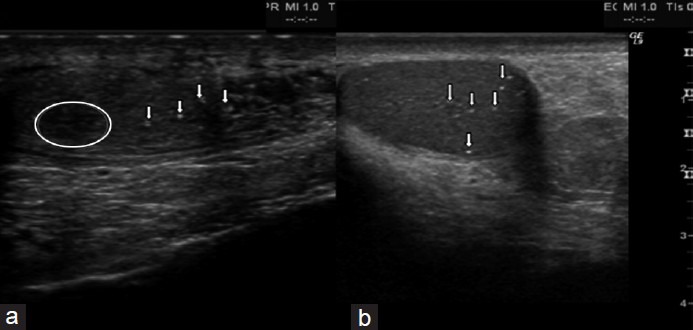
Longitudinal scrotal ultrasonography of two Klinefelter syndrome subjects (a) widespread inhomogeneous testicular structure with micro-calcifications (arrows) and small hypo-echoic areola (circled). (b) Various testicular micro-calcifications (arrows) in an inhomogeneous testicular echo-structure.
Figure 4.
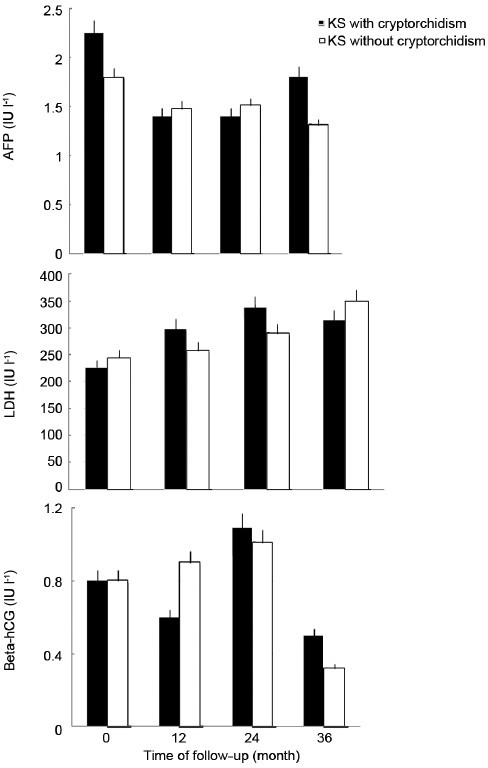
Time course of serum alpha-fetoprotein, β-human chorionic gonadotropin and lactate dehydrogenase before and after 3 years of follow-up in Klinefelter syndrome men with and without cryptorchidism.
DISCUSSION
The current study shows that in our series of subjects with KS, with a mean age of 29.8 ± 4.0 years, which is described to be an age with high risk of testicular cancer, this malignancy was excluded in spite of the higher incidence of cryptorchidism and gonadal congenital alterations. MR and specific tumor markers, together with testicular US were useful tools for differential diagnosis. Our result is of interest because it may contribute to the puzzle of testicular cancer risk in KS patients. Nevertheless, to exclude the appearance of testicular cancer, a longer time of follow-up is needed. Moreover, an adequate use of instrumental and biochemical diagnostic tools has several advantages, including lower medical expenses for testicular cancer suspected in KS men. The sample size of 40 consecutive KS was calculated by considering the prevalence of testicular cancer in the general population of 0.4% and the prevalence of KS of 1–2/1000 males. Currently a man's risk of testicular cancer is about one in 250 (0.4%) over his lifetime and it is the most common tumor in men aged 29–39 years. Boys with isolated cryptorchidism are 3 times more likely to develop testicular cancer; and cryptorchidism is 6 times more frequent in the KS than the normal population.27,28,29 We selected 40 KS men with a mean age of 29.8 ± 4 years, when the incidence rate for testicular cancer peak is around 17 or 18/100000 in the normal population. In our series 11 out of 40 KS subjects has a history of undescended testicle (28%), a higher incidence than in the general population, which falls within the range of 0.9%–9%, comparable with that of the KS population,1,30 nevertheless we did not find in our subjects any case of testicular cancer. In a Danish cohort of KS men, there was one testicular cancer when 1.75 testicular cancers were expected.14 Testicular biopsy samples from 35 KS subjects revealed no cases of carcinoma in situ of the testis.31 The absence of an increased risk of testicular cancer demonstrated by Swerdlow et al., with a narrower confidence interval than in previous data, suggests that testicular atrophy or elevated gonadotropin levels, etiologically associated with risk of this malignancy, were not involved in testicular cancer development in KS and our results are consistent with these data.
