Abstract
Mammalian spermatogenesis is a well-organized process of cell development and differentiation. Meiosis expressed gene 1 (MEIG1) plays an essential role in the regulation of spermiogenesis. To explore potential mechanisms of MEIG1's action, a yeast two-hybrid screen was conducted, and several potential binding partners were identified; one of them was membrane occupation and recognition nexus repeat containing 3 (MORN3). MORN3 mRNA is only abundant in mouse testis. In the testis, Morn3 mRNA is highly expressed in the spermiogenesis stage. Specific anti-MORN3 polyclonal antibody was generated against N-terminus of the full-length MORN3 protein, and MORN3 expression and localization was examined in vitro and in vivo. In transfected Chinese hamster ovary cells, the antibody specifically crossed-reacted the full-length MORN3 protein, and immunofluorescence staining revealed that MORN3 was localized throughout the cytoplasm. Among multiple mouse tissues, about 25 kDa protein, was identified only in the testis. The protein was highly expressed after day 20 of birth. Immunofluorescence staining on mixed testicular cells isolated from adult wild-type mice demonstrated that MORN3 was expressed in the acrosome in germ cells throughout spermiogenesis. The protein was also present in the manchette of elongating spermatids. The total MORN3 expression and acrosome localization were not changed in the Meig 1-deficient mice. However, its expression in manchette was dramatically reduced in the mutant mice. Our studies suggest that MORN3 is another regulator for spermatogenesis, probably together with MEIG1.
Keywords: acrosome, manchette, membrane occupation and recognition nexus repeat containing 3, spermiogenesis
INTRODUCTION
Spermatogenesis is a complex process, including mitotic renewal of spermatogonia, meiosis of spermatocytes, and spermiogenesis.1,2 During the final stage, haploid round spermatids differentiate into spermatozoa with dramatic morphological changes, including nuclear condensation, acrosome and flagellum formation, and extrusion of residual cytoplasm to yield testicular spermatozoa.3,4,5 Although the list of proteins specifically expressed in spermiogenesis has grown in recent years, many remain uncharacterized, and their roles await further investigation.6
Mouse meiosis expressed gene 1 (MEIG1) was originally identified in the search for mammalian genes potentially involved in meiosis.7,8 A bioinformatic analysis revealed that MEIG1 is most abundantly expressed in tissues rich in ciliated cells, such as testis, lung, olfactory sensory neurons; and is, therefore, predicted to be important for cilia/flagella function.9 MEIG1-deficient mice have been generated in our laboratory, and our studies demonstrated that MEIG1 plays a critical role in maintaining manchette stability and functioning, and is essential for the mouse spermiogenesis.10 MEIG1 is a protein with only 88 amino acids, and there is no conserved domain predicting its function. To identify potential binding proteins are interacting with MEIG1 protein, a yeast two-hybrid screening was conducted, and several possible candidates were found such as PACRG10 and transcription factor-like 5.11 Membrane occupation and recognition nexus repeat-containing protein 3 (MORN3, GenBank accession number: NM_029112.1) was identified to be another potential binding partner in the same screen.
Membrane occupation and recognition nexus repeat-containing domain were first identified as a plasma membrane-binding module in junctophilin-1 (JP-1).12 In JP-1, a motif (MORN motif) containing 14 residues with consensus sequences “Tyr-Gln/Glu-Gly-Glu/Gln-Trp-x-Asn-Gly-Lys-x-His-Gly-Tyr-Gly repeated 8 times in the amino-terminal region. BLAST searches using the domain with MORN motif found additional hypothetical proteins carrying MORN motif sequences in Caenorhabditis elegans (JP homologue), Arabidopsis thaliana (PIP 5-kinase), and Cyanobacterium (hypothetical protein); thus, MORN motif is a novel protein-folding module shared by functionally different proteins, and might have a specific physiological role.12,13,14
Besides junctophilin, MORN domain has been reported to be present in several other proteins involved in membrane fission.15,16 In Arabidopsis, the only protein reported containing MORN motifs is ARC3.15 MORN1 is conserved across the Apicomplexa. Toxoplasma gondii has two MORN-containing proteins (MORN1 and MORN2) that contain only multiple MORN motifs.16 MORN1 consists of 14 MORN motifs of 23-amino acids; it is present near the flagellar basal bodies and mitochondrion. It is associated with several structures that have a role in cell division.17 These observations suggest a conserved role for MORN1 in both asexual and sexual development across the Apicomplexa. MORN1 is also required for basal complex assembly and loss of MORN1 resulting in defects in the apicoplast division and daughter segregation.18 Recent proteomics studies demonstrated that it is associated with proteins in the cytoskeleton.19
Mouse MORN4 is the ortholog of Drosophila melanogaster retinophilin (rtp). Retinophilin encodes a MORN domain-containing protein, which protects axons from degeneration in the presence of taxol.20 Similarly, MORN4 promotes axonal degeneration in mouse sensory axons following axotomy, illustrating the conservation of function.21 MORN5 was identified in a work analyzing the human chromosome 9.22
Several MORN-motif proteins have been reported to be expressed in male germ cells, including mouse meichroacidin (MCA),23 its human ortholog, radial spoke protein 44 (previously testis specific gene A2, or TSGA2).24 Here we characterized the mouse Morn3 gene. We discovered that Morn3 mRNA is abundant in mouse testis, and is highly expressed in the spermiogenesis stage, the translated protein is localized in the acrosome in germ cells throughout spermiogenesis; it is also present in the manchette of elongating spermatids. The total MORN3 expression and acrosome localization were not changed in the Meig1-deficient mice. However, its expression in manchette was dramatically reduced in the mutant mice. Our studies suggest that MORN3 is a novel regulator for spermatogenesis, probably together with MEIG1.
