Abstract
New concerns have been raised regarding cardiovascular (CV) risks with testosterone (T) therapy (TTh). These concerns are based primarily on two widely reported retrospective studies. However, methodological flaws and data errors invalidate both studies as credible evidence of risk. One showed reduced adverse events by half in T-treated men but reversed this result using an unproven statistical approach. The authors subsequently acknowledged serious data errors including nearly 10% contamination of the dataset by women. The second study mistakenly used the rate of T prescriptions written by healthcare providers to men with recent myocardial infarction (MI) as a proxy for the naturally occurring rate of MI. Numerous studies suggest T is beneficial, including decreased mortality in association with TTh, reduced MI rate with TTh in men with the greatest MI risk prognosis, and reduced CV and overall mortality with higher serum levels of endogenous T. Randomized controlled trials have demonstrated benefits of TTh in men with coronary artery disease and congestive heart failure. Improvement in CV risk factors such as fat mass and glycemic control have been repeatedly demonstrated in T-deficient men treated with T. The current evidence does not support the belief that TTh is associated with increased CV risk or CV mortality. On the contrary, a wealth of evidence accumulated over several decades suggests that low serum T levels are associated with increased risk and that higher endogenous T, as well as TTh itself, appear to be beneficial for CV mortality and risk.
Keywords: cardiovascular, mortality, myocardial infarction, risk, stroke, testosterone
INTRODUCTION
As the use of testosterone (T) therapy (TTh) has increased substantially over the last decade,1 there has been increased attention paid to the health consequences of testosterone deficiency (TD), as well as to the potential risks of its treatment. One of the key topics in this area is the relationship of TD to cardiovascular (CV) health, particularly CV mortality. The purpose of this article is to review the literature regarding this issue.
In the 1970s and 1980s, as epidemiologic studies demonstrated that men suffered from higher rates of myocardial infarctions (MIs) and CV deaths than women for every decade of life, it was speculated that T played a significant role in CV disease, as a clear difference between men and women was the presence of substantial serum concentrations of T in men.2 However, this belief largely disappeared once studies investigating the relationship of T and CV disease began to show that atherosclerosis in men was more prevalent in men with low T concentrations, and men with higher T concentrations appeared to be protected.3
Over the last 20 years, the major focus of potential risks of TTh has been its impact on prostate cancer (PCa). Whereas it had been long assumed that raising serum T would increase the risk of PCa and would cause any existing foci of PCa to grow rapidly,4 data accumulated over nearly 20 years failed to support this belief.5 Curiously, just as concerns regarding PCa have declined, we are once again faced with concerns regarding CV risk.
As reviewed in greater detail below, a sizable number of observational studies have investigated the association of serum T concentrations and mortality, and more specifically, CV mortality, in men. The majority of those studies have shown a significant association between lower serum T concentrations and mortality, with a smaller number showing no association. None of these observational studies has shown increased mortality of normal serum T compared with low serum T concentrations. However, two recent retrospective studies reported increased adverse CV events, including mortality, in men who received T prescriptions compared with men who did not.6,7 Those studies have created a firestorm of media attention as well as scrutiny from regulatory bodies regarding the issue of T and CV risk. Despite serious methodological and data management concerns regarding those two studies (see below), their impact on the medical “conversation” regarding T and CV issues has been enormous. It is thus particularly timely, then, to review the literature on this topic so as to provide context and perspective on this important issue.
A number of excellent systematic reviews and meta-analyses of mortality and other CV adverse effects with TTh have been published over the last several years.2,8,9,10 The purpose of this article is not to repeat those analyses, but rather to review the literature, highlighting notable results and providing an overview of those analyses. Most of these studies investigated overall mortality, whereas others provided specific information on CV mortality. However, it should be noted that overall mortality is often used as a nonspecific surrogate for CV risk since CV disease is the greatest single cause of mortality in industrialized countries.11
TESTOSTERONE AND CARDIOVASCULAR MORTALITY
The association of serum T and CV mortality has been extensively studied (Table 1).
Table 1.
