Abstract
It is no exaggeration to say that our conceptualization of the (patho-) physiological functions of testosterone has undergone a revolutionary development over the last three decades. The traditional thinking was that the biological functions of testosterone were restricted mainly to the area of reproduction and male sexuality. However, scientific research has clearly demonstrated that testosterone is a multi-system hormone serving a wide range of hitherto unsuspected biological functions.
In line with this, it will be argued in this contribution that the physiological role of testosterone has been underestimated, while the risks of testosterone administration have been overstated. Space does not permit to elaborate extensively on all new insights of the role of testosterone in the biology of the male. Three areas will be addressed: (1) the role that testosterone can play in body weight management of hypogonadal men; (2) the role of testosterone in inflammatory processes; (3) the strategy required to let patients benefit from the recent insights that testosterone is a multi-system hormone whose use should not be limited to reproductive/sexual medicine.
TESTOSTERONE AND WEIGHT MANAGEMENT
Obesity is a worldwide epidemic both of the developed and of the developing world. It is associated with a strong increase of mortality and a wide range of morbidity. Its economic costs, not only medical but also with regard to disability, are overwhelming. The obvious remedies, reduction of caloric intake and exercise, the latter also to prevent loss of lean body mass, while dieting, may be successful in the short term, but maintenance of weight loss is disappointing. Pharmacotherapy, even with the outlook of great profitability, has largely been unsuccessful. There is an urgent need to develop new ways of approaching the problem of obesity. Obesity is strongly associated with adverse cardiometabolic events, even at younger age. In a cohort of men included at the age of 22 years in a 33 years follow-up study in Denmark, young obese men, compared with those of normal weight, had an absolute risk increase for Type 2 diabetes, cardiovascular morbidity or premature death of almost 30% before the age of 55 years.1 Epidemiological research shows that obesity increases with aging. It has equally been established that serum testosterone levels in men decline with aging. More detailed analysis has shown that though calendar age per se may be a factor, obesity is a major determinant in the decline of serum testosterone at all ages.2 Conversely, weight loss induces a rise of bound and unbound serum testosterone levels. Testosterone appears to play a critical role in regulating energy utilization including nitrogen retention, carbohydrate and fat metabolism and adipogenesis, and testosterone deficiency, best exemplified in androgen deprivation treatment of prostate cancer, impacts negatively on these processes. Androgen deprivation treatment decreases lean mass and increases fat mass. It also decreases insulin sensitivity while increasing low-density lipoprotein cholesterol and triglycerides and has inconsistent effects on high-density lipoprotein cholesterol. In a number of studies of hypogonadal men whose serum testosterone was restored to normal,3,4 it could be demonstrated that over the duration of the study (up to 6 years) there was a progressive decline of body weight and waist circumference and an increase in lean mass and thereby metabolic rate, with parallel improvements of metabolic parameters3,4 (Figures 1 and 2). We interpret this to indicate that for successful weight loss, serum testosterone should be in the normal range. Another effect of testosterone administration could be improved energy, motivation and behavioral changes, which are difficult to achieve with other interventions. The successful achievement of weight loss, as well as the consistent increase in lean mass, contribute, although not exclusively, to beneficial effects on Type 2 diabetes.5
Figure 1.
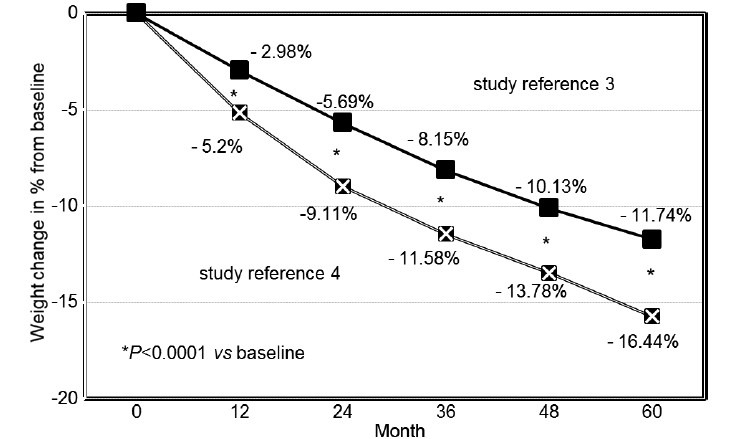
Weight change (% from baseline) in 343 obese hypogonadal men receiving long-term treatment with testosterone undecanoate.
Figure 2.