Given that male factor infertility affects a large sector of the population, however, many of its etiologies are unknown. By elucidating the underlying genetic basis of infertile phenotypes, may be possible to discover the causes of infertility and determine effective treatments for patients. Our studies demonstrate that the dysfunction of MEIG1/MORN3 genes might be novel genetic factors for male infertility, and these genes might be targets for effective treatments for infertile males.
MATERIALS AND METHODS
Ethics statement
All rodent work was approved by Virginia Commonwealth University's Institutional Animal Care and Use Committee (protocol permit #AM10297 and AD10000167) in concordance with all federal and local regulations regarding the use of nonprimate vertebrates in scientific research.
Identification of the membrane occupation and recognition nexus repeat containing 3 cDNA by yeast two-hybrid screen
The mouse Morn3 cDNA (NM_029112.1) was identified from a yeast two-hybrid screen using full-length MEIG1 as bait; the clone appeared 3 times in the screen.
Mammalian expression constructs
A complementary DNA covering the full-length mouse Morn3 cDNA was amplified by reverse transcription-polymerase chain reaction (RT-PCR), in which the specific primers were designed for PCR amplification that is 5’-gaattcagaggcagccagcatgccggtc-3’ (forward) and 5’-ggatccgtctgacctcagccctcctcttc-3’ (reverse). After sequencing, the PCR products were cloned into EcoRI/BamHI sites of the p3 × FLAG-CMV™-7.1 vector (Sigma, St. Louis, MO, USA), creating the construct expressing full-length MORN3/FLAG fusion protein.
To make the construct expressing MORN3/GFP protein, PCR was conducted using the following primer set: forward: 5’-gaattctatgccggtcactaagtgtccaag-3’ (EcoRI) and reverse: 5’-ggtacctcagccctcctcttcctcgggctt-3’ (BamHI). The correct PCR product was ligated into pEGFP-C1 vector (Clontech, Mountain View, CA, USA).
Cell culture and transient transfection
Chinese hamster ovary (CHO) and COS-1 cells were cultured with DMEM/F12 or DMEM (with 10% fetal bovine serum) at 37°C. The cells were transfected with indicated plasmids using Fugene6 transfection reagent (Promega, Madison, WI, USA). Forty-eight hours after transfection, the cells were processed either for co-immunoprecipitation/Western blot analysis or immunofluorescence staining.
Co-immunoprecipitation
Co-immunoprecipitation experiments using transfected cells were performed as described in our previous study.11 Briefly, COS-1 cells were co-transfected with a total of 6 μg of indicated plasmids: MORN3/FLAG and MEIG1/pTarget plasmids. Forty-eight hours after transfection, cells were washed twice with ice-cold phosphate-buffered saline (PBS) following with harvesting into immunoprecipitation buffer. After centrifugation at 11 600 g for 5 min, the supernatants were precleaned with protein A beads mixture (50% v/v, GE Healthcare, Uppsala, Sweden) at 4°C for 30 min. Immunoprecipitate were then incubated with 1 μl (1 μg μl−1) of anti-MEIG1 antibody, or preimmune rabbit serum as a control at 4°C for 2 h; protein A beads were added with a further incubation at 4°C overnight. The collected samples were used for Western blot with monoclonal anti-FLAG antibody (Sigma, St. Louis, MO, USA). Co-immunoprecipitation using testicular extracts of wild-type mice was conducted with the same procedure as described above except that 1 mg of testicular protein was incubated with an anti-MORN3 polyclonal antibody or preimmune rabbit serum, and Western blot was conducted using an anti-MEIG1 antibody.