Association between levels of endogenous testosterone and mortality
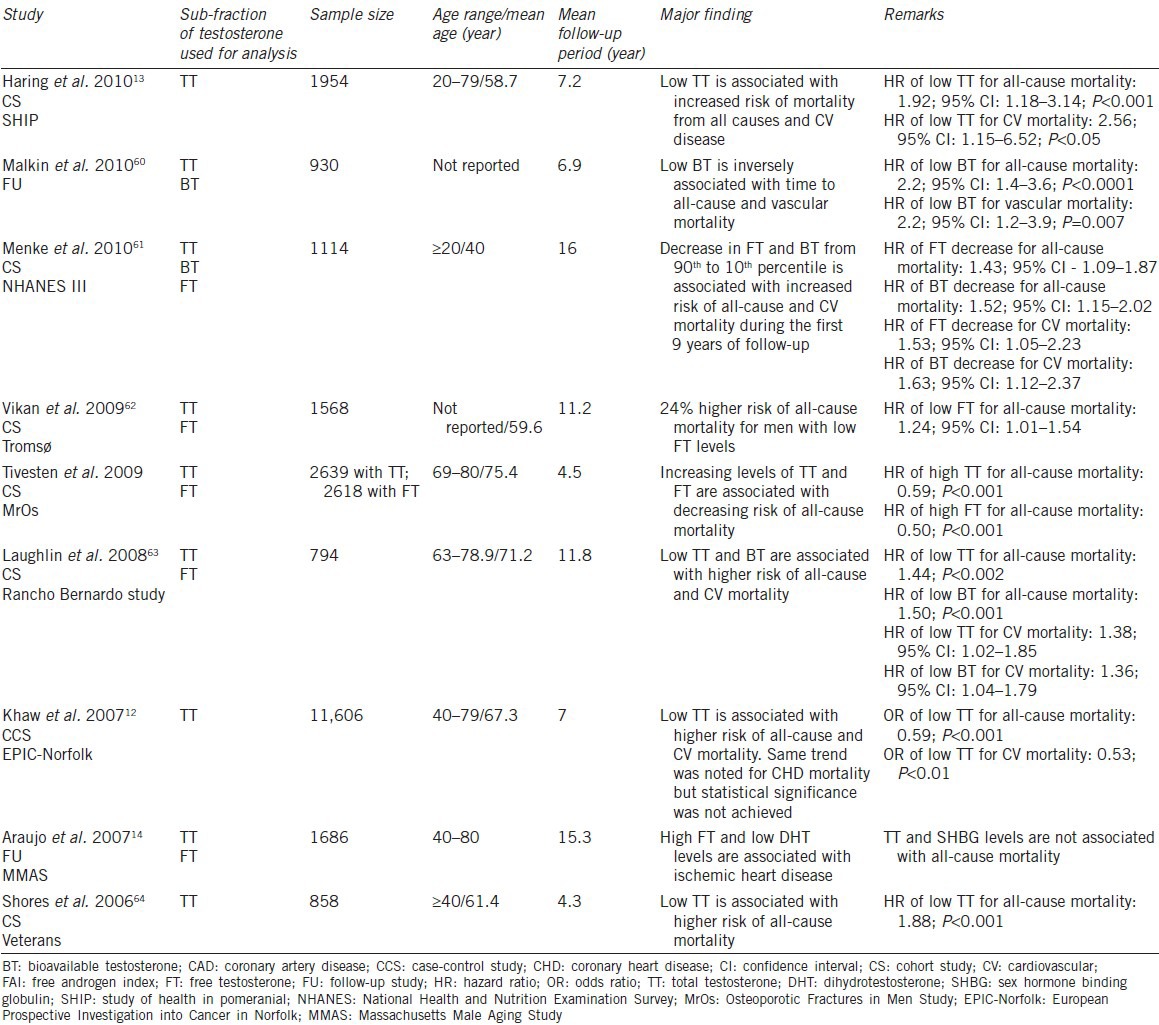
Several studies of community-dwelling men have been performed to investigate the relationship of endogenous serum T to CV mortality. One of these was the European Prospective Investigation into Cancer in Norfolk by Khaw et al.12 This was a nested case-control study based on 11 606 men aged 40–79 years, surveyed in 1993–1997, and followed-up to 2003. The study examined the prospective relationship between endogenous serum T concentrations and mortality due to all causes, CV disease, as well as cancer. The study population was comprised of 825 men without cancer or known CV disease at baseline who subsequently died, and these results were compared with a control group of 1489 men who were still alive, matched for age and date of baseline visit.12
The primary result was that overall mortality and CV mortality were inversely associated with serum T concentrations.12 Analysis by quartiles for serum T showed that men in the lowest quartile were at greatest risk for CV and overall mortality, whereas men with in the upper quartiles were at reduced risk of death. Odds ratios (with 95% confidence interval [CI]) for mortality for increasing quartiles of endogenous T compared with the lowest quartile were 0.75 (0.55–1.00), 0.62 (0.45–0.84), and 0.59 (0.42–0.85). The statistical significance associated with all of these was P < 0.001. These last results were based on the adjustment for multiple variables, including age, body mass index (BMI), blood pressure (BP), smoking history, and diabetes.12
Another study by Haring et al. investigated overall mortality as well as CV mortality using data from the prospective population-based Study of Health in Pomerania.13 Among 1954 men with serum T concentrations at baseline, there were 195 deaths, with a mean follow-up period of 7.2 years. The authors reported that men with low serum T, defined as < 8.7 nmol l−1 (250 ng dl−1), demonstrated significantly greater all-cause mortality than men with higher serum T (hazard ratio [HR]: 2.24; 95% CI: 1.41–3.57), as well as greater CV mortality (HR: 2.56; 95% CI: 1.15–6.52). Increased risk of low T for mortality was seen in younger (20–59 years) and older (60–79 years) age groups, with the greatest risk noted in the older population.13
