Abstract
Urotensin-II (U-II) is a cyclic peptide that acts through a G protein-coupled receptor (urotensin-II receptor [UTR]) mainly involved in cardiovascular function in humans. The urotensinergic system is also implicated in the urogenital tract. Indeed, U-II relaxes human corpus cavernosum strips and causes an increase in intracavernous pressure (ICP) in rats. In light of this, the U-II/UTR pathway can be considered a new target for the treatment of erectile dysfunction. On this hypothesis, herein we report on two new UTR high affinity-agonists, P5U (H-Asp-c[Pen-Phe-Trp-Lys-Tyr-Cys]-Val-OH) and UPG84(H-Asp-c[Pen-Phe-DTrp-Orn-(pNH2) Phe-Cys]-Val-OH). The effects of P5U and UPG84 were each compared separately with U-II by monitoring the ICP in anesthetized rats. Intracavernous injection of U-II (0.03–1 nmol), P5U (0.03–1 nmol) or UPG84 (0.03–1 nmol) caused an increase in ICP. P5U, in particular, elicited a significant increase in ICP as compared to U-II. The observed effect by using P5U at a dose of 0.1 nmol per rat was comparable to the effect elicited by U-II at a dose of 0.3 nmol. Moreover, UPG84 at the lowest dose (0.03 nmol) showed an effect similar to the highest dose of U-II (1 nmol). Furthermore, UPG84 was found to be more effective than P5U. Indeed, while the lowest dose of P5U (0.03 nmol) did not affect the ICP, UPG84, at the same dose, induced a prominent penile erection in rat. These compounds did not modify the blood pressure, which indicates a good safety profile. In conclusion, UPG84 and P5U may open new perspectives for the management of erectile dysfunction.
Keywords: erectile dysfunction, intracavernous pressure, rat, urotensin-II, urotensin-II ligands
INTRODUCTION
Urotensin-II (U-II) is a cyclic peptide hormone initially isolated from the caudal neurosecretory system of the teleostean fish Gillichthys mirabilis. The peptide was identified as the natural ligand of an orphan G-protein coupled receptor 14, referred as urotensin-II receptor (UTR).1 The wide distribution of the U-II/UTR pathway, as well as its high interspecies homology, indicates that the urotensinergic system plays a role in the regulation of many physiological functions in vertebrates.1 In this context, it has been shown that both U-II and UTR are expressed in the brain, cardiovascular system, liver, kidney, endocrine gland, intestine, prostate, and corpus cavernosum.2,3,4,5 Interestingly, U-II can be also measured in the human plasma of healthy volunteers suggesting that this pathway has a physiological role in vascular homeostasis.6 The effect of U-II on the vascular system is variable, depending on species, vascular bed and the caliber of vessels. The net effect on vascular tone is a balance between endothelium-independent vasoconstriction and endothelium-dependent vasodilatation.7,8 In many ways, U-II exhibits actions similar to other key neurohormonal factors, that is, angiotensin-II and endothelin-1, in driving a variety of cardiac and vascular disease processes.9 These include vasoconstriction as well as mitogenic, trophic and pro-fibrotic effects.10
However, strong evidence indicates that UTR stimulation on endothelial cells can trigger the release of nitric oxide (NO) and prostaglandins that would serve to balance the contractile effects of U-II on smooth muscle.11,12,13,14,15,16,17,18 In this framework, it has been reported that U-II acts as vasodilator in human and rat corpus cavernosum suggesting a physiological role in erectile function.5 Indeed, the intracavernous administration of U-II in rats elicits a dose-dependent increase in intracavernous pressure (ICP), that is, penile erection.5 Moreover, U-II relaxes human corpus cavernosum in endothelium-and NO-dependent manner.5 The activation of the urotensinergic system in human corpus cavernosum causes endothelial NO synthase (eNOS) phosphorylation and heat shock protein 90 recruitment, shifting eNOS to a more activate state.19 This in turn leads to a sustained NO release, which contributes to the maintenance of the ongoing erection.19 Thus, the U-II/UTR pathway may represent an important novel target in order to find new pharmacological approaches in erectile dysfunction. As new synthetic UTR peptide agonists have been developed,20,21 we have evaluated the activity of two super-agonists, P5U (H-Asp-c[Pen-Phe-Trp-Lys-Tyr-Cys]-Val-OH) and the newest UPG84(H-Asp-c[Pen-Phe-DTrp-Orn-(pNH2) Phe-Cys]-Val-OH), following intracavernous injection in anesthetized rats. Both compounds have been designed and developed in an attempt to produce a high affinity-agonist capable of providing a superior activity in vivo versus U-II.