Reverse transcription-polymerase chain reaction
Membrane occupation and recognition nexus repeat containing 3 transcript was analyzed by RT-PCR. Mouse total RNAs from the indicated tissues (testis, brain, liver, kidney, heart, skeletal muscle, spleen, and lung) were isolated using TRIzol reagent (Invitrogen, Grand Island, NY, USA), and RT was performed using the first-stand cDNA synthesis kit from Fermentas (Beijing, China). Briefly, the same amount of total RNA (1 μg) from each tissue was pretreated with DNase I (Promega, Madison, WI, USA) and reverse transcribed with Moloney murine leukemia virus in the presence of random primers. To extract total RNAs from purified germ cells, germ cells were separated by the StaPut method.25 To purify populations of pachytene spermatocytes, round spermatids and condensing spermatids, the mixed germ cells were separated at unit gravity in a 2%–4% bovine serum albumin gradient in Eagle's Minimum Essential Medium with Earle's Salts. Examination of the purity of isolated fractions under Nomarski optics revealed that the pachytene spermatocyte, round spermatid, and condensing spermatid fractions were 85%, 90%, and 60% respectively. One microliter of the RT product was used for PCR. PCR amplification with 35 or 40 cycles was conducted by using the PCR master Mix system (Thermo Scientific Fermentas, Beijing, China) to examine the expression of the MORN3 transcript using the following primers: 5’-GGGGATTGGAAGTTTGGG-3’ (forward) and 5’-CCTCGTAATACT CCTTGGGT-3’ (reverse). Gapdh was served as a loading control.
Generation of the anti-membrane occupation and recognition nexus repeat containing 3 specific antibody
The coding sequence of the N-terminal portion from MORN3 was PCR-amplified from a Morn3 cDNA clone. The two primers used were: 5’-GCTAGCGAGGCAGCCAGC ATGCCGGTC-3’ (forward) and 5’-GAATTCGAAAAACTGGATCCCATAGCC-3’ (reverse), adding an NdeI restriction site at the 5’ ends and an EcoRI at the 3’ end, respectively. The PCR product was inserted into NdeI/EcoRI restriction sites of the pET28a expression vector (Novagen, Madison, WI, USA). Notably, His6 tags at both the N and C termini were contained in the resulting fusion protein. The construct was transformed into Escherichia coli strain BL-21 (DE3) cells, and the fusion protein was induced and subsequently purified as reported previously.26 As a control of expression, the plasmid pET28a was used without any insert. The purified recombinant protein was used to generate polyclonal antisera in rabbits by a commercial organization (Antibody Research Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China).
Western blot analysis
Equal amounts of protein (50 μg per lane) were heated to 95°C for 10 min in sample buffer, samples were resolved on a 10% sodium dodecyl sulfate-polyacrylamide gels and electrotransferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were then blocked in a Tris-buffered saline (TBST) solution containing 5% nonfat dry milk powder and 0.05% Tween 20 (TBST), and incubated with either rabbit anti-MORN3 polyclonal antibody or anti-His tag antibody at 4°C for 16 h. After washing 3 times with TBST, the blots were immersed in horseradish peroxidase-labeled goat anti-rabbit IgG with dilution of 1:10 000 at room temperature for 1 h. Following by washing with TBST, the bound antibodies were detected with SuperSignal Chemiluminescent Substrate (Pierce, Rockford, IL, USA). To examine the specificity of the antibody, the antibody was mixed with the purified protein that was used to generate the antibody, and Western blot was performed with the blocked antibody.
Enzyme-dissociated testicular cell preparations and immunofluorescence
Enzyme-dissociated testicular cells were prepared using a previously described method.27 Briefly, a pair of testes from an adult, wild-type mouse were decapsulated and pretreated with 5 ml DMEM (containing 0.5 mg ml−1 collagenase IV and 1.0 mg ml−1 DNase I) for 30 min at 32°C. After centrifuging for 5 min at 200 g, the dispersed mixture of testicular cells were fixed with 4% paraformaldehyde/PBS solution (containing 4% sucrose) for 15 min; then washed 3 times with PBS. After re-suspending in 12.5 ml PBS, 100 μL of mixed germ cells were loaded on SuperFrost/Plus microscope slides, (Fisher Scientific, Pittsburgh, PA, USA) allowing to natural withering. The cells were permeabilized with 1% Triton X-100 for 5 min at 37°C; blocked 1 h at room temperature with 10% goat serum in PBS. After the cells were incubated overnight with the primary antibodies against MORN3 or α-tubulin at a final dilution as 1:100 at 4°C in a moist chamber, cells were washed 3 times 0.05% Tween 20 in PBS and incubated 1 h with the same secondary antibodies as used for the tissue sections. Some cells were double-stained with an acrosome marker, peanut-lectin (Invitrogen, Grand Island, NY, USA. Cat number: L21409).28 Briefly, after the cells were stained with secondary antibody, the cells were washed 3 times in PBS, and incubated with peanut-lectin (20 μg ml−1 final concentration) at room temperature for 15 min, washed again 3 times in PBS, mounted with VectaMount (Vector laboratories, Burlingame, CA, USA), and sealed with a cover slip. Images were captured by confocal laser-scanning microscopy as before.