Testicular parenchymal abnormalities and testicular cancers are more frequent in infertile men; hence the guidelines recommend a systematic scrotal US examination. Currently, diagnostic US serve to confirm the presence of a testicular mass, and its sensitivity in detecting a testicular tumor is close to 100%. US can distinguish intrinsic from extrinsic testicular lesions, and can identify masses within the testicle. Data from the literature show that in men with KS, sonography of the testicular parenchyma detects a heterogeneous irregular pattern with spread hyper- and hypo-echoic foci.24,32 We found by testicular US the presence of micro-calcifications in eight subjects (20%) and in three subjects (11.1%) nodules < 1 cm. Ekerhovd and Westlander24 showed that testicular sonography in KS men is able to identify intra-testicular tumors in one KS man out of 26 studied. The subject was treated by unilateral orchiectomy and histopathological diagnosis showed a benign Sertoli cell tumor. In our study, all KS men with nodules and micro-calcifications underwent MR, which offers higher sensitivity and specificity than US for differential diagnosis of tumors. MR excluded the presence of testicular tumors, avoiding false positive and costly and unnecessary surgical procedures. Serum tumor markers are prognostic factors and contribute to diagnosis and staging of testicular tumors: the markers AFP, HCG, and LDH should be determined. Globally, there is an increased level of these markers in 51% of cases of testicular tumor.33 However, unraised marker levels do not exclude the diagnosis of a GCT.15 About 90% of non-seminomatous tumors appear with an increase in one or two of the markers. Up to 30% of seminomas can present or develop an elevated β-HCG level during the course of the disease.34,35 LDH is a less specific marker, and its serum concentration is related to cancer volume. Its level may be elevated in 80% of subjects with advanced testicular cancer.34 Following the guidelines29 for screening testicular cancer, we measured AFP, LDH, and β-HCG in our subjects and no significant increase above the normal values at first observation were found after 3 years.35 We observed a significant increase, but within the normal range, of serum LDH levels, when the basal value was compared with the serum level detected at 12, 24, and 36 months of follow-up. The significance of this observation is not clear, probably a longer period of follow-up is needed to clarify this result. No other data are present in the literature about this and our observation is useful because it indicates that it may be unnecessary to screen KS men for testicular cancer only with testicular US. While the prevalence of micro-calcifications amongst symptomatic men has been quoted as being between 0.6% and 9%, we detected testicular micro-calcifications in 20% of KS men.36 The association between testicular cancer and testicular micro-calcifications is still under debate.36,37 Scrotal US could be recommended at the first assessment visit, and when nodules and micro-calcifications are present the measurement of testicular tumor markers and performing abdominal MR can be considered.12,38 Investigations for suspected cancer are stressful and costly, so it is important to know when they are really indicated.39
One open question is: why is testicular cancer less frequent in the KS population despite the increased incidence of cryptorchidism? We can speculate that the reduced level of testosterone during embryonic life is associated with the increased incidence of cryptorchidism in KS patients. In fact, the process of testicular descent is dependent on gubernacular growth and reorganization, which is regulated by the Leydig cell hormones insulin-like peptide 3 and testosterone.40 Complete testicular descent is a sign of, and a prerequisite for, normal testicular function in adult life. Measured cord-blood testosterone in two 47, XXY infants and one with mosaicism (46, XX/47, XXY) revealed significantly lower levels than in three control infants,41 and the peak of testosterone during the period between 30 and 80 days after the birth when testosterone and gonadotropin surges occur in male infants, called mini-puberty, is lower in KS men than in a control population, indicating an early damage of Leydig cell function.42 A low risk of death from prostate cancer in KS subjects has been described, which could be linked to lower testosterone levels.12 The influence of hypogonadism and testosterone replacement therapy on the incidence of testicular cancer is not clear. Our data suggest that prepubertal testosterone levels and their normalization during replacement therapy do not increase the risk for this malignancy.