MATERIALS AND METHODS
Peptides
The human U-II and the analogues P5U and UPG84 were obtained by solid-phase peptide synthesis as previously reported.22 Purification was achieved using a semi-preparative reversed-phase high-performance liquid chromatography (HPLC) C18 bonded silica column (Vydac 218TP1010; The Separations Group Inc., Hesperia, CA, USA). The purified peptide was 99% pure as determined by analytical reversed-phase HPLC. The correct molecular weights were confirmed by mass spectrometry and amino acid analysis.
Binding experiments
All experiments were performed on membranes obtained from stable CHO-K1 cells expressing the recombinant human UTR (Euroscreen ES-440-M, Bruxelles, Belgium). Assay conditions were: tris-buffer (20 mmol l−1, pH 7.4 at 37°C) added with MgCl2 (5 mmol l−1) and 0.5% bovine serum albumin (BSA). Final assay volume was 0.1 ml, containing 1 μg membrane proteins. The radioligand used for competition experiments was [125I] U-II (specific activity 2000 Ci mmol−1; Amersham Biosciences, Buckinghamshire, UK) in the range 0.07–1.4 nmol l−1 (corresponding to 1/10–1/5 of its KD). Nonspecific binding was determined in the presence of 1 μmol l−1 of unlabelled U-II, and ranged between 10% and 20% of total binding. Competing ligands were tested in a wide range of concentrations (1 pmol l−1 to 10 μmol l−1). The incubation period (120 min at 37°C) was terminated by rapid filtration through UniFilter-96 plates (Packard Instrument Company, Meriden, CT, USA), presoaked for at least 2 h in BSA 0.5%, and using a MicroMate 96 Cell Harvester (Packard Instrument Company). The filters were then washed 4 times with 0.2 ml aliquots of Tris-HCl buffer (20 mmol l−1, pH 7.4, 4°C). Filters were dried and soaked in Microscint 40 (50 μl in each well, Packard Instrument Company) and bound. Radioactivity was counted by a TopCount Microplate Scintillation Counter (Packard Instrument Company). Determinations were performed in duplicate. All binding data were fitted by using GraphPad Prism 4.0 (San Diego, CA, USA) in order to determine the equilibrium dissociation constant (Kd) from homologous competition experiments and the ligand concentration inhibiting the radioligand binding of the 50% (IC50) from heterologous competition experiments. Ki values were calculated from IC50 using the Cheng-Prusoff equation (Ki = IC50/(1+ [radioligand]/Kd) according to the concentration and Kd of the radioligand.23
Animals
This study was performed in accordance with the guidelines of Italian law (D.L. 116/1992) which complies with European Union guidelines (CEE Directive 86/609) for experimental animal care and use. Male Wistar rats (200–250 g) were used in the experiments (Harlan Laboratories, Udine, Italy). The experimental procedures were approved by the Institutional Animal Ethics Committee. Animals were kept under laboratory conditions (temperature 23 ± 2°C, humidity range 40%–70%, 12-h light/dark cycle). Food and water were fed ad libitum.