RESULTS
Membrane occupation and recognition nexus repeat containing 3 associates with meiosis expressed gene 1
Meiosis expressed gene 1 was used as bait for a yeast two-hybrid screening, MORN3 was identified to be a potential binding partner. After generation of expression plasmids for MORN3 (MORN3/FLAG) and MEIG1 (MEIG1/pTarget), a potential interaction of both proteins was investigated in co-transfected COS-1 cells. The MORN3/FLAG was transfected into COS-1 cells, and the protein was expressed (Figure 1a). In order to test the interaction of MORN3 and MEIG1 in the transfected COS-1 cells, MORN3/FLAG and MEIG1/pTarget plasmids were co-transfected, and Co-IP was performed, an anti-MEIG1 antibody was used for a pull-down experiment, MORN3/FLAG protein was co-precipitated from the lysate (Figure 1b). To further investigate if the two proteins interacts in vivo, testicular extracts from adult wild-type mice was pulled down using an anti-MORN3 polyclonal antibody generated, and Western blot was conducted with the anti-MEIG1 polyclonal antibody, MEIG1 protein was co-precipitated by the MORN3 antibody (Supplementary Figure 1 (915.1KB, tif) ).
Figure 1.
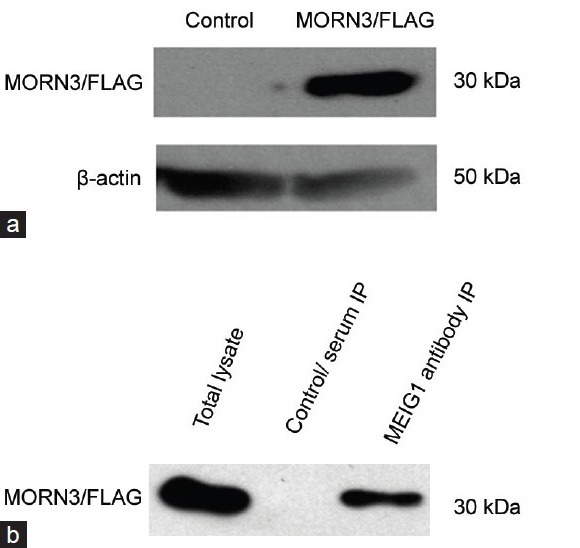
Membrane occupation and recognition nexus repeat containing 3 (MORN3) associates with meiosis expressed gene 1 (MEIG1) in vitro, (a) Western blot analysis of MORN3/FLAG fusion protein expressed in COS-1 cells transfected with Flag-tagged mouse MORN3 expression plasmid using an anti-FLAG antibody (upper panel). The membrane was re-probed with an anti-β-actin antibody as a loading control (lower panel). (b) Co-immunoprecipitation using cell lysates from COS-1cells co-transfected by MEIG1/pTarget and MORN3/FLAG plasmids.
MORN3 associates with MEIG1 in vivo
Membrane occupation and recognition nexus repeat containing 3 mRNA is highly expressed in the mouse testis
The tissue-specific expression pattern of was analyzed by RT-PCR analyses of total RNA derived from different mouse tissues. Mouse Morn3 mRNA was abundantly expressed in the testis, and to much weaker extent expressed in the brain and in the oviduct (Figures 2 and 3).
Figure 2.
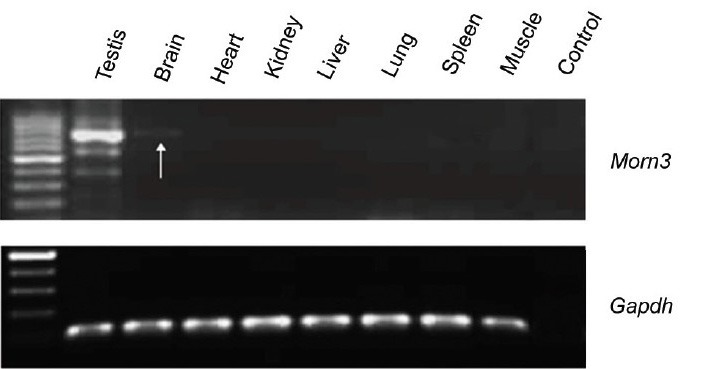
Mouse membrane occupation and recognition nexus repeat containing 3 (Morn3) mRNA is abundant in the testis. Reverse transcription-polymerase chain reaction (RT-PCR) was conducted using cDNAs from indicated mouse tissues; Morn3 cDNA was amplified from testicular cDNA using 40 cycles. Beside in the testis, Morn3 cDNA was also detected in the brain (arrow) (upper panel). Gapdh cDNA was examined as a control (lower panel). Notice that in the testis, beside the major band at the correct size, several other PCR products were also co-amplified, they may represent nonspecific PCR products, or other Morn3 isoforms.
