Abstract
The study was to compare treatment preference, efficacy, and tolerability of sildenafil citrate (sildenafil) and tadalafil for treating erectile dysfunction (ED) in Chinese men naïve to phosphodiesterase 5 (PDE5) inhibitor therapies. This multicenter, randomized, open-label, crossover study evaluated whether Chinese men with ED preferred 20-mg tadalafil or 100-mg sildenafil. After a 4 weeks baseline assessment, 383 eligible patients were randomized to sequential 20-mg tadalafil per 100-mg sildenafil or vice versa for 8 weeks respectively and then chose which treatment they preferred to take during the 8 weeks extension. Primary efficacy was measured by Question 1 of the PDE5 Inhibitor Treatment Preference Questionnaire (PITPQ). Secondary efficacy was analyzed by PITPQ Question 2, the International Index of Erectile Function (IIEF) erectile function (EF) domain, sexual encounter profile (SEP) Questions 2 and 3, and the Drug Attributes Questionnaire. Three hundred and fifty men (91%) completed the randomized treatment phase. Two hundred and forty-two per 350 (69.1%) patients preferred 20-mg tadalafil, and 108/350 (30.9%) preferred 100-mg sildenafil (P < 0.001) as their treatment in the 8 weeks extension. Ninety-two per 242 (38%) patients strongly preferred tadalafil and 37/108 (34.3%) strongly the preferred sildenafil. The SEP2 (penetration), SEP3 (successful intercourse), and IIEF-EF domain scores were improved in both tadalafil and sildenafil treatment groups. For patients who preferred tadalafil, getting an erection long after taking the medication was the most reported reason for tadalafil preference. The only treatment-emergent adverse event reported by > 2% of men was headache. After tadalafil and sildenafil treatments, more Chinese men with ED naïve to PDE5 inhibitor preferred tadalafil. Both sildenafil and tadalafil treatments were effective and safe.
Keywords: erectile dysfunction, patient preference, phosphodiesterase 5 inhibitors
INTRODUCTION
Erectile dysfunction (ED) affects millions of men worldwide,1 and can be distressing because of its effect on self-esteem, quality of life, and interpersonal relationships.2,3 From the relational component, the quality of the relationship represents another important determinant that the impairment of relational factors independently predicted ED severity.4 Therefore, it is not easy to evaluate all these aspects for a correct diagnosis and essential step for the right ED treatment prescription. Besides correcting lifestyle changes and risk factors whenever possible, and providing sexual education and counseling, a first-line treatment strategy usually includes a phosphodiesterase 5 (PDE5) inhibitor either administered on-demand or on a daily basis.5 Currently, PDE5 inhibitors are as the first-line treatment choice for ED in Western society.1 Although traditional Chinese medicine has been widely used in China for the treatment of impotence, more men are switching to PDE5 inhibitors for ED treatment.2 Although, it is still a matter of debate if and how any information on the patient and any characteristic of his disease should lead to the selection of one treatment rather than another, a particular PDE5 inhibitor often relies more on personal beliefs than on solid evidence.5
In China, there are three PDE5 inhibitors: sildenafil citrate (sildenafil), tadalafil, and verdanafil hydrochloride (verdanafil) as needed (pro re nata [PRN]). Some preference studies have shown patients3,6,7 and partners3,8 prefer tadalafil over sildenafil in the treatment of ED, but no preference studies have been conducted in men with ED in China. At the moment this study was conceived, the majority of patients in China were prescribed sildenafil or tadalafil. Hence, we designed a crossover randomized, open-label trial to compare patient's preference of tadalafil 20-mg with sildenafil 100-mg over 8 weeks of each drug. Patients who completed both treatment arms chose either tadalafil or sildenafil as the treatment of choice to be continued for gratis during an 8 weeks extension phase. At the end of the extension period, patient's sexual quality of life was assessed. The primary objective of this study was to evaluate the treatment preference of tadalafil as the treatment of choice compared with the most widely prescribed PDE5 inhibitor, sildenafil, in Chinese men with ED.9 This choice of continued therapy was a behavioral indicator of which drug the patient prefers. In addition, this study also described efficacy, safety, tolerability, and sexual quality-of-life with treatment.
MATERIALS AND METHODS
This was a multicenter, randomized, open-label, crossover study to evaluate whether men with ED preferred 20-mg tadalafil or 100-mg sildenafil and to compare their efficacy and tolerability. Eligible patients were men in China who were at least 18 years of age, who were in a stable relationship with a female partner, and who had a history of ED for at least 3 months, but were naïve to any treatment with a PDE5 inhibitor. Both tadalafil and sildenafil were administered as needed before sexual activity, but at no more than one dose per day. Written informed consent was obtained from all patients and Local Institutional Review Committees approved the study.