Not all population-based studies have demonstrated increased overall or CV mortality with lower serum T concentrations. Araujo et al. analyzed results for 1709 men in the Massachusetts Male Aging Study with ages ranging from 40 to 79 years.14 There were 395 deaths during 15.3 years of follow-up. No significant association was noted with serum T concentrations for overall or CV mortality.
Separately, Araujo et al.15 performed a meta-analysis of serum T concentrations and mortality, both overall and specific to CV disease. They selected 12 studies of serum T concentrations in community-dwelling men, 11 of which were eligible for all-cause mortality assessment, involving 16 184 subjects, and 7 of which were eligible for CV mortality assessment, involving 11 831 subjects. A clear, significant association was noted for increased overall mortality, with a relative risk of 1.35 (95%CI: 1.13–1.62), and strongly suggestive but nonsignificant relative risk for CV mortality of 1.25 (95%CI: 0.97–1.60).15
Corona et al.16 performed a meta-analysis of a broader literature set of 70 studies, including men with co-morbidities and clinic populations, consisting of 54 cross-sectional studies, 10 longitudinal studies, and 6 randomized, controlled intervention studies. In cross-sectional studies, lower T levels were significantly associated with the presence of any CV disease using a logistic regression model with adjustment for age and BMI (HR = 0.837; 95%CI: 0.823–0.852), and also when diabetes and hypertension were added as covariates (HR = 0.536; 95%CI: 0.447–0.606). Longitudinal studies involving 12 375 men revealed lower T levels in men with overall and CV mortality. The six randomized controlled trials were performed in men with documented coronary artery disease, with the primary endpoint of time to development of 1 mm ST depression on electrocardiogram (EKG) during exercise. Men treated with T compared with men who received placebo were able to exercise longer before demonstration of coronary ischemia as represented by ST segment depression.16
TESTOSTERONE PRESCRIPTIONS AND CARDIOVASCULAR EVENTS AND MORTALITY
Against a longstanding backdrop of more than 30 years of studies suggesting that low levels of T represent an increased risk for CV and overall mortality,15 and evidence that lower serum T concentrations also are associated with CV disease, including incident coronary artery disease17,18,19 and atherosclerosis,3,20 two recent studies have gained considerable media and scientific attention for reporting increased CV events and/or mortality in men who received a T prescription.