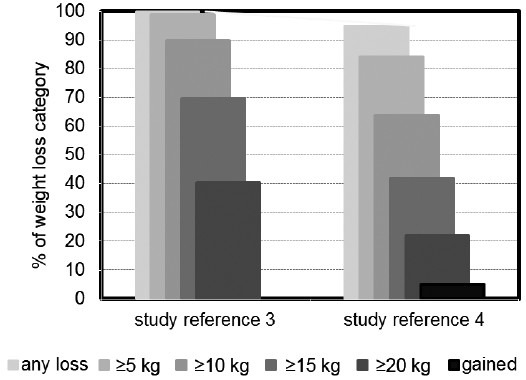
Distribution of weight loss categories in 343 obese hypogonadal men from two separate registry studies receiving long-term treatment with testosterone undecanoate.
TESTOSTERONE AND INFLAMMATION
Inflammation is the body's response to cellular injury, and it is accompanied by a pro-inflammatory state expressed by the increasing levels of inflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β). There is evidence that IL-6, TNF-α and IL-1β inhibit testosterone secretion by their influence on the central (hypothalamic-pituitary) and peripheral (testicular) components of the gonadal axis. Androgen deprivation treatment has shown that testosterone deficiency is associated with a pro-inflammatory state. Further support for this contention comes from a study of men with hypogonadism in whom an increase of levels of TNF-α and IL-6 were observed upon withdrawal of androgen replacement therapy.6 Several studies document the immunosuppressive effect of testosterone administration. This may open a new avenue of treatment of immunopathology with androgens.
There are a number of disease entities of which inflammation now appears to be a core element.
Over the last two decades, the role of inflammation in cardiovascular disease has become clear.7 There is a well-recognized role of low-testosterone levels in atherosclerosis and plaque vulnerability, inducing low-grade systemic inflammation evidenced by increased serum levels of pro-inflammatory cytokines and chemokines. Observational evidence suggests that several pro-inflammatory cytokines (including IL-1β, IL-6, TNF-α, and highly sensitive C-reactive protein [CRP]) and serum testosterone levels are inversely associated in patients with coronary artery disease and Type 2 diabetes mellitus.8 Several studies have documented that administration of testosterone reduces levels of inflammatory factors (for review9,10).
Studies linking low testosterone to the metabolic syndrome (with its sequelae cardiovascular disease and diabetes mellitus) and inflammation have been predominantly performed in elderly men. However in relatively young men, low serum testosterone concentrations were significantly associated with elevated levels of the pro-inflammatory cytokine TNF-α as well as the pro-inflammatory chemokines.11
There is a number of disease entities more evidently characterized by inflammation, and a link of these conditions with testosterone is becoming apparent. Inflammatory bowel disease is a chronic inflammatory disorder that is comprised of both Crohn's disease and ulcerative colitis and has alternating phases of clinical relapse and remission. Their etiologies are unknown, but they are characterized by an imbalanced production of pro-inflammatory mediators, e.g. TNF-α, as well as increased recruitment of leukocytes to the site of inflammation.12,13 In a pilot study of men with Crohn's disease who were hypogonadal, we were able to demonstrate a beneficial effect of normalization of their serum testosterone.14
Multiple sclerosis is a disease characterized by inflammation and demyelination. Currently, the cause of multiple sclerosis is unknown. Experimental autoimmune encephalomyelitis is the most common mouse model of multiple sclerosis. Treatments with the sex hormones, estrogens and androgens, are capable of offering disease protection in animal experiments and are currently being used in clinical trials of multiple sclerosis.15
Another piece of evidence indicating relation between sex hormone levels and inflammation is the recent finding of low-testosterone levels predicting increased risk of rheumatoid factor negative rheumatoid arthritis in men.16
Chronic inflammation is a common finding in benign prostate hyperplasia (BPH). In the last decade, cross-sectional and longitudinal observations of several large cohorts have confirmed that chronic inflammation is a crucial component of BPH. Although androgens are involved in prostate growth during developmental age, their role in the pathogenesis of BPH/lower urinary tract symptoms is now debated.17 Recent data indicate that low, rather than high testosterone and high estradiol favor disease progression. In addition, dihydrotestosterone exerts an immune regulatory role on human prostatic stromal cells, inhibiting their potential actively to induce and/or sustain autoimmune and inflammatory responses.17
TESTOSTERONE FROM “NICHE” HORMONE TO MULTI-SYSTEM HORMONE
The challenge is to convince the medical profession at large that testosterone is not a “niche” hormone, but a multi-system hormone with much wider range of actions than reproduction or sexual functions. Very salient is the wrongful association most physicians assume to exist between testosterone and the development of prostate pathology (prostate cancer and BPH) and cardiovascular disease. It has been in particular the work of Dr. Abraham Morgentaler dispelling the historical fear that raising testosterone levels will result in more prostate cancer. Studies have failed to show increased risk of prostate cancer in men with higher serum testosterone, and supraphysiological testosterone fails to increase prostate volume or prostate-specific antigen in healthy men. This apparent paradox is explained by the “saturation model,” which posits a finite capacity of androgen to stimulate growth of prostate cancer. Modern studies indicate no increased risk of prostate cancer among men with serum testosterone in the therapeutic range.18 Follow-up of elderly men receiving testosterone is necessary because age is a strong prognosticator of prostate malignancy.