Patients
The study included men (≥18 years and < 65 years of age) with ED who were in a steady exclusive relationship (at least 3 months) with a female partner. All patients were naïve to treatment for ED with medications that inhibit PDE5. ED was defined as a consistent change in the quality of erection that adversely affects the patient's satisfaction with sexual intercourse.
Men with these criteria were excluded: untreated endocrine disease (e.g. hypogonadism); premature ejaculation; a history of radical prostatectomy (except nerve-sparing with residual erectile function [EF]) or other pelvic surgery (with subsequent failure to achieve erection); clinically significant penile deformity; a history of penile implant; significant renal or hepatobiliary disease; a hemoglobin A1C of > 11%; unstable angina or congestive heart failure within the preceding 6 months; a history of myocardial infarction, coronary artery bypass graft surgery, or percutaneous coronary intervention within the preceding 90 days; a history of sudden cardiac arrest, clinically significant arrhythmia, or conduction defect within the preceding 90 days; a systolic blood pressure > 170 or < 90 mmHg or diastolic blood pressure > 100 or < 50 mmHg; malignant hypertension; retinitis pigmentosa; significant central nervous system injuries within the preceding 6 months; current treatment with nitrites, cancer chemotherapy, or antiandrogens; history of human immunodeficiency virus infection; and history of substance abuse (drug or alcohol) within the preceding 6 months.
Study objectives
The primary objective was to evaluate whether, after two 8 weeks treatment periods, male patients in China with ED prefer 20-mg tadalafil PRN orally or 100-mg sildenafil PRN orally as measured by Question 1 of the PDE5 Inhibitor Treatment Preference Questionnaire (PITPQ), “Which medication do you prefer to take for the next 8 weeks of treatment, tadalafil or sildenafil?”
The secondary objectives included the degree of patient treatment preference from Question 2 of the PITPQ (“for the treatment you selected in question one, what is your degree of preference”), a comparison of the efficacy of sildenafil and tadalafil using the International Index of Erectile Function (IIEF) EF domain and sexual encounter profile (SEP) diary questions SEP2 (successful penetration) and SEP3 (successful intercourse), and the Drug Attribute Questionnaire (DRAQ). The safety and tolerability of sildenafil and tadalafil were evaluated by patient-reported adverse events (AEs).
Study design
The study had a minimum of 8 visits, comprising 7 phases. Study duration was approximately 29–30 weeks. Approximately, 370 patients were randomly assigned to treatment (185 patients per treatment sequence). Approximately, 15 sites in China participated in this study. An 8 weeks extension phase was incorporated in this study. Patients who completed this study through visit 7 might be eligible to participate in the extension phase. There was no expectation of any further extension. The extension provided a behavioral indicator of the patient's drug preference for the treatment of ED. In addition, the extension provided the opportunity to obtain additional safety data on tadalafil and sildenafil (Figure 1).
Figure 1.
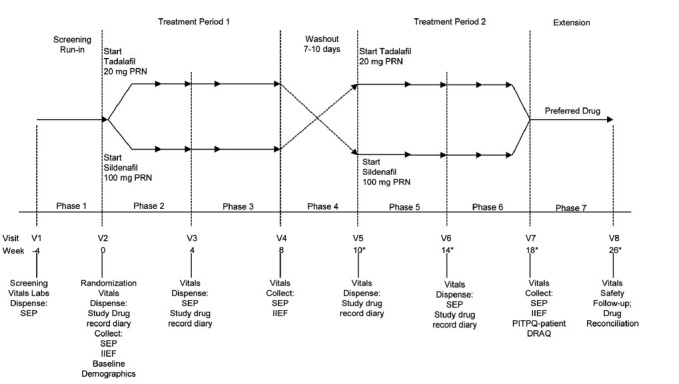
The study design. DRAQ, IIEF, PITPQ, PRN (as needed), SEP, and V. *Wash-out period was 7–10 days, up to 10 days have been included in the timeline (i.e., 1.5 weeks). The number of weeks had been rounded up to the nearest integer. DRAQ: Drug Attributes Questionnaire; IIEF: International Index of Erectile Function; PITPQ: Phosphodiesterase 5 Inhibitor Treatment Preference Questionnaire; PRN: pro re nata; SEP: sexual encounter profile; V: visit.