Organ bath experiments
Rats were euthanized under anesthesia. The thoracic aorta was cleared of surrounding tissue and excised from the aortic arch to the diaphragm. From each vessel, a helically cut strip was prepared, and was then cut into two parallel strips. The endothelium was removed by gently rubbing the vessel intimal surface with a cotton-tip applicator before cutting the strips; the effectiveness of this maneuver was assessed by the loss of the relaxation response to acetylcholine (Ach, Sigma, Milan, Italy, 1 μmol l−1) in noradrenaline (Sigma, Milan, Italy, 1 μmol l−1) precontracted preparations. All preparations were placed in 5 ml organ baths filled with normal Krebs solution of the following composition (mmol l−1): NaCl 119; NaHCO3 25; KH2PO4 1.2; MgSO4 1.5; CaCl2 2.5; KCl 4.7 and glucose 11, warmed at 37°C and oxygenated with 95% O2, 5% CO2. The tissues were connected to isotonic force transducers (UgoBasile, VA, Italy) under a constant load of 5 mN and motor activity was digitally recorded by an Octal Bridge Amplifier connected to PowerLab/8SP hardware system and analyzed using the Chart 4.2 software (ADInstruments Ltd, Oxford, UK).20,24 After 60 min equilibration, tissue responsiveness was assessed by the addition of noradrenaline (1 μmol l−1) followed by a further equilibration of 60 min. In order to assess the agonist activity cumulative concentration-response curves to U-II and to the peptide, agonists P5U and UPG84 were constructed so as to determine the molar concentration of the agonist producing the 50% (EC50) of its maximal effect. Concentration-response curves were analyzed by sigmoidal nonlinear regression fit, using the GraphPad Prism 4.0 program. Agonist activity of all compounds was expressed as pD2 (−log EC50).
Monitoring of intracavernous pressure in anesthetized rats
Rats were anesthetized with an intraperitoneal injection of urethane (1 g kg−1). For continuous systemic blood pressure measurements, a heparinized (5 IU ml−1) polyethylene catheter was introduced into the carotid artery connected to a pressure transducer (BLPR-2, 2Biological Instruments, Milan, Italy) as previously reported.25 Briefly, a midline perineal incision, followed by blunt dissection of the overlying striated muscles was performed and the proximal part of the right corpus cavernous was exposed after dissection of the ischiocavernous muscle. A 26-gauge needle (Delta Ven, Italy) flushed with heparinized (10 IU ml−1) solution was placed through the right crus of the penis into the cavernosal space. The ICP was monitored with a pressure transducer (2Biological Instruments, Milano, Italy). These parameters were continuously recorded and data acquisition and calculation were performed using a computer system (Biopac, 2Biological Instruments, Milan, Italy).26 For pharmacological evaluation via the intracavernous route as described above, a 26-gauge needle (Delta Ven, Italy) was placed at the other crus for drug injection. P5U or UPG84 were intracavernously administrated at doses of 0.03, 0.1, 0.3 and 1 nmol dissolved in 50 μl saline. In order to compare the P5U as well as the UPG84 effect with a referred compound, U-II (0.03, 0.1, 0.3 and 1 nmol) was administered as previously demonstrated.5 Saline served as the control vehicle (50 μl). After each administration we waited for the signal had returned to the baseline for at least 5 min before the next dose was given. As a positive control, Ach was used at a dose of 5 nmol administrated intracavernously at the end of each experiment. Data were calculated as delta (mmHg) of ICP as well as area under the curve (mmHg × s). Results were expressed as mean ± standard error of mean (s.e.m.) from 5 to 7 separate experiments. The changes in systemic blood pressure following intracavernous injection were calculated as the difference from basal value (delta mmHg) and expressed as mean ± s.e.m. Data were analyzed using ANOVA followed by Bonferroni as post test. P < 0.05 were considered as significant.
RESULTS
Binding and activity studies of urotensin-II, P5U and UPG84
The binding study performed on membranes obtained from stable CHO-K1 cells expressing the recombinant human UTR clearly showed that P5U has the highest affinity when compared to endogenous ligand, U-II or UPG84 (Table 1). Although UPG84 possesses a lower value of pKi, it showed the highest activity compared with the endogenous ligand, U-II, or P5U (Table 1). Indeed, the molar concentration of the agonists producing the 50% (EC50) of its maximal effect, pD2, was 8.31, 9.344 and 10.040 for U-II, P5U and UPG84 respectively (Table 1).