Figure 3.
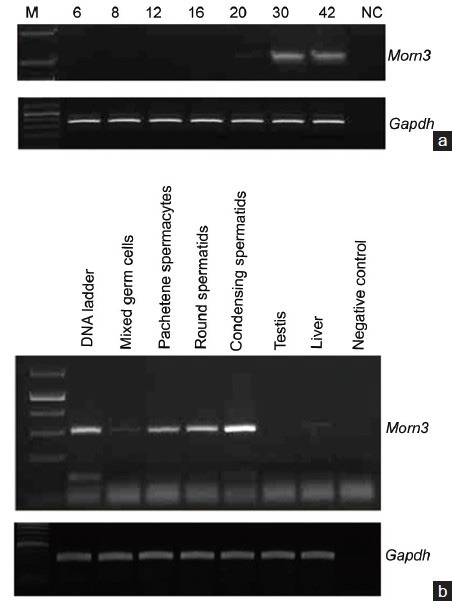
Mouse membrane occupation and recognition nexus repeat containing 3 (Morn3) mRNA is expressed in the late stage of spermatogenesis, (a) Reverse transcription-polymerase chain reaction (RT-PCR) was conducted for 35 cycles using cDNAs prepared from testes of wild-type mice at indicated ages. Notice that Morn3 mRNA is highly expressed at day 30 and 42 after birth. (b) RT-PCR for 35 cycles was conducted using cDNAs prepared from mixed germ cells and indicated purified male germ cells. Notice that Morn3 mRNA is highly expressed in round and condensing spermatids. The weak signal in pachytene spermatocytes might be from other cell populations co-purified with pachytene spermatocytes during germ cell separation.
Membrane occupation and recognition nexus repeat containing 3 mRNA is abundant in the late stage of spermatogenesis
Expression of the mouse Morn3 gene was examined in the testis during the first wave of spermatogenesis by RT-PCR. The message was undetectable from day 6 to day 16 after birth. Morn3 mRNA was detectable at day 20 after birth; the expression strongly increased at day 30 and day 42 after birth (Figure 3a).
To further examine Morn3 mRNA abundance during spermatogenesis stages, mixed germ cells were isolated from adult mouse testes, and pachytene spermatocytes, round spermatids, and condensing spermatids were separated from the isolated mixed germ cells by StaPut method. Morn3 mRNA was abundant in the round and condensing spermatids, but not in the pachytene spermatocytes (Figure 3b).
Membrane occupation and recognition nexus repeat containing 3 is present in the cytoplasm of transfected mammalian cells
A polyclonal antibody was generated against N-terminus of the full-length MORN3 protein. To test the antibody, Western blot was conducted using lysates from COS-1 cells transfected with MORN3/Flag and MORN3/pEGFP-C1 plasmids. The antibody cross-reacted with the MORN3 fusion proteins (Supplementary Figure 2a (841.7KB, tif) ). Localization of MORN3/FLAG in the transfected mammalian cells was examined by immunofluorescence staining; the protein is present throughout the cytoplasm (Supplementary Figure 2b (841.7KB, tif) ).
Examination of anti-MORN3 antibody by Western blot analysis and immunofluorescence
Tissue distribution of membrane occupation and recognition nexus repeat containing 3 protein and its expression during the first wave of spermatogenesis
With the anti-MORN3 antibody generated, Western blotting was performed using tissue lysates prepared from multiple tissues, including brain, heart, lung, liver, spleen, muscle, kidney, oviduct and testis. In tissues, it was examined that the antibody cross-reacted with about 25 kDa protein only in the testis (Figure 4a).
Figure 4.