Adverse events
Patient-reported treatment-emergent adverse events (TEAEs) were defined as events that first occurred or became worse after baseline, and were categorized as mild, moderate, or severe. AEs were recorded using Preferred Terms from the Medical Dictionary for Regulatory Activities (MedDRA, Version 7.0).
Statistical analysis
A sample size of 370 patients (185 patients per sequence group) was estimated to achieve 90% power to detect an increased preference for tadalafil over sildenafil of 10% (60% vs 50%) using a two-sided chi-square test with a significance level of 0.05 assuming 30% of Chinese patients have a missing treatment preference.
The baseline visit for efficacy and safety measures (where applicable) was prior to starting of the first treatment period, the randomization visit (visit 2). Change from baseline to end of each treatment period was defined as the value at the end of each treatment period minus the baseline value.
All efficacy analyses were conducted on an intent-to-treat (ITT) basis. The primary ITT analysis included all randomized patients who completed both treatment periods (until visit 7) Wilson score method was used to calculate the 95% confidence interval (CI) of the preference of tadalafil over sildenafil.10 The secondary efficacy analyses for Question 2 of the PITPQ, the IIEF-EF domain, SEP2 and SEP3, and the DRAQ included all randomized patients with a baseline observation and at least one postbaseline observation. Changes from baseline to endpoint were analyzed using a mixed effect analysis of covariance model for crossover designs for a change from baseline to end of each treatment period. The model included treatment, period, sequence, and pooled site as fixed effects; centered baseline value of the efficacy measure (defined as the baseline value for a patient minus the overall baseline mean value) as a covariate; patient within sequence as a random effect; and centered baseline-by-treatment interaction (if P < 0.1) as a fixed effect. Since the wash-out period was planned for more than 7 days (7–10 days), no carryover effect was included in the mixed model. Frequency tables using counts and percentages were generated for the DRAQ.
The safety analyses for AEs and vital signs included all randomized patients who received at least one dose of the study drugs (either tadalafil or sildenafil) in any of the two treatment periods.
Statistical analyses were performed using SAS® Drug Development.
RESULTS
Patient disposition and demographics
Figure 2 illustrates the patient disposition and reasons for study discontinuation. While 418 patients signed consent to participate the study, 35 failed the initial screening. Of the remainders, 383 patients completed run-in phase and randomization; 190 men were randomized to treatment with tadalafil, followed by sildenafil (IC/S) and 193 to treatment with sildenafil followed by tadalafil (S/IC). In the IC/S group, 26 men discontinued treatment, and in the S/IC group, 20 men discontinued treatment. The reasons for discontinuation in a majority of patients were lost of follow-up and/or patient's withdrawal. Other reasons for discontinuation were AEs (3 patients), physician decision (1 patient), entry criteria not met (1 patient), and lack of efficacy (1 patient) in IC/S group (Figure 2). A total of 337 (88.0%) of randomized patients completed the study through the extension phase. The baseline patient demographics were comparable between the two treatment sequences (i.e. S/IC and IC/S) (Table 1).
Figure 2.
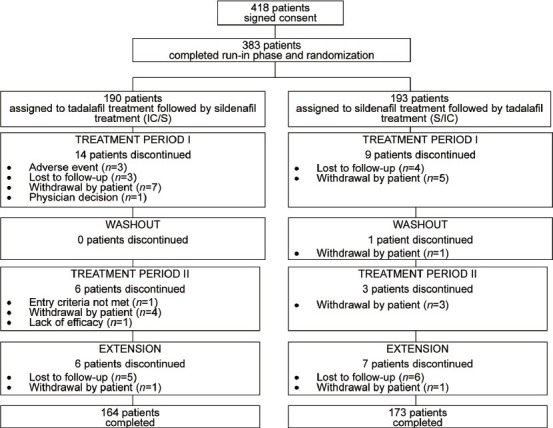
Patient disposition and reasons for discontinuation from the study.
Table 1.