Table 1.
Binding affinity and activity of U-II, P5U and UPG84 peptides (H-Asp-c[Pen-Phe-Xaa-Yaa-R-Cys]-Val-OH)
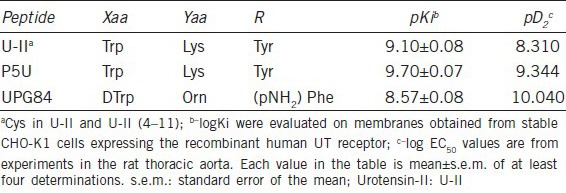
The effects of the intracavernous injection of urotensin-II on penile erection in rats
A typical trace showing the increase in ICP following the U-II intracavernous injections (0.01–1 nmol) is reported in Figure 1a. Intracavernous injection of U-II (0.03–1 nmol), as already reported,5 increases ICP as area under the curve (Figure 1b) and as delta of ICP increase (Figure 1c). The U-II effect is already detectable at the dose of 0.1 nmol reaching an area of 8.75 ± 2.6, 65.78 ± 3.7 and 268.3 ± 13.5 mmHg × s (n = 5) for the doses of 0.1, 0.3 and 1 nmol per rat, respectively.
Figure 1.
(a) A typical trace showing the increase in intracavernous pressure (ICP) followed by urotensin-II (U-II) intracavernous injection (0.1–1 nmol). The arrows indicate the corresponding time to each intracavernous injection (b). Intracavernous injection of U-II (0.03–1 nmol) causes an increase in ICP expressed as area under the curve(mmHg × s). ***P < 0.001 versus vehicle, °°°P < 0.001 versus U-II 0.03 nmol, §§§P < 0.001 versus U-II 0.1 nmol, ###P < 0.001 versus U-II 0.3 nmol or (c) Delta of increase (mmHg) ***P < 0.001 and **P < 0.01 versus vehicle, °°°P < 0.001 versus U-II 0.03 nmol; §§§P < 0.001 versus U-II 0.1 nmol. Data are calculated as mean ± standard error of mean of n = 5 experiments and analyzed by ANOVA followed by Bonferroni as posttest.
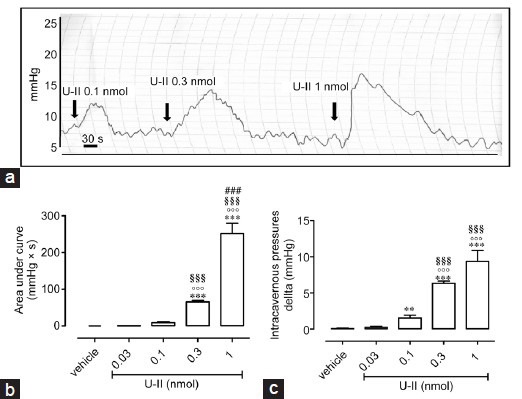
Effects of intracavernous injection of P5U on penile erection in rats
The intracavernous injection of P5U induces a significant increase in ICP (Figure 2). In particular, P5U significantly raises ICP at doses of 0.1, 0.3 and 1 nmol (Figure 2a). P5U-induced effect, at a dose of 0.1 nmol of (73.95 ± 7.312 mmHg × s, n = 7) is not statistically different from the effect elicited by U-II at a dose of 0.3 nmol per rat (65.78 ± 3.7 mmHg × s, n = 5). In addition, the increase in ICP calculated as delta displays the same profile of the area under the curve (Figure 2b).
Figure 2.