The first of these was published in November 2013 by Vigen et al.7 This was a retrospective analysis of a database accumulated from the Veterans Administration healthcare system of 8709 men who had undergone coronary angiography and had a documented serum T concentration < 300 ng dl−1. The authors looked at rates of MI, stroke, and death in men who eventually received a prescription for TTh and compared those adverse event rates to men who were untreated. Although no statistically significant differences were noted at years 1, 2, or 3, the overall rate of events over the course of the study was reported to be significantly higher in T-treated men by 29%. The authors concluded that TTh was associated with an increased risk of MI, stroke and death.7
However, it soon came to light that the actual rate of adverse events was only half as great in the T group (123 events in 1223 men at risk = 10.1%) as in the untreated group (1587 events in 7486 men = 21.2%).21 The authors never acknowledged this fact, and came to their opposite interpretation of the data based on complex statistics that included adjustment for more than 50 variables. The methodology in this study, that is, stabilized inverse propensity treatment weighting (IPTW) applied to Kaplan–Meier curves with time as a covariate, had only been described 1-year earlier in a separate article by senior author Michael Ho in which the novelty and lack of documented accuracy was underscored by the following statement: “clearly, assessing and confirming adequate covariate balance in IPTW time-varying models is challenging and needs further study. Further work with simulations and contrasts to other methods and other study applications would help elucidate the advantages and disadvantages of this approach.”22
The study by Vigen et al.7 has already undergone two published corrections,23,24 the most recent for a series of data errors in which the numbers of men in one group was decreased by > 1000 individuals, the numbers in a second group were increased by > 900 individuals, and nearly 10% of the all-male dataset was found to be contaminated by women.24 Due to these errors, 29 medical societies have called for retraction of the article, asserting “gross data mismanagement and contamination,” that rendered the study “no longer credible.”25
The second study, published in January 2014 by Finkle et al. was an analysis of health insurance claims data that reported a 36% increased rate of nonfatal MI in the 90d following receipt of a T prescription compared with the 12 prior months.6 Comparison with men who received a prescription for a phosphodiesterase type 5 inhibitor (PDE5i) revealed no increased rate of MI following the prescription. The authors’ concluded that T prescriptions were associated with an increased risk of nonfatal MI.
The strength of the study was the very large population size of 55 593 men. However, important weaknesses render the results highly questionable. Methodologically, the comparison of postprescription MI rates to MI rates prior to the prescription are meaningless, since this was not an experiment with a run-in period, but rather a retrospective look at what actually happened in clinical practice. The post prescription MI rate, with some caveats, may provide a reasonable estimate of what actually occurred in this population. However, since these men were included only because they had already been prescribed T, the preprescription MI rate reflects only how often healthcare providers were willing to write a prescription for men with a history of MI within the prior 12 months. Any reluctance to prescribe this medication would result in low value, and the ratio of post- to pre-prescription MI rates will falsely appear to be increased.
In addition, there was no information available regarding any standard CV risk factors, such as BP, smoking history, or obesity, and no information for any blood test result, such as serum T, or lipid profiles. Moreover, the reported rates of MI before the T prescription (3.48 per 1000 person-years) and after (4.75 per 1000 person-years) were both substantially lower than would be predicted based on the NIH risk calculator for similar-aged US men using favorable parameters (13 per 1000 person-year), specifically age 54 years (same as mean age of study participants), nonsmoker, total cholesterol 230 mg dl−1, high-density lipoprotein 40 mg dl−1, systolic BP 140 mm Hg−1.26 Finally, the comparison with men who received a PDE5i prescription was inappropriate. These were two dissimilar groups treated with dissimilar medications for dissimilar indications. This is a classic case of apples and oranges that provides no useful information. Despite the considerable attention given to this article, this dataset provides no credible data indicating that TTh is associated with increased risk of MI.21