Patient demographics and baseline comorbidities
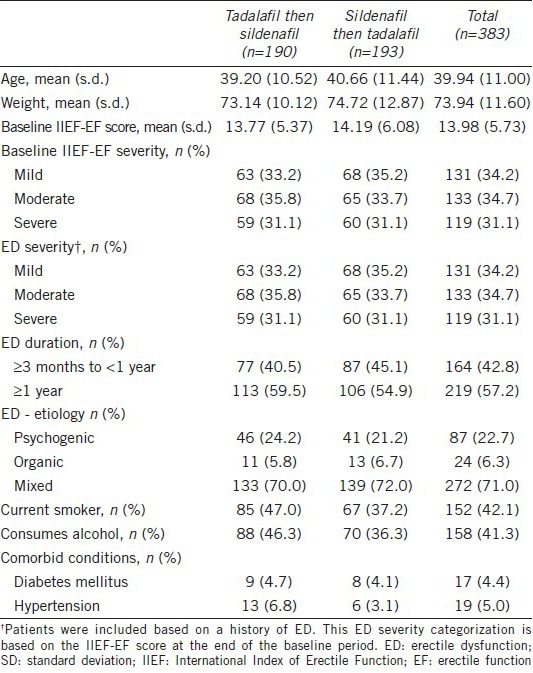
Table 1 shows demographics and other baseline characteristics: age, weight, IIEF-EF domain and severity, duration and etiology of ED, smoking and alcohol use, and comorbid conditions. The baseline patient demographics were similar for the two treatment sequences. Patients had a mean age of 39.94 years with a range of 21.38–65.22 years. At baseline, the percentage of men who had a history of ED between 3 months and 1 year was 42.8%, and the percentage of those with ED more than 1 year was 57.2%. The ED severity was moderate or severe in 65.8% of men, and the cause of ED was “organic” or “mixed” in 77.3% (“organic” 6.3%, “mixed” 71.0%) of men. The most common comorbidities included diabetes mellitus (4.4%) and hypertension (5%).
Efficacy
A total of 350 patients completed both treatment sequences. After the 8 weeks extension, 100% patients responded to Question 1 of the PITPQ. The number of patients who preferred 20-mg tadalafil (242/350 [69.1%]) was twice than that of patients who preferred 100-mg sildenafil (108/350 [30.9%]) for ED therapy. Significantly more patients chose tadalafil treatment over sildenafil treatment (95% CI: 0.64–0.74; P < 0.001) (Figure 3 and Table 2).
Figure 3.
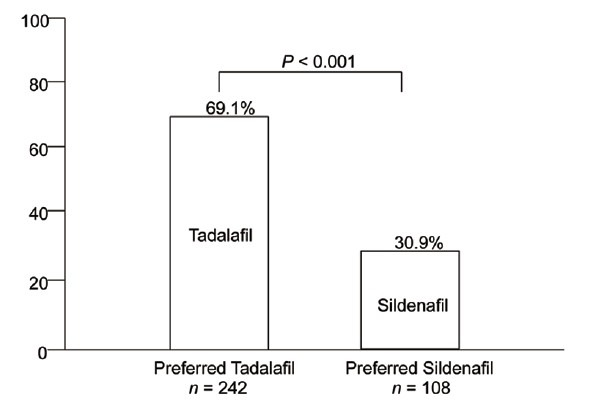
Patient preference treatment.
Table 2.
Summary of PDE5 inhibitor treatment preference for questions 1 and 2
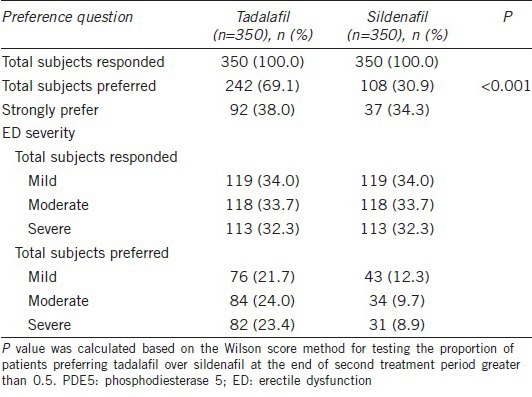
Among the patient who answered Question 2 of the PITPQ and preferred tadalafil, 38.0% (92/242) of them showed a strong preference. It was relatively higher than the 34.3% (37/108) of patients who strongly preferred the sildenafil. When divided ED severity patients into three sub-groups (mild, moderate and severe), it was found that the responded patients in each sub-group was similar, but patient preference from ED mild, moderate and severe sub-groups was higher in the tadalafil group than that in the sildenafil group (Table 2).
Figure 4 demonstrates the point estimates and 95% CIs for the differences (tadalafil minus sildenafil) in the least-square mean change from baseline on each of the five IIEF domains.
Figure 4.