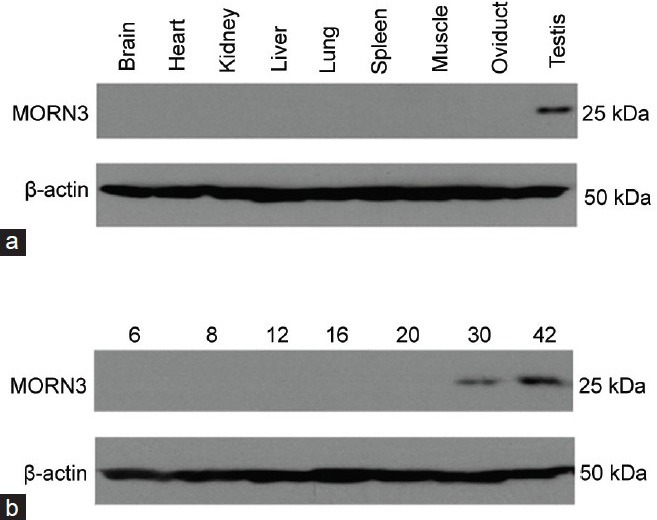
Analysis of membrane occupation and recognition nexus repeat containing 3 (MORN3) protein expression by Western blot in multiple mouse tissues and testes at first wave of spermatogenesis. MORN3 protein expression in the indicated mouse tissues from an adult wild-type mouse (a) and testes of wild-type mice at indicated ages (b) was examined by Western blot analysis. Notice that the antibody cross-reacted to a 25 kDa protein only in the testis, and that MORN3 is only present in the testes of the mice at day 30 and 42 after birth, but not in early stage. The 25 kDa signal was absent when the antibody was blocked with purified MORN3 protein, indicating that the 25 kDa protein was specific for MORN3 (data not shown).
To further examine expression of MORN3 protein in the testis during the first wave of spermatogenesis, Western blotting was conducted using testicular extracts from mice at day 6, 8, 12, 16, 20, 30 and 42 after birth. The 25 kDa protein was expressed at day 30 through today 42 after birth (Figure 4b).
Membrane occupation and recognition nexus repeat containing 3 is localized in the acrosome and manchette of spermatids, and the manchette localization is dependent on meiosis expressed gene 1 protein
Localization of endogenous MORN3 protein was examined by using mixed testicular cells prepared as described in the method. No specific signal was detected in the germ cells by the preimmune serum (Supplementary Figure 3 (1.1MB, tif) ). In primary spermatocytes of the pachytene stage, no specific signal was detected (Figure 5a). However, the specific signal was observed to be largely restricted on the apical region surrounding the nuclear membrane in round spermatids and elongating spermatids, and co-localized with lectin, an acrosome marker (Figure 5b–5d).
Figure 5.
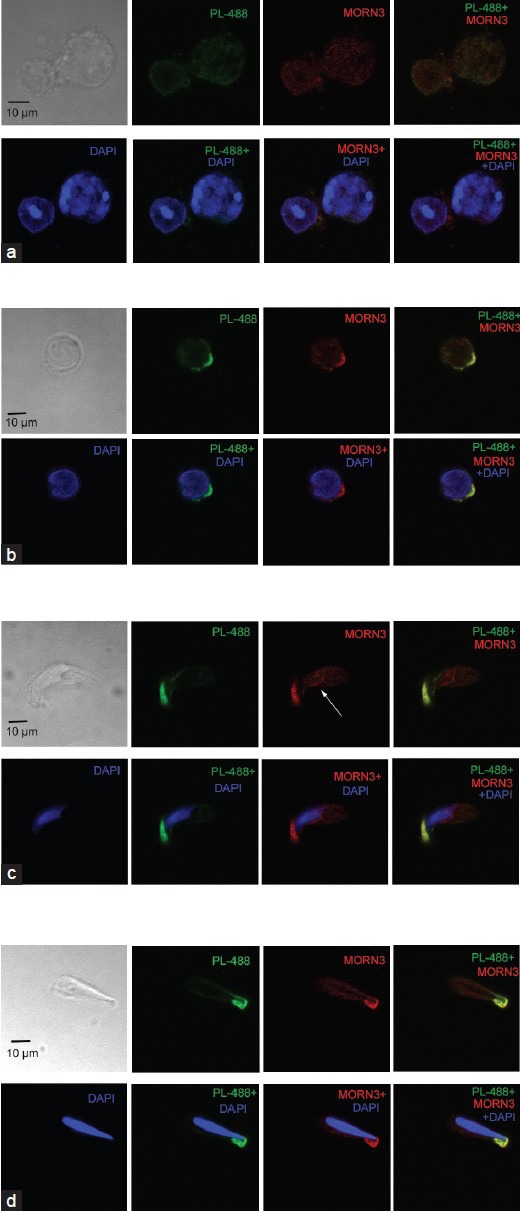
Co-localization of membrane occupation and recognition nexus repeat containing 3 (MORN3) with acrosome marker, lectin in round and elongating spermatids of wild-type mice. Isolated, mixed germ cells were double stained with anti-MORN3 antibody and an acrosome marker, lectin. MORN3 was not present in spermatocytes (a), but was co-localized with lectin in round spermatids and elongating spermatids (b-d). Notice that in the elongating spermatids, MORN was also present in the cauda region that closely associated with a nuclear membrane (arrows in c and d). PL-488: peanut-lectin-488 labeled. The arrowhead in A points to a round spermatid that appears to express MORN3 at low level. The red signals were absent when the anti-MORN3 antibody was blocked with purified MORN3 protein, indicating that these red signals were specific for MORN3 (data not shown).