It is important to note that two prior studies have examined the effect of T prescriptions on mortality in men with TD.27,28 The first, by Shores et al.28 investigated 1031 men in the VA healthcare system with documented serum T < 250 ng dl−1. The mean age was 62 year, and mean follow-up was 40.5 months. Mortality in T-treated men was reduced by approximately half in treated men compared with untreated men, at 10.3% versus 20.7%, respectively (P = 0.0001). The mortality rate for men who received TTh was 3.4 deaths per 100 person-years, and 5.7 deaths per 100 person-years in untreated men. Multivariate adjustment for age, BMI, T level, medical morbidity, diabetes, and coronary heart disease yielded a HR of 0.61 (95%CI: 0.42–0.88; P = 0.008), indicating a significant reduction in mortality with TTh.28
A second study demonstrating reduced mortality with TTh was performed by Muraleedharan et al. in a group of 581 diabetic men followed for a mean of 5.8 years.27 Men with low T, defined as serum T < 10.4 nmol l−1 (300 ng dl−1), demonstrated increased mortality compared with men with T values above this threshold. Adjusted mortality in the low T group was 17.2% compared with 9% in the normal T group (P = 0.003). In these populations, the multivariate-adjusted HR for decreased survival was 2.02 (P = 0.009, 95%CI: 1.2–3.4). Among men who received TTh, mortality was reduced to 8.4% compared with 19.2% in the men with low T who were untreated. The HR for decreased survival in the untreated group was 2.3 after multivariate adjustment (95% CI: 1.3–3.9, P = 0.004).27
Although these two studies provide reassuring and suggestively beneficial results for CV effects and mortality related to T prescriptions, neither gained the media attention of the articles alleging increased risks. Ironically, the validity of these results is supported by the absolute event rates in the study by Vigen et al. in that study as well as those by Shores et al.28 and Muraleedharan et al.27 the event rate were lower by approximately half in T-deficient men who received a T prescription compared with untreated men.
OTHER REPORTS OF INCREASED CARDIOVASCULAR RISK WITH TESTOSTERONE THERAPY
In addition to the articles by Vigen et al.7 and Finkle et al.6 reporting increased CV risk with T prescriptions, there appear to be only two additional studies in the literature that reported increased CV risks with TTh. The first, conducted by Basaria et al. was a prospective randomized trial designed to investigate whether T gel provided greater muscular and functional benefits over placebo in an elderly, frail population of men.29 The study was terminated early due to the observation of increased adverse events categorized as “CV” in the T arm. There were 23 of these events in the T arm and 5 in the placebo arm.
It is important to note that this study was not designed to investigate CV events, and none of the reported events were primary or secondary endpoints. A large majority of reported “events” were anecdotal and of questionable clinical importance, including palpitations, pedal edema, and premature ventricular contractions noted on EKG. None of these items was defined prior to study enrollment, and no attempt was made to assess these in both groups. Although there were 5 major adverse cardiac events (1 death, 2 MI's, one coronary revascularization, one stroke), all in the T group, one must be extremely cautious in drawing conclusions from rare events. In a similar study in frail elderly men performed in the UK by Srinivas-Shankar et al.30 there were two major CV events (1 death, 1 MI), both occurring in the placebo group. As the authors Basaria et al.29 themselves stated “the lack of a consistent pattern in these events and the small number of overall events suggest the possibility that the differences detected between the two trial groups may have been due to chance alone.”
In the absence of predefined endpoints or any attempt to systematically investigate both groups for these adverse events, this study provides no meaningful information regarding CV risks.
The second article frequently cited as support for increased CV risks with T administration is the meta-analysis by Xu et al.31 The authors assessed CV events in 27 placebo-controlled T studies of 12 weeks duration or longer, and reported that CV events were greater in men who received T compared with placebo. Just two of the 27 studies contributed 35% of all CV events in the T arm. One is a study by Basaria et al.29 discussed above, in which 18 of 23 events (incorrectly reported as 25 events by Xu et al.31) would not normally be included in reporting of CV events. The other is a 1986 Copenhagen study in which a nonapproved oral formulation of micronized T was administered at a remarkably high dose of 600 mg daily to men with cirrhosis of the liver, resulting in serum T concentrations exceeding 4000 ng dl−1 (approximately 140 nmol l−1) in a quarter of the T group, and with levels reaching as high as 21 000 ng dl−1 (745 nmol l−1), a value approximately 20 times the upper limit of the normal range. Since oral T is known to cause liver toxicity via a first-pass effect, it should be no surprise that markedly supraphysiologic T doses in a hepatically compromised population would prove harmful. This study provides no clinically relevant information, and inclusion of its results in the meta-analysis is misleading.