In summary, KS men, in spite of testicular parenchyma abnormalities and an increased occurrence of cryptorchidism, did not present a high incidence of testicular cancer. Our results suggest that, apart from physical examinations, routine measurement of serum tumor markers and abdominal MR can be applied safely in KS men only for differential diagnosis of benign and malignant lesions. Genetic alteration linked to the extra X-protective mechanisms underlying this observation need to be known, and the KS model can help to provide a better understanding of the genetic basis of testicular tumors.
AUTHOR CONTRIBUTIONS
GA, DE, FB, AR and DP participated in the conception and design of the study. GA and DE acquired all relevant data. KE, GC, and GD critically interpreted and analyzed the data. GV performed all testicular ultrasound analyses. DP, KE, GC, and GD all participated in the critical revision of the manuscript for intellectual content. DP supervised the study. All authors read and approved the manuscript.
COMPETING INTERESTS
All authors declare that there are no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Grant PRIN 2008 prot. 2008LFK7J5_002 to DP from MIUR (Ministero dell’Istruzione dell’Università e della Ricerca), Italy.
REFERENCES
- 1.Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91:1254–60. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- 2.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet. 2004;364:273–83. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- 3.Radicioni AF, Ferlin A, Balercia G, Pasquali D, Vignozzi L, et al. Consensus statement on diagnosis and clinical management of Klinefelter syndrome. J Endocrinol Invest. 2010;33:839–50. doi: 10.1007/BF03350351. [DOI] [PubMed] [Google Scholar]
- 4.Ferlin A, Schipilliti M, Foresta C. Bone density and risk of osteoporosis in Klinefelter syndrome. Acta Paediatr. 2011;100:878–84. doi: 10.1111/j.1651-2227.2010.02138.x. [DOI] [PubMed] [Google Scholar]
- 5.Shanmugam VK, Tsagaris KC, Attinger CE. Leg ulcers associated with Klinefelter's syndrome: a case report and review of the literature. Int Wound J. 2012;9:104–7. doi: 10.1111/j.1742-481X.2011.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotondi M, Fallerini C, Pirali B, Longo I, Pasquali D, et al. A unique patient presenting with concomitant Klinefelter syndrome, Alport syndrome, and craniopharyngioma. J Androl. 2012;33:1155–9. doi: 10.2164/jandrol.111.016204. [DOI] [PubMed] [Google Scholar]
- 7.Groth KA, Skakkebæk A, Høst C, Gravholt CH, Bojesen A. Clinical review: Klinefelter syndrome – A clinical update. J Clin Endocrinol Metab. 2013;98:20–30. doi: 10.1210/jc.2012-2382. [DOI] [PubMed] [Google Scholar]
- 8.Rotondi M, Coperchini F, Renzullo A, Accardo G, Esposito D, et al. High circulating levels of CCL2 in patients with Klinefelter's syndrome. Clin Endocrinol (Oxf) 2014;80:465–7. doi: 10.1111/cen.12245. [DOI] [PubMed] [Google Scholar]
- 9.Giugliano G, Pasquali D, Notaro A, Brongo S, Nicoletti G, et al. Verapamil inhibits interleukin-6 and vascular endothelial growth factor production in primary cultures of keloid fibroblasts. Br J Plast Surg. 2003;56:804–9. doi: 10.1016/s0007-1226(03)00384-9. [DOI] [PubMed] [Google Scholar]
- 10.Pasquali D, Arcopinto M, Renzullo A, Rotondi M, Accardo G, et al. Cardiovascular abnormalities in Klinefelter Syndrome. Int J Cardiol. 2013;168:754–9. doi: 10.1016/j.ijcard.2012.09.215. [DOI] [PubMed] [Google Scholar]