The other barrier to overcome is the “hormonophobia” that had led to discussions about the safety of testosterone administration with regard to cardiovascular disease. Studies showing a relationship between testosterone administration and cardiovascular disease in elderly men have been heavily criticized for methodological flaws and should invite a much needed critical analysis of pro and con arguments.19 Enlightening is the paper by Grimes and Schulz20 stating that reported associations in observational clinical research are not rarely false or, if true, are overstated. There is often no due recognition of the inherent limitations of statistical power of these studies.
Prescription of testosterone has traditionally been the domain of endocrinologists and urologists. A large number of convincing clinical studies will be needed to persuade other disciplines to include testosterone in their therapeutical armamentarium.
REFERENCES
- 1.Schmidt M, Johannesdottir SA, Lemeshow S, Lash TL, Ulrichsen SP, et al. Obesity in young men, and individual and combined risks of type 2 diabetes, cardiovascular morbidity and death before 55 years of age: a Danish 33-year follow-up study. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2013-002698. doi: 10.1136/bmjopen-2013-002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 3.Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational cohort study. Obes Res Clin Pract. 2014;8:e339–49. doi: 10.1016/j.orcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes. 2013;3:73–83. doi: 10.1111/cob.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol 2014. 2014:683515. doi: 10.1155/2014/683515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–9. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 7.Herring MJ, Mesbah Oskui P, Hale SL, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the basic science literature. J Am Heart Assoc. 2013;2:e000271. doi: 10.1161/JAHA.113.000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nettleship JE, Pugh PJ, Channer KS, Jones T, Jones RD. Inverse relationship between serum levels of interleukin-1beta and testosterone in men with stable coronary artery disease. Horm Metab Res. 2007;39:366–71. doi: 10.1055/s-2007-976543. [DOI] [PubMed] [Google Scholar]
- 9.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217:R25–45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- 10.Vodo S, Bechi N, Petroni A, Muscoli C, Aloisi AM. Testosterone-induced effects on lipids and inflammation. Mediators Inflamm 2013. 2013:183041. doi: 10.1155/2013/183041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One. 2013;8:e61466. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20:64–77. doi: 10.3748/wjg.v20.i1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haider A, Kurtz W, Giltay EJ, Gooren LJ, Saad F. Administration of testosterone to elderly hypogonadal men with Crohn's disease improves their Crohn's Disease Activity Index: a pilot study. Horm Mol Biol Clin Investig. 2010;2:287–92. doi: 10.1515/HMBCI.2010.033. [DOI] [PubMed] [Google Scholar]
- 15.Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33:105–15. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pikwer M, Giwercman A, Bergström U, Nilsson JÅ, Jacobsson LT, et al. Association between testosterone levels and risk of future rheumatoid arthritis in men: a population-based case-control study. Ann Rheum Dis. 2014;73:573–9. doi: 10.1136/annrheumdis-2012-202781. [DOI] [PubMed] [Google Scholar]
- 17.Vignozzi L, Cellai I, Santi R, Lombardelli L, Morelli A, et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol. 2012;214:31–43. doi: 10.1530/JOE-12-0142. [DOI] [PubMed] [Google Scholar]
- 18.Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65:115–23. doi: 10.1016/j.eururo.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Morgentaler A, Kacker R. Andrology: testosterone and cardiovascular risk - Deciphering the statistics. Nat Rev Urol. 2014;11:131–2. doi: 10.1038/nrurol.2014.24. [DOI] [PubMed] [Google Scholar]
- 20.Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120:920–7. doi: 10.1097/AOG.0b013e31826af61a. [DOI] [PubMed] [Google Scholar]