Without the contributions of the Copenhagen study and the nonmajor CV events in the study by Basaria et al.29 the rates of adverse CV events in the T and placebo groups are similar, with a slightly lower rate in the T group (78 events in 1599 men, 4.88% vs 60 events in 1174 men, 5.1%, respectively). It should be underscored that several other meta-analyses have also been published, with none indicating increased CV risk with T administration versus placebo.8,9,10,32
Reassuringly, the latest study to investigate this issue revealed no increased MI risk among men who received TTh. On the contrary, in this study of 25 420 US Medicare recipients 65 years and older, a significant trend towards reduced MI rates were noted with increasing quartiles of risk.33 For men in the highest prognostic MI risk quartile, treatment with TTh was associated with reduced risk (HR = 0.69; 95% CI = 0.53–0.92).
DISCUSSION
The issue of CV safety with TTh has suddenly become a major healthcare issue. Although TTh has been controversial for many years, the rapid increase in prescriptions over the last decade and direct-to-consumer marketing in the US has led to a parallel increase in scrutiny for adverse effects. The issue is confounded by opinion pieces in medical and lay publications criticizing pharmaceutical company for profits for what some have asserted is not a real medical condition. A tripling in T prescriptions in the US over the last decade has been cited as ipso facto evidence that TTh is overused and abused.33 With this as a background, two retrospective studies of questionable merit have been widely hailed as evidence that TTh is risky, contradicting a wealth of data accumulated over several decades.
Any objective review must consider the evidence as it exists, based on the quality of the methodology and results, rather than what has been asserted about those studies, including interpretations by the authors. Although all new information merits consideration, this does not mean that results from every new study must be accepted as credible, particularly if they contradict prior existing information. This is the situation in which we find ourselves with publication of the two studies, by Vigen et al.7 and by Finkle et al.6 that reported increased CV risks with TTh in contrast to a wealth of prior literature that strongly pointed in the opposite direction. When surprising or contrary results are obtained, the explanation is often found in the methodology.
This is the case here. The absolute rate of events in the study by Vigen et al.7 demonstrated a reduced risk of stroke, MI, and death in men who received a T prescription compared with men who did not. The authors reported opposite results using a new statistical methodology-stabilized inverse propensity weighting applied to Kaplan–Meier curves-that is still in development without adequate confirmation by large published studies. As the authors explained, this methodology in this study resulted in an adverse event in the T group being counted as greater than one event, whereas a similar event in the untreated group was counted as less than one event.34 Specifically, one death, MI, or stroke in the T group counted as approximately 3 events,35 whereas the same event in the untreated group counted as approximately 0.9 events. This is clearly a troubling statistical approach.
Moreover, the series of data errors acknowledged by the authors, including errors of > 1000 men in one category, >900 in another, and contamination by nearly 10% women in one part of the dataset indicates serious problems with data management. In response to these errors, 29 medical societies from around the world have called for retraction of this article, citing “gross data mismanagement and contamination,” rendering the study “no longer credible.”25
The article by Finkle et al.6 actually revealed low rates of MI in men treated with T. The report of an increased risk following receipt of a prescription was only in comparison to an artificially reduced prescribing rate of T to men with prior history of MI within the last 12 months.
In contrast, two prior studies have shown that mortality is reduced, by approximately half, in men who received a T prescription compared with those who did not,27,28 findings consistent with the actual rates reported by Vigen et al.7 In addition, a majority of observational studies have found that low endogenous serum T levels are associated with increased mortality. Although a few studies showed no association with serum T concentrations, it must also be noted that none have shown that normal serum T concentrations are associated with increased risk.
Is it possible that the recent worrisome studies uncovered a population at risk? This also appears not to be the case. English et al. performed a T versus placebo-controlled trial of exercise in men with known coronary artery disease, and investigated time to 1 mm ST depression by EKG as an indication of ischemia.36 Men who received TTh were able to exercise significantly longer without ischemia compared with men who received placebo. In men with congestive heart failure, those who received T demonstrated greater walking distance and other functional endpoints compared with those who received placebo.38
It should also be noted that TTh has been shown uniformly and repeatedly to improve several known CV risk factors, including reduced fat mass, body fat percent, and waist circumference, and increased lean mass.37,38,39,40,41,42,43,44,45,46,47,48 Numerous studies have also shown improved glycemic control39,40,41,42,47,48,49,50,51 and reductions in insulin resistance.38,39,48,49,51,52,53,54,55,56,57,58,59 Taken as a whole, then, the evidence strongly points to improved CV status with normal serum T or treatment with TTh in men with TD, and most observational studies regarding T and mortality appear to support this concept.