(a) Intracavernous injection of P5U (0.03–1 nmol) causes an increase in intracavernous pressure expressed as area under the curve (mmHg × s). ***P < 0.001 and **P < 0.01 versus vehicle, °P < 0.05 and °°°P < 0.001 versus P5U 0.03 nmol, §§§P < 0.001 versus P5U 0.1 nmol, ###P < 0.001 versus P5U 0.3 nmol or (b) Delta of increase (mmHg) ***P < 0.001 and **P < 0.01 versus vehicle, °P < 0.05 and °°°P < 0.001 versus P5U 0.03 nmol, §§§P < 0.001 versus P5U 0.1 nmol. Data are calculated as mean ± standard error of mean of n = 7 experiments and analyzed by ANOVA followed by Bonferroni as posttest.
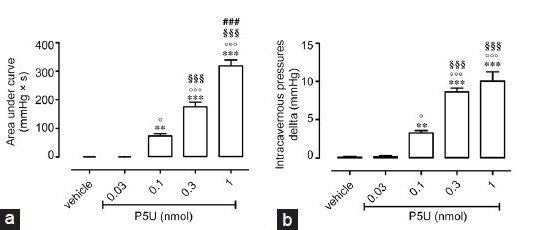
Effects of intracavernous injection of UPG84 on penile erection in rats
UPG84 (0.03–1 nmol) increases ICP in a dose-dependent manner either as area or delta (Figure 3a and 3b). At the lowest tested dose used, UPG84 already elicited a marked increase in ICP, expressed as area, compared with vehicle (Figure 3a). The intracavernously injection of UPG84 at the lowest dose (0.03 nmol) showed a comparable profile to that elicited by the highest dose of U-II (1 nmol). Figure 3b reports the UPG84 effect expressed as delta of increase in ICP showing a similar profile of the area under the curve. As a positive control we used Ach (5 nmol). Intracavernous injection of Ach causes an increase in ICP expressed as area under the curve of 265 ± 24 mmHg × s (n = 19).
Figure 3.
(a) Intracavernous injection of UPG84 (0.03–1 nmol) causes an increase in intracavernous pressure expressed as area under the curve (mmHg × s). *P < 0.05 and **P < 0.01 versus vehicle, °P < 0.05 versus UPG84 0.03 nmol, §§§P < 0.001 versus UPG84 0.1 nmol or (b) Delta of increase *P < 0.05 and **P < 0.01 versus vehicle, °P < 0.05 and °°P < 0.01 versus UPG84 0.03 nmol. Data are calculated as mean ± standard error of mean of n = 7 experiments and analyzed by ANOVA followed by Bonferroni as posttest.
Effects of intracavernous injection of urotensin-II, P5U and UPG84 on systemic blood pressure
The intracavernous injection of U-II as previously demonstrated5 does not significantly modify the systemic blood pressure (Figure 4a). Similarly, the intracavernous injection of P5U or UG84 did not cause significant changes in blood pressure (Figure 4b and 4c).
Figure 4.
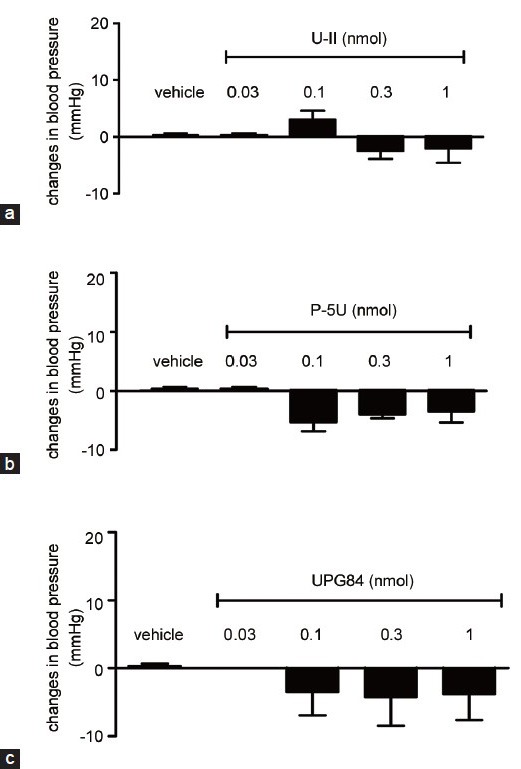
(a) Intracavernous injection of urotensin-II (0.03–1 nmol), (b) P5U (0.03–1 nmol) or (c) UPG84 (0.03–1 nmol) did not modify systemic blood pressure. Data are calculated as mean ± standard error of mean of n = 5–7 and analyzed by ANOVA followed by Bonferroni as posttest.