- 11.Weakley SM, Wang H, Yao Q, Chen C. Expression and function of a large non-coding RNA gene XIST in human cancer. World J Surg. 2011;35:1751–6. doi: 10.1007/s00268-010-0951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA, et al. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516–22. doi: 10.1210/jc.2005-1077. [DOI] [PubMed] [Google Scholar]
- 13.Aetiology of testicular cancer: association with congenital abnormalities, age at puberty, infertility, and exercise. United Kingdom Testicular Cancer Study Group. BMJ. 1994;308:1393–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Hasle H, Mellemgaard A, Nielsen J, Hansen J. Cancer incidence in men with Klinefelter syndrome. Br J Cancer. 1995;71:416–20. doi: 10.1038/bjc.1995.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin YS, Kim HJ. Current management of testicular cancer. Korean J Urol. 2013;54:2–10. doi: 10.4111/kju.2013.54.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannuel HD, Mitikiri N, Khan M, Hussain A. Testicular germ cell tumors: biology and clinical update. Curr Opin Oncol. 2012;24:266–71. doi: 10.1097/CCO.0b013e32835167fc. [DOI] [PubMed] [Google Scholar]
- 17.Isurugi K, Imao S, Hirose K, Aoki H. Seminoma in Klinefelter's syndrome with 47, XXY, 15s+karyotype. Cancer. 1977;39:2041–7. doi: 10.1002/1097-0142(197705)39:5<2041::aid-cncr2820390521>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Reddy SR, Svec F, Richardson P. Seminoma of the testis in a patient with 48, XXYY variant of Klinefelter's syndrome. South Med J. 1991;84:773–5. doi: 10.1097/00007611-199106000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Poster RB, Katz DS. Leydig cell tumor of the testis in Klinefelter syndrome: MR detection. J Comput Assist Tomogr. 1993;17:480–1. doi: 10.1097/00004728-199305000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Kurabayashi A, Furihata M, Matsumoto M, Sonobe H, Ohtsuki Y, et al. Primary intrapelvic seminoma in Klinefelter's syndrome. Pathol Int. 2001;51:624–8. doi: 10.1046/j.1440-1827.2001.01246.x. [DOI] [PubMed] [Google Scholar]
- 21.Fishman MD, Eisenberg DA, Horrow MM. Klinefelter syndrome with leydig cell tumor/hyperplasia. Ultrasound Q. 2010;26:101–2. doi: 10.1097/RUQ.0b013e3181dd27d0. [DOI] [PubMed] [Google Scholar]
- 22.Soria JC, Durdux C, Chrétien Y, Sibony M, Damotte D, et al. Malignant Leydig cell tumor of the testis associated with Klinefelter's syndrome. Anticancer Res. 1999;19:4491–4. [PubMed] [Google Scholar]
- 23.Elzinga-Tinke JE, Sirre ME, Looijenga LH, van Casteren N, Wildhagen MF, et al. The predictive value of testicular ultrasound abnormalities for carcinoma in situ of the testis in men at risk for testicular cancer. Int J Androl. 2010;33:597–603. doi: 10.1111/j.1365-2605.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- 24.Ekerhovd E, Westlander G. Testicular sonography in men with Klinefelter syndrome shows irregular echogenicity and blood flow of high resistance. J Assist Reprod Genet. 2002;19:517–22. doi: 10.1023/A:1020959818687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollmannsberger C, Tyldesley S, Moore C, Chi KN, Murray N, et al. Evolution in management of testicular seminoma: population-based outcomes with selective utilization of active therapies. Ann Oncol. 2011;22:808–14. doi: 10.1093/annonc/mdq466. [DOI] [PubMed] [Google Scholar]
- 26.De Bellis A, Colao A, Savoia A, Coronella C, Pasquali D, et al. Effect of long-term cabergoline therapy on the immunological pattern and pituitary function of patients with idiopathic hyperprolactinaemia positive for antipituitary antibodies. Clin Endocrinol (Oxf) 2008;69:285–91. doi: 10.1111/j.1365-2265.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 27.Lip SZ, Murchison LE, Cullis PS, Govan L, Carachi R. A meta-analysis of the risk of boys with isolated cryptorchidism developing testicular cancer in later life. Arch Dis Child. 2013;98:20–6. doi: 10.1136/archdischild-2012-302051. [DOI] [PubMed] [Google Scholar]