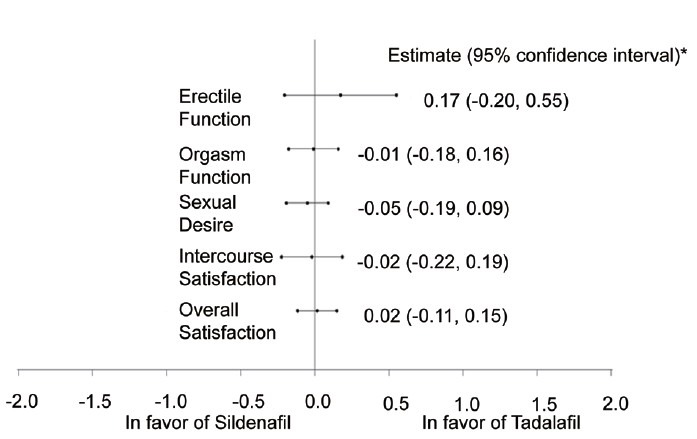
IIEF domain change (LS mean). *Analyses were conducted on an intent-to-treat basis for all randomized patients with baseline and at least one postbaseline outcome. Note: the estimate and 95% confidence interval for difference between tadalafil and sildenafil on IIEF domain score change were from a crossover analysis of variance model, which contained terms for centered baseline score, treatment, pooled site, period, sequence and centered baseline-by-treatment interaction (if P < 0.1). IIEF: International Index of Erectile Function.
The CIs contain zero for all five domains of EF, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction, indicating that the changes in domain scores are similar for men took tadalafil compared with sildenafil. Table 3 shows that the mean changes from baseline to endpoint were similar for men that took tadalafil compared with sildenafil on the five IIEF domains. For the IIEF-EF domains, the mean changes from baseline were improved in both men treated with tadalafil (12.03) and those treated with sildenafil (11.86) (P = 0.364).
Table 3.
The IIEF domain scores
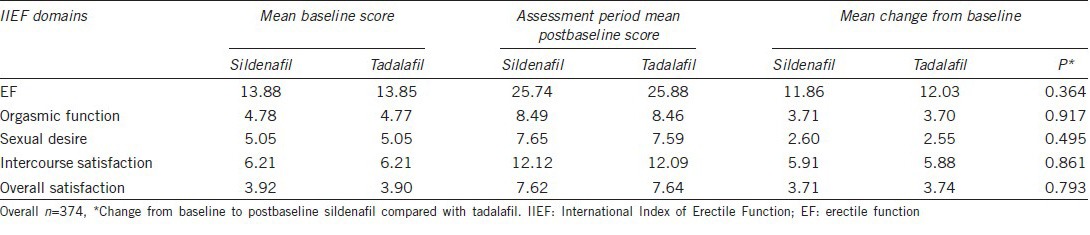
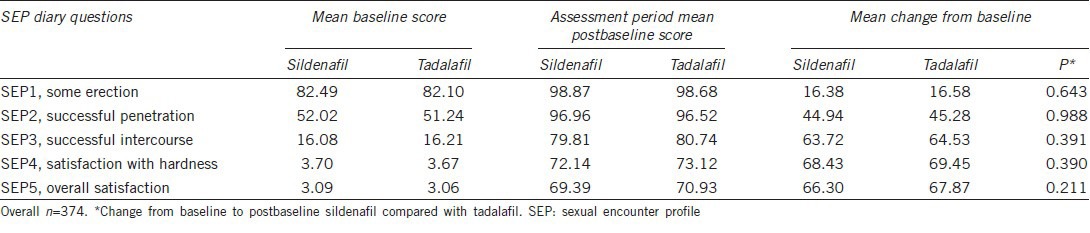
Table 4 shows the mean score per patient percentage of “yes” responses to the SEP diary questions at baseline and after the sildenafil and tadalafil treatment assessment phases. The mean changes from baseline were similar after sildenafil and tadalafil on all five questions. For SEP2 (successful penetration), an increase from baseline in the mean per patient percentage of “yes” responses was 44.94% after sildenafil versus 45.28% after tadalafil (P = 0.988). For SEP3 (successful intercourse), an increase from baseline in the mean per patient percentage of “yes” responses was 63.72% after sildenafil versus 64.53% after tadalafil (P = 0.391). The improvements were obvious for both treatments, but there was no significant difference between them.
Table 4.