DISCUSSION
Urotensin-II was originally isolated from the goby urophysis in the 1960s as a vasoactive peptide with a prominent role in cardiovascular homeostasis.27 During the last decade, the urotensinergic system has drawn the attention of the scientific community due to its marked involvement in various physiological and pathological states, including cardiovascular diseases. The identification and characterization of U-II and its specific receptor UTR by Ames et al.3 in 1999 led to investigating the putative role of the U-II/UTR pathway in multiple pathophysiological effects in humans. Since, U-II is widely expressed in several peripheral tissues, including human prostate and corpus cavernosum, the design and development of novel U-II analogs can improve knowledge structure-activity relationships leading to the development of lead compounds that can be used as therapeutics. To date, human U-II (H-Glu-Thr-Pro-Asp-c[Cys-Phe-Trp-Lys-Tyr-Cys]-Val-OH) has been described as the most potent vasoconstrictor compound identified, but it acts also as a vasodilator in some vascular districts.28
We have previously established that U-II relaxes human corpus cavernosum strips in endothelium-and NO-dependent manner.5 Simultaneously, by using an in vivo rat model, it has been shown that intracavernous administration of U-II elicits a dose-dependent increase in ICP.5 This pro-erectile effect of U-II involves the generation of eNOS-derived NO.
We can therefore conclude that U-II contributes to the induction and maintenance of full penile erection19 by way of triggering the eNOS pathway. It is widely accepted that neurogenic and endothelial NO is a key factor in initiating erection.29 Besides this role, the increased eNOS activity, through its phosphorilation, has a key role in the maintenance of tumescence.30 During the course of our study, we have evaluated the intracavernous effect of a recently identified super-agonist of U-II termed P5U (H-Asp-c[Pen-Phe-Trp-Lys-Tyr-Cys]-Val-OH)20 and of a newly identified UTR ligand, named UPG84(H-Asp-c[Pen-Phe-DTrp-Orn-(pNH2) Phe-Cys]-Val-OH).
Our in vitro data demonstrated that P5U has a higher affinity (pKi) for UTR and a higher activity (pD2) when compared to the endogenous ligand U-II.20,22 Interestingly, experiments performed on membranes of CHO-K1 cells expressing the recombinant human UTR showed that UPG84 has a good binding strength for UTR. In addition, UPG84 proved to be more potent than U-II or P5U as demonstrated by in vitro experiments. This latter result is consistent with our data in vivo. Indeed, UPG84, characterized by a (pNH2) Phe residue at position eight of U-II sequence, caused a significant increase in ICP compared to the endogenous ligand, U-II, and to P5U. In fact, the lowest dose (0.03 nmol) of UPG84 shows an effect (242.5 ± 33.8 mmHg × s) comparable to the highest tested dose of U-II (1 nmol). Moreover, UPG84 was found to be even more effective than P5U. While the lowest dose (0.03 nmol) of P5U did not affect the ICP, UPG84, at the same dose, induced a prominent penile erection. This effect was comparable to the penile erection induced by P5U at the dose of 1 nmol. It worth noting, however, that all the compounds tested had no influence on systemic blood pressure implying that the effect is restricted to the corpus cavernosum. The analysis of the response elicited by the intracavernous injection reported as area under the curve, also gave some important hints of the efficacy within the time frame of the experiments. Indeed, the difference among the molecules tested is markedly evident and can be taken as an index of erection maintenance. In other words, the new ligands tested have a more pronounced effect over time when compared to the endogenous agonist U-II.
Until date, treatment of erectile dysfunction by phosphodiesterase-5 inhibitors (PDE5i) is widely accepted. Therefore, in this era of PDE5i therapy, the development of UTR ligands may represent a new therapeutical approach for the management of erectile dysfunction and requires further investigation in this direction.