Immunofluorescence on mouse mixed germ cells using preimmune serum
Besides in the acrosome, MORN3 signal was also detected in the cauda region that closely associates with a nuclear membrane of elongating spermatids (arrows in Figure 5c and 5d). Since this region represents a unique structure of elongating spermatids, the manchette, thus double staining was conducted with anti-MORN3 antibody and a monoclonal anti-α-tubulin antibody; which specifically decorates manchette. In the elongating spermatids, MORN3 protein that associates with a nuclear membrane in the cauda region co-localizes with α-tubulin (Figure 6a and 6b).
Figure 6.
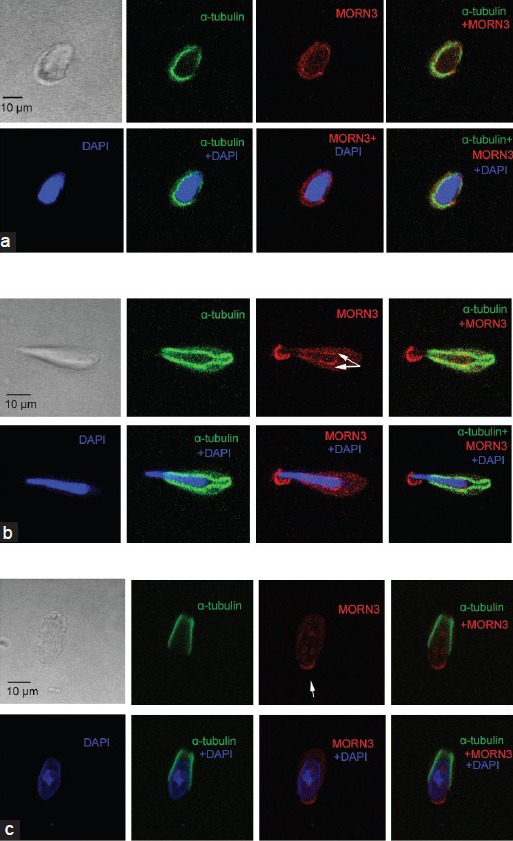
Membrane occupation and recognition nexus repeat containing 3 (MORN3) is localized on the manchette of the elongating spermatids and is dependent on meiosis expressed gene 1 (MEIG1). Isolated mixed germ cells were double stained with anti-MORN3 antibody and a manchette marker, α-tubulin. Notice that MORN3 that was in the cauda region of the nuclear membrane and co-localized with α-tubulin (arrows in a and b). Arrowheads point to the acrosome. (a) an elongating spermatid at earlier stage of a wild-type mouse; (b) an elongating spermatid at later stage of a wild-type mouse; (c) MORN3 localization in a remaining elongating spermatid of an MEIG1-deficient mouse. Notice that MORN3 is no longer present in the manchette. The red signals were absent when the anti-MORN3 antibody was blocked with purified MORN3 protein, indicating that these red signals were specific for MORN3 (data not shown).
Membrane occupation and recognition nexus repeat containing 3 associates with MEIG1 in vivo, its protein expression level and localization were examined in the MEIG1-deficient mice. Testicular MORN3 protein expression level was examined in adult wild-type and MEIG1 knockout mice by Western blot analysis. There was no obvious difference in MORN3 protein expression level between wild-type and MEIG1 mutant mice (Supplementary Figure 4 (953.5KB, tif) ). Even though, MORN3 was present in the elongating spermatids of wild-type mice, MORN3 signal appeared to be missing from the manchette of MEIG1-deficient mice (Figure 6c). However, localization of MORN3 in the acrosome remains unchanged in all steps of spermatids of MEIG1-deficient mice (Figure 7a–7d).
Figure 7.