The SEP diary question responses–mean per patient percentage of “yes” responses
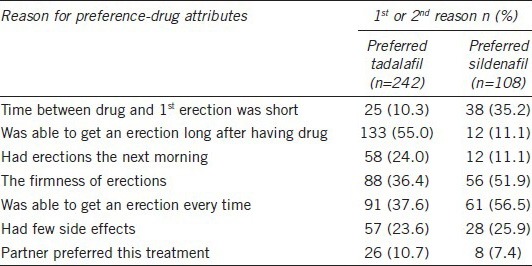
The DRAQ was administered at the end of the second treatment assessment period for the patients who had answered the PITPQ. Table 5 illustrates the 1st and 2nd best reasons chosen by patients to explain why they preferred either tadalafil or sildenafil. Among the answers to the DRAQ, “was able to get an erection long after having drug” was the main reason patients chose tadalafil treatment, with 133/242 (55.0%) patients indicating this response as the 1st or 2nd reason of the preference while only 12/108 (11.1%) patients who preferred the sildenafil treatment indicated the same reason. Of the 108 patients who preferred sildenafil, 61/108 (56.5%) identified “was able to get an erection every time” as the 1st or 2nd reason for their preference.
Table 5.
Drug attribute questionnaire results from patients who preferred tadalafil and sildenafil
Adverse events
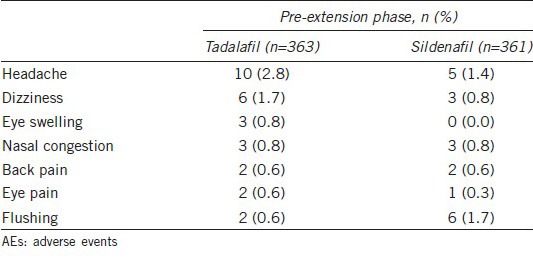
Table 6 shows the overview of AEs in both pre-extension and extension phases. In the pre-extension phase, total 363 patients received tadalafil treatment, 30 (8.3%) reported TEAEs, and of the 361 patients that received sildenafil treatment, 26 (7.2%) reported TEAEs. The most frequently reported TEAEs were headache, dizziness, eye swelling, nasal congestion, back pain, eye pain, and flushing. The only TEAE reported in ≥ 2% of patients was headache (Table 7). AEs leading to discontinuation occurred for 3 (0.8%) patients in tadalafil treatment group, including 2 (0.6%) patients with headache and 1 (0.3%) patient with alanine aminotransferase increased.
Table 6.
Adverse events overview
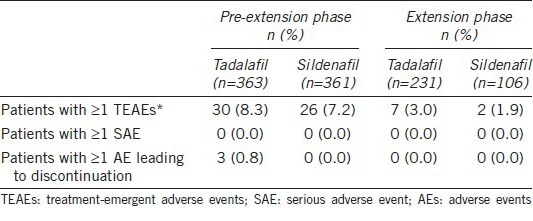
Table 7.
Treatment emergent AEs (pre-extension)
In the extension phase, a total of 231 patients chose to take tadalafil treatment, 7 (3.0%) reported TEAEs and of the 106 patients that chose to take sildenafil treatment, 2 (1.9%) reported TEAEs. There were no AEs leading to discontinuation reported in the extension phase.
No serious AE was thought by the investigator to be related to tadalafil or sildenafil treatment. No death was reported during the study.
DISCUSSION
Massachusetts Male Aging Study shows that transient ED and inadequate erection effect were observed in as many as 50% of males between the ages of 40 and 70 years,11 and China has the similar ED prevalence in men over 40 years old.12,13,14 Eardley et al.15 did a preference study in European countries to demonstrate most ED patients prefer tadalafil, and this study evaluated the preferences of Chinese patients. In Yuan's comparative study,16 from the practitioners, the finding shows that in the recommended dosage, oral PDE5 inhibitors are more effective than placebo for ED. For the three traditional PDE5 inhibitors, sildenafil, vardenafil and tadalafil, their efficacy has been confirmed. Among the three inhibitors, tadalafil is likely to be the most effective one for ED because tadalafil also has many other advantages in additional to safety and well toleration, such as patient preference, which may be considered the first choice for ED patients.16
In this study, after receiving both treatments, 69.1% patients preferred tadalafil, and 30.9% patients preferred sildenafil (Figure 3). Of the patients who preferred tadalafil, 38% strongly preferred it; of those who preferred sildenafil, 34.3% patients strongly preferred the sildenafil. The preference for tadalafil in Chinese patients is similar to that in the previous Eardley study.15
In the answers to the DRAQ, more patients (55.0%) responded that when using tadalafil treatment, they were able to get an erection long after having drug than those treated with sildenafil (11.1%) and it is the main reason patients preferred tadalafil. The IIEF domain scores were obviously improved in both the tadalafil and sildenafil treatments, and there is no significant difference between the two treatments. In SEP2 and SEP3, there were obviously improved scores in both the tadalafil and sildenafil treatments. The incidences of TEAEs (most were mild) were low in both the tadalafil and sildenafil treatments in the pre-extension (tadalafil: 8.3%; sildenafil: 7.2%) and extension periods (tadalafil: 3.0%; sildenafil: 1.9%). No severe TEAEs reported in tadalafil treatment.