AUTHOR CONTRIBUTIONS
RDVB participated in the design and coordination of this study and helped to draft the manuscript. EM carried out the in vivo studies and contributed to the draft of the manuscript. ED collaborated with the in vivo studies, FF, NL, VM participated in the draft of the manuscript, GD, PG and EN carried out the peptides synthesis, the binding affinity and in vitro activity assay. GC analyzed data and helped to draft the manuscript. RS conceived of the study and participated in the draft of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Vaudry H, Do Rego JC, Le Mevel JC, Chatenet D, Tostivint H, et al. Urotensin II, from fish to human. Ann N Y Acad Sci. 2010;1200:53–66. doi: 10.1111/j.1749-6632.2010.05514.x. [DOI] [PubMed] [Google Scholar]
- 2.Conlon JM, Yano K, Waugh D, Hazon N. Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J Exp Zool. 1996;275:226–38. [PubMed] [Google Scholar]
- 3.Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–6. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 4.Grieco P, Franco R, Bozzuto G, Toccacieli L, Sgambato A, et al. Urotensin II receptor predicts the clinical outcome of prostate cancer patients and is involved in the regulation of motility of prostate adenocarcinoma cells. J Cell Biochem. 2011;112:341–53. doi: 10.1002/jcb.22933. [DOI] [PubMed] [Google Scholar]
- 5.d’Emmanuele di Villa Bianca R, Cirino G, Mitidieri E, Coletta C, Grassia G, et al. Urotensin II: a novel target in human corpus cavernosum. J Sex Med. 2010;7:1778–86. doi: 10.1111/j.1743-6109.2009.01450.x. [DOI] [PubMed] [Google Scholar]
- 6.Ong KL, Lam KS, Cheung BM. Urotensin II: its function in health and its role in disease. Cardiovasc Drugs Ther. 2005;19:65–75. doi: 10.1007/s10557-005-6899-x. [DOI] [PubMed] [Google Scholar]
- 7.Douglas SA, Ohlstein EH. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc Med. 2000;10:229–37. doi: 10.1016/s1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- 8.Camarda V, Rizzi A, Calò G, Gendron G, Perron SI, et al. Effects of human urotensin II in isolated vessels of various species; comparison with other vasoactive agents. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:141–9. doi: 10.1007/s00210-001-0503-0. [DOI] [PubMed] [Google Scholar]
- 9.Maguire JJ, Kuc RE, Davenport AP. Orphan-receptor ligand human urotensin II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br J Pharmacol. 2000;131:441–6. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimoto T, Matsushita M, Hirata Y. Role of urotensin II in peripheral tissue as an autocrine/paracrine growth factor. Peptides. 2004;25:1775–81. doi: 10.1016/j.peptides.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Bottrill FE, Douglas SA, Hiley CR, White R. Human urotensin-II is an endothelium-dependent vasodilator in rat small arteries. Br J Pharmacol. 2000;130:1865–70. doi: 10.1038/sj.bjp.0703513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray GA, Jones MR, Sharif I. Human urotensin II increases coronary perfusion pressure in the isolated rat heart: potentiation by nitric oxide synthase and cyclooxygenase inhibition. Life Sci. 2001;69:175–80. doi: 10.1016/s0024-3205(01)01101-8. [DOI] [PubMed] [Google Scholar]
- 13.Gardiner SM, March JE, Kemp PA, Maguire JJ, Kuc RE, et al. Regional heterogeneity in the haemodynamic responses to urotensin II infusion in relation to UT receptor localisation. Br J Pharmacol. 2006;147:612–21. doi: 10.1038/sj.bjp.0706503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishihata A, Ogaki T, Aita T, Katano Y. Role of prostaglandins in urotensin II-induced vasodilatation in the coronary arteries of aged rats. Eur J Pharmacol. 2005;523:119–26. doi: 10.1016/j.ejphar.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Katano Y, Ishihata A, Aita T, Ogaki T, Horie T. Vasodilator effect of urotensin II, one of the most potent vasoconstricting factors, on rat coronary arteries. Eur J Pharmacol. 2000;402:R5–7. doi: 10.1016/s0014-2999(00)00506-9. [DOI] [PubMed] [Google Scholar]