CONCLUSIONS
A long history of studies investigating T and CV risks, specifically mortality, reveals important associations with low T and mortality with higher serum T appearing to be protective in a majority of studies. Several retrospective observational studies have now shown reduced mortality and CV adverse events in men who received T compared with untreated men. Although no definitive, large, prospective studies have yet been performed, a substantial literature accumulated over several decades fails to provide any credible evidence that TTh is associated with increased CV mortality or major adverse CV events.
ACKNOWLEDGMENTS
Monica Caliber expertly assisted with manuscript preparation.[64]
REFERENCES
- 1.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–6. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly DM, Jones TH. Testosterone and cardiovascular risk in men. Front Horm Res. 2014;43:1–20. doi: 10.1159/000360553. [DOI] [PubMed] [Google Scholar]
- 3.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–9. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 4.Morgentaler A. Goodbye androgen hypothesis, hello saturation model. Eur Urol. 2012;62:765–7. doi: 10.1016/j.eururo.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65:115–23. doi: 10.1016/j.eururo.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vigen R, O’Donnell CI, Barón AE, Grunwald GK, Maddox TM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–75. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 9.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Carson CC, 3rd, Rosano G. Exogenous testosterone, cardiovascular events, and cardiovascular risk factors in elderly men: a review of trial data. J Sex Med. 2012;9:54–67. doi: 10.1111/j.1743-6109.2011.02337.x. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, et al. Executive summary: heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 12.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 13.Haring R, Völzke H, Steveling A, Krebs A, Felix SB, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010;31:1494–501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 14.Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, et al. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med. 2007;167:1252–60. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 15.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, et al. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–19. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corona G, Rastrelli G, Monami M, Guay A, Buvat J, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 17.Dobrzycki S, Serwatka W, Nadlewski S, Korecki J, Jackowski R, et al. An assessment of correlations between endogenous sex hormone levels and the extensiveness of coronary heart disease and the ejection fraction of the left ventricle in males. J Med Invest. 2003;50:162–9. [PubMed] [Google Scholar]
- 18.Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, et al. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res. 2007;19:176–82. doi: 10.1038/sj.ijir.3901504. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Rui L, Zhu T, Xia H, Yang X, et al. Low testosterone level in middle-aged male patients with coronary artery disease. Eur J Intern Med. 2011;22:e133–6. doi: 10.1016/j.ejim.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Mäkinen J, Järvisalo MJ, Pöllänen P, Perheentupa A, Irjala K, et al. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol. 2005;45:1603–8. doi: 10.1016/j.jacc.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Morgentaler A. ASG Urges FDA to Deny Petition for Black Box Warning. Androgen Study Group (ASG) 2014. [Last accessed on 2014 Jul 02]. Available from: http://www.androgenstudygroup.org/initiatives/letter-to-fda-asking-to-deny-black-box-petition .
- 22.Xu S, Shetterly S, Powers D, Raebel MA, Tsai TT, et al. Extension of Kaplan-Meier methods in observational studies with time-varying treatment. Value Health. 2012;15:167–74. doi: 10.1016/j.jval.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correction. “Incorrect Language.“. [Last accessed on 2014 Apr 26];JAMA. 2014 311:306. Available from: http://www.jama.jamanetwork.com/article.aspx?articleid=1814192 . [Google Scholar]
- 24.“Incorrect Number of Excluded Patients Reported in the Text and Figure.”. [Last accessed on 2014 Apr 26];JAMA. 2014 311:967. Available from: http://www.jama.jamanetwork.com/article.aspx?articleid=1835478 . [Google Scholar]
- 25.Morgentaler A. Letter to JAMA Asking for Retraction of Misleading Article on Testosterone Therapy, Androgen Study Group (ASG) 2014. [Last accessed on 2014 Jul 02]. Available from: http://www.androgenstudygroup.org/initiatives/letter-to-jama-asking-for-retraction-of-misleading-article-on-testosterone-therapy .