Previous studies suggested that tadalafil was efficacious, safe, and well tolerated in the treatment of ED. The reason why patients preferred tadalafil compared to sildenafil was mainly because of the longer duration of action that increased the patient's freedom in sexual life.17 The preference for tadalafil was established in an open-label study that switched patients who had previously used 25, 50, or 100-mg sildenafil to treatment with 20-mg tadalafil.15
In this China preference study, the doses selected were 20-mg tadalafil and 100-mg sildenafil represented the approved maximum label doses. The selection of sildenafil as the primary comparator was appropriate because it was the most commonly prescribed oral PDE5 inhibitor worldwide. The highest dose of the comparator was selected to minimize any difference in preference due to efficacy.
Tadalafil differs from the other two PDE5 inhibitors in its’ pharmacokinetic characteristics. The mean half-life of both sildenafil and vardenafil is approximately 4 h,18 with demonstrated improvement in EF up to 8–12 h postdose;19,20 whereas the mean half-life of tadalafil is 17.5 h,21 with demonstrated improvement in EF up to 36 h postdose.22,23 The difference between the tadalafil and sildenafil pharmacokinetic profiles grants patients more freedom to perform sexual intercourse with less need to plan ahead.
Considering the limitations of an open-label study design, investigators and/or patients could have been influenced by marketing messages of both tadalafil and sildenafil creating initial preconceptions. However, the use of 4 weeks treatment periods and the randomization by sequential 20 mg tadalafil per 100 mg sildenafil or vice versa and a 4 weeks assessment phase was expected to minimize this potential bias. In addition, the study population included only men who were naïve to treatment with PDE5 inhibitors for the treatment of ED, removing any influence of including patients who were previous responders or nonresponders to PDE5 inhibitors.24
CONCLUSIONS
Twenty milligram tadalafil and 100-mg sildenafil were effective and safe treatments for men with ED naïve to PDE5 inhibitor treatment who had a broad range of demographic characteristics and comorbid conditions that are representative of the general patient population of men with ED in China. After experiencing both tadalafil and sildenafil treatments, more patients and partners preferred to choose the tadalafil during the study extension phase because the patients were able to get an erection long after tadalafil dosing and the patients had less time concerns and more spontaneity.
AUTHOR CONTRIBUTIONS
WJB, HJL, YTD, XYH, YRH, JHL and XFW contributed to the data collection and intellectual revision of this manuscript. WJB, HJL, SS, CJ and JJJ contributed to draft the manuscript. XFW, SS, JJJ and CJ participated in the study design, coordination. CJ, SS and JJJ performed the statistical analysis. All authors read, reviewed and approved the final manuscript.
COMPETING INTERESTS
All Authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by Eli Lilly and Company, who was responsible for design and conduct of the study, for monitoring the study, and for verification and analysis of the data. The identifier label for this study is H6D-CR-LVIZ. The authors would like to thank the investigators who participated in this study, clinical research physician, Wen-ping Xu, who reviewed and provided comments in this manuscript, as well as the medical writer, Yong Joy Zhou, who polished the manuscript.