- 16.Lacza Z, W Busija D. Urotensin-II is a nitric oxide-dependent vasodilator in the pial arteries of the newborn pig. Life Sci. 2006;78:2763–6. doi: 10.1016/j.lfs.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Zhang AY, Chen YF, Zhang DX, Yi FX, Qi J, et al. Urotensin II is a nitric oxide-dependent vasodilator and natriuretic peptide in the rat kidney. Am J Physiol Renal Physiol. 2003;285:F792–8. doi: 10.1152/ajprenal.00342.2002. [DOI] [PubMed] [Google Scholar]
- 18.Chen ZW, Yang Q, Huang Y, Fan L, Li XW, et al. Human urotensin II in internal mammary and radial arteries of patients undergoing coronary surgery. Vascul Pharmacol. 2010;52:70–6. doi: 10.1016/j.vph.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 19.d’Emmanuele di Villa Bianca R, Mitidieri E, Fusco F, D’Aiuto E, Grieco P, et al. Endogenous urotensin II selectively modulates erectile function through eNOS. PLoS One. 2012;7:e31019. doi: 10.1371/journal.pone.0031019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Grieco P, Carotenuto A, Campiglia P, Gomez-Monterrey I, Auriemma L, et al. New insight into the binding mode of peptide ligands at Urotensin-II receptor: structure-activity relationships study on P5U and urantide. J Med Chem. 2009;52:3927–40. doi: 10.1021/jm900148c. [DOI] [PubMed] [Google Scholar]
- 21.Merlino F, Di Maro S, Munaim Yousif A, Caraglia M, Grieco P. Urotensin-II ligands: an overview from peptide to nonpeptide structures. J Amino Acids. 2013;2013:979016. doi: 10.1155/2013/979016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grieco P, Carotenuto A, Campiglia P, Zampelli E, Patacchini R, et al. A new, potent urotensin II receptor peptide agonist containing a Pen residue at the disulfide bridge. J Med Chem. 2002;45:4391–4. doi: 10.1021/jm025549i. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 24.Caliendo G, Perissutti E, Santagada V, Fiorino F, Severino B, et al. Synthesis and vasorelaxant activity of new 1,4-benzoxazine derivatives potassium channel openers. Bioorg Med Chem. 2002;10:2663–9. doi: 10.1016/s0968-0896(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 25.d’Emmanuele di Villa Bianca R, Lippolis L, Autore G, Popolo A, Marzocco S, et al. Dexamethasone improves vascular hyporeactivity induced by LPS in vivo by modulating ATP-sensitive potassium channels activity. Br J Pharmacol. 2003;140:91–6. doi: 10.1038/sj.bjp.0705406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.d’Emmanuele di Villa Bianca R, Sorrentino R, Maffia P, Mirone V, Imbimbo C, et al. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc Natl Acad Sci U S A. 2009;106:4513–8. doi: 10.1073/pnas.0807974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell FD. Emerging roles of urotensin-II in cardiovascular disease. Pharmacol Ther. 2004;103:223–43. doi: 10.1016/j.pharmthera.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Stirrat A, Gallagher M, Douglas SA, Ohlstein EH, Berry C, et al. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280:H925–8. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]
- 29.Cirino G, Fusco F, Imbimbo C, Mirone V. Pharmacology of erectile dysfunction in man. Pharmacol Ther. 2006;111:400–23. doi: 10.1016/j.pharmthera.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Hurt KJ, Sezen SF, Lagoda GF, Musicki B, Rameau GA, et al. Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2012;109:16624–9. doi: 10.1073/pnas.1213790109. [DOI] [PMC free article] [PubMed] [Google Scholar]