- 28.Hayes-Lattin B, Nichols CR. Testicular cancer: a prototypic tumor of young adults. Semin Oncol. 2009;36:432–8. doi: 10.1053/j.seminoncol.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilic D, Misso ML. Screening for testicular cancer. Cochrane Database Syst Rev 2011. 2011;2:CD007853. doi: 10.1002/14651858.CD007853.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Boisen KA, Kaleva M, Main KM, Virtanen HE, Haavisto AM, et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363:1264–9. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- 31.Müller J, Skakkebaek NE. Gonadal malignancy in individuals with sex chromosome anomalies 1991. In: Evans JA, Hamerton JL, Robinson A, editors. Children and Young Adults with Sex Chromosome Aneuploidy: Follow-up, Clinical, and Molecular Studies. Proceedings of the 5th International Workshop on Sex Chromosome anomalies, Minaki, Ontario, Canada, March of Dimes Birth Defects Foundation. Wiley Liss for the National Foundation; 1989. Jun 7-10, [Google Scholar]
- 32.Westlander G, Ekerhovd E, Granberg S, Hanson L, Hanson C, et al. Testicular ultrasonography and extended chromosome analysis in men with nonmosaic Klinefelter syndrome: a prospective study of possible predictive factors for successful sperm recovery. Fertil Steril. 2001;75:1102–5. doi: 10.1016/s0015-0282(01)01793-9. [DOI] [PubMed] [Google Scholar]
- 33.Stenman UH, Lamerz R, Leendert H. Tumor markers in testicular cancers. Looijenga laboratory medicine practice guidelines. In: Sturgeon CM, Diamandis E, editors. Use of Tumor Markers in Testicular, Prostate, Colorectal, Breast, and Ovarian Cancers. Washington, DC: The National Academy of Clinical Biochemistry; 2009. pp. 3–14. [Google Scholar]
- 34.Peyret C. Testicular tumors. Summary and recommendations in onco-urology. Prog Urol. 1993;2:60–4. [Google Scholar]
- 35.Javadpour N. The role of biologic tumor markers in testicular cancer. Cancer. 1980;45:1755–61. [PubMed] [Google Scholar]
- 36.Richenberg J, Brejt N. Testicular microlithiasis: is there a need for surveillance in the absence of other risk factors? Eur Radiol. 2012;22:2540–6. doi: 10.1007/s00330-012-2520-4. [DOI] [PubMed] [Google Scholar]
- 37.Cast JE, Nelson WM, Early AS, Biyani S, Cooksey G, et al. Testicular microlithiasis: prevalence and tumor risk in a population referred for scrotal sonography. AJR Am J Roentgenol. 2000;175:1703–6. doi: 10.2214/ajr.175.6.1751703. [DOI] [PubMed] [Google Scholar]
- 38.Aganovic L, Cassidy F. Imaging of the scrotum. Radiol Clin North Am. 2012;50:1145–65. doi: 10.1016/j.rcl.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Motzer RJ, Agarwal N, Beard C, Bhayani S, Bolger GB, et al. Testicular cancer. J Natl Compr Canc Netw. 2012;10:502–35. doi: 10.6004/jnccn.2012.0050. [DOI] [PubMed] [Google Scholar]
- 40.Aksglaede L, Wikström AM, Rajpert-De Meyts E, Dunkel L, Skakkebaek NE, et al. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update. 2006;12:39–48. doi: 10.1093/humupd/dmi039. [DOI] [PubMed] [Google Scholar]
- 41.Sørensen K, Nielsen J, Wohlert M, Bennett P, Johnsen SG. Serum testosterone of boys with karyotype 47, XXY (Klinefelter's syndrome) at birth. Lancet. 1981;2:1112–3. doi: 10.1016/s0140-6736(81)91314-3. [DOI] [PubMed] [Google Scholar]
- 42.Ratcliffe SG, Bancroft J, Axworthy D, McLaren W. Klinefelter's syndrome in adolescence. Arch Dis Child. 1982;57:6–12. [PMC free article] [PubMed] [Google Scholar]