REFERENCES
- 1.Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7:524–40. doi: 10.1111/j.1743-6109.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang EY. Switching between traditional Chinese medicine and Viagra: cosmopolitanism and medical pluralism today. Med Anthropol. 2007;26:53–96. doi: 10.1080/01459740601184448. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Pommerville P, Brock G, Gagnon R, Mehta P, et al. Physician-rated patient preference and patient- and partner-rated preference for tadalafil or sildenafil citrate: results from the Canadian ‘Treatment of Erectile Dysfunction’ observational study. BJU Int. 2006;98:623–9. doi: 10.1111/j.1464-410X.2006.06384.x. [DOI] [PubMed] [Google Scholar]
- 4.Corona G, Petrone L, Mannucci E, Magini A, Lotti F, et al. Assessment of the relational factor in male patients consulting for sexual dysfunction: the concept of couple sexual dysfunction. J Androl. 2006;27:795–801. doi: 10.2164/jandrol.106.000638. [DOI] [PubMed] [Google Scholar]
- 5.Corona G, Mondaini N, Ungar A, Razzoli E, Rossi A, et al. Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: the proper drug for the proper patient. J Sex Med. 2011;8:3418–32. doi: 10.1111/j.1743-6109.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- 6.Tolrà JR, Campaña JM, Ciutat LF, Miranda EF. Prospective, randomized, open-label, fixed-dose, crossover study to establish preference of patients with erectile dysfunction after taking the three PDE-5 inhibitors. J Sex Med. 2006;3:901–9. doi: 10.1111/j.1743-6109.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 7.Brock G, Chan J, Carrier S, Chan M, Salgado L, et al. The treatment of erectile dysfunction study: focus on treatment satisfaction of patients and partners. BJU Int. 2007;99:376–82. doi: 10.1111/j.1464-410X.2006.06586.x. [DOI] [PubMed] [Google Scholar]
- 8.Conaglen HM, Conaglen JV. Investigating women's preference for sildenafil or tadalafil use by their partners with erectile dysfunction: the partners’ preference study. J Sex Med. 2008;5:1198–207. doi: 10.1111/j.1743-6109.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 9.Hu JL, Chen B. Tadalafil and patients preference in the treatment of erectile dysfunction. Zhonghua Nan Ke Xue. 2006;12:1145–8. [PubMed] [Google Scholar]
- 10.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–12. [Google Scholar]
- 11.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 12.Bai Q, Xu QQ, Jiang H, Zhang WL, Wang XH, et al. Prevalence and risk factors of erectile dysfunction in three cities of China: a community-based study. Asian J Androl. 2004;6:343–8. [PubMed] [Google Scholar]
- 13.Leng J, Wang YJ, Huang XY, Bo JJ, Han YF. Epidemiology survey in 1582 middle-aged men with erectile dysfunction in Shanghai. Chin Journal of Androl. 2000;14:29–31. [Google Scholar]
- 14.Zhang ZC, Sun B, Liu YS, Zuo C, Zhongcheng X, et al. The prevalence of erectile dysfuncrion in 1247 married men in Beijing urban area. Chin J Urol. 2003;12:855–7. [Google Scholar]
- 15.Eardley I, Mirone V, Montorsi F, Ralph D, Kell P, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naïve to phosphodiesterase 5 inhibitor therapy. BJU Int. 2005;96:1323–32. doi: 10.1111/j.1464-410X.2005.05892.x. [DOI] [PubMed] [Google Scholar]
- 16.Yuan J, Zhang R, Yang Z, Lee J, Liu Y, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol. 2013;63:902–12. doi: 10.1016/j.eururo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Mirone V, Fusco F, Rossi A, Sicuteri R, Montorsi F. Tadalafil and vardenafil vs sildenafil: a review of patient-preference studies. BJU Int. 2009;103:1212–7. doi: 10.1111/j.1464-410X.2008.08267.x. [DOI] [PubMed] [Google Scholar]
- 18.Hatzimouratidis K, Porst H, Sharlip ID, Hatzichristou D, Rubio-Aurioles E, et al. Extended duration of efficacy of vardenafil when taken 8 hours before intercourse: a randomized, double-blind, placebo-controlled study (Editorial comment) Eur Urol. 2006;50:1095. doi: 10.1016/j.eururo.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Porst H, Sharlip ID, Hatzichristou D, Rubio-Aurioles E, Gittelman M, et al. Extended duration of efficacy of vardenafil when taken 8 hours before intercourse: a randomized, double-blind, placebo-controlled study. Eur Urol. 2006;50:1086–94. doi: 10.1016/j.eururo.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Gingell C, Sultana SR, Wulff MB, Gepi-Attee S. Duration of action of sildenafil citrate in men with erectile dysfunction. J Sex Med. 2004;1:179–84. doi: 10.1111/j.1743-6109.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 21.Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257–80. doi: 10.1111/j.1464-410X.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- 22.Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, et al. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology. 2003;62:121–5. doi: 10.1016/s0090-4295(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 23.Young JM, Feldman RA, Auerbach SM, Kaufman JM, Garcia CS, et al. Tadalafil improved erectile function at twenty-four and thirty-six hours after dosing in men with erectile dysfunction: US trial. J Androl. 2005;26:310–8. doi: 10.2164/jandrol.04126. [DOI] [PubMed] [Google Scholar]
- 24.Mulhall JP. Understanding erectile dysfunction medication preference studies. Curr Opin Urol. 2004;14:367–73. doi: 10.1097/00042307-200411000-00013. [DOI] [PubMed] [Google Scholar]