- 26.NIH. Risk Assessment Tool for Estimating Your 10-year Risk of Having a Heart Attack. [Last accessed on 2014 Apr 21]. Available from: http://www.cvdrisk.nhlbi.nih.gov/calculator.asp .
- 27.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–33. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 28.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–8. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 29.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–7. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173:1460–2. doi: 10.1001/jamainternmed.2013.7579. [DOI] [PubMed] [Google Scholar]
- 34.Ho PM, Barón AE, Wierman ME. Deaths and cardiovascular events in men receiving testosterone – reply. JAMA. 2014;311:964–5. doi: 10.1001/jama.2014.401. [DOI] [PubMed] [Google Scholar]
- 35.Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311:961–2. doi: 10.1001/jama.2014.392. [DOI] [PubMed] [Google Scholar]
- 36.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–11. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 37.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63:280–93. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 38.Aversa A, Bruzziches R, Francomano D, Rosano G, Isidori AM, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7:3495–503. doi: 10.1111/j.1743-6109.2010.01931.x. [DOI] [PubMed] [Google Scholar]
- 39.Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol. 2014;2014:527470. doi: 10.1155/2014/527470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, et al. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11:840–56. doi: 10.1111/jsm.12404. [DOI] [PubMed] [Google Scholar]
- 41.Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract. 2014;8:e339–49. doi: 10.1016/j.orcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515. doi: 10.1155/2014/683515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013;21:1975–81. doi: 10.1002/oby.20407. [DOI] [PubMed] [Google Scholar]
- 44.Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–29. doi: 10.1111/ijcp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–98. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- 46.Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes. 2013;3:73–83. doi: 10.1111/cob.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–29. doi: 10.1111/ijcp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 49.Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–37. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7. [PubMed] [Google Scholar]
- 51.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 52.Mårin P, Krotkiewski M, Björntorp P. Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissues. Eur J Med. 1992;1:329–36. [PubMed] [Google Scholar]
- 53.Marin P. Testosterone and regional fat distribution. Obes Res. 1995;3(Suppl 4):609S–12. doi: 10.1002/j.1550-8528.1995.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 54.Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, et al. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167:531–41. doi: 10.1530/EJE-12-0525. [DOI] [PubMed] [Google Scholar]
- 55.Naharci MI, Pinar M, Bolu E, Olgun A. Effect of testosterone on insulin sensitivity in men with idiopathic hypogonadotropic hypogonadism. Endocr Pract. 2007;13:629–35. doi: 10.4158/EP.13.6.629. [DOI] [PubMed] [Google Scholar]
- 56.Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–27. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 57.Malkin CJ, Jones TH, Channer KS. The effect of testosterone on insulin sensitivity in men with heart failure. Eur J Heart Fail. 2007;9:44–50. doi: 10.1016/j.ejheart.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Strollo F, Strollo G, Morè M, Magni P, Macchi C, et al. Low-intermediate dose testosterone replacement therapy by different pharmaceutical preparations improves frailty score in elderly hypogonadal hyperglycaemic patients. Aging Male. 2013;16:33–7. doi: 10.3109/13685538.2013.773305. [DOI] [PubMed] [Google Scholar]
- 59.Juang PS, Peng S, Allehmazedeh K, Shah A, Coviello AD, et al. Testosterone with dutasteride, but not anastrazole, improves insulin sensitivity in young obese men: a randomized controlled trial. J Sex Med. 2014;11:563–73. doi: 10.1111/jsm.12368. [DOI] [PubMed] [Google Scholar]
- 60.Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, et al. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96:1821–5. doi: 10.1136/hrt.2010.195412. [DOI] [PubMed] [Google Scholar]
- 61.Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, et al. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 2010;171:583–92. doi: 10.1093/aje/kwp415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vikan T, Schirmer H, Njølstad I, Svartberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromsø Study. Eur J Endocrinol. 2009;161:435–42. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- 63.Tivesten A, Vandenput L, Labrie F, Karlsson MK, Ljunggren O, et al. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009;94:2482–8. doi: 10.1210/jc.2008-2650. [DOI] [PubMed] [Google Scholar]
- 64.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–5. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]