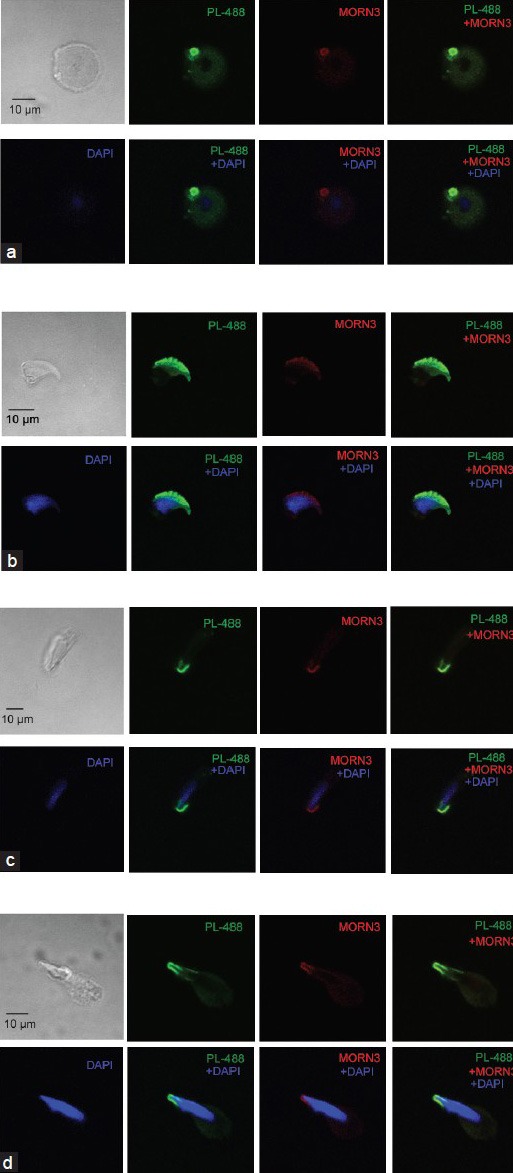
Membrane occupation and recognition nexus repeat containing 3 (MORN3) remained in the acrosome of spermatids in the meiosis expressed gene 1 (Meig1)-deficient mice. MORN3 localization was examined in the isolated mixed germ cells of MEIG1-deficient mice. MEIG1 remained in the acrosome of round (a) and elongating spermatids (b-d). PL-488: peanut-lectin-488 labeled.
MORN3 protein expression level was not changed in the testis of Meig1-deficient mice
DISCUSSION
We characterized Morn3 gene in mouse. Both Morn3 messenger RNA and protein are highly expressed in the testis, and it is expressed postmeiotically, suggesting that the gene might be involved in morphological changes of male germ cells. MORN3 protein was not expressed in somatic testicular cells in our studies (data not shown), indicating that it only executes roles in germ cells.
Even though Morn3 mRNA is highly abundant in the testis, the message was also detectable in the brain and oviduct. Given that these tissues have motile flagella and cilia respectively, we hypothesize that MORN3 might not only be involved in spermiogenesis, it might play a general role in ciliogenesis, particularly for motile cilia; this hypothesis is supported by the following published observations: (1) MORN3 is enriched in the ciliated tissues;9 (2) MORN3 is present in the proteomic database derived from cilia;29 and (3) Morn3 mRNA is significantly up-regulated by multicilin during ciliogenesis.30
The potential role of MORN3 in ciliogenesis is not only supported by its tissue distribution and stage when it is activated during spermatogenesis, but also by its cellular localization. Even though exogenous MORN3 is present throughout the cytoplasm in the transfected CHO cells, endogenous MORN3 protein is localized in the acrosome and manchette; both structures are unique in male germ cells and are essential for spermiogenesis/flagellogenesis.31,32
The anterior part of the sperm nucleus is highly conserved through evolution, where the acrosome, which forms during the beginning of spermiogenesis, as a unique membranous organelle located over it.33 Early studies established that the acrosome is a Golgi derived secretory vesicle. During acrosome and maturation phase, spermatids appear to possess an extra-Golgi pathway, permitting direct protein transport from the endoplasmic reticulum to the acrosome;34 recent studies also suggested that the acrosome is a novel lysosome-related organelle,33 which has many Rab family proteins, small GTPases critical for vesicle fusion and transport.35,36,37
We have previously reported that RC/BTB2, another MEIG1 associated protein, is also localized in the acrosome of spermatids.38 However, endogenous RC/BTB2 is also expressed in spermatocytes and localized in the Golgi (unpublished observation). In transfected CHO cells, RC/BTB2 was present as small vesicles in the beginning, and then fused to form a large one and attached to the nuclear membrane. These suggest that the RC/BTB2 might be involved in acrosome biosynthesis. This is different from MORN3. Even though weak Morn3 message is detected in spermatocytes (Figure 3b), the signal might be from other cell populations co-purified with pachytene spermatocytes during germ cell separation as no specific signal was detected in spermatocytes by immunofluorescent experiment using specific anti-MORN3 antibody (Figure 5a). RT-PCR using testicular cDNAs from mouse testes at different ages during the first wave of spermatogenesis also suggests that Morn3 mRNA is only abundant in spermatids (Figure 3a). MORN3 is not expressed in spermatocytes, and in transfected cells, it is localized in the whole cytoplasm. However, it is not yet known whether MORN3 is also located in the Golgi and if any other protein (s) are involved in MORN3 acrosomal enrichment. Our own data suggest that this is not depended on MEIG1, as MORN3 acrosome localization was not changed in MEIG1-deficient mice. Even though the anti-MORN3 antibody is highly specific, it only works for isolated mixed testicular cells for immunofluorescent staining, not for testicular sections and immunogold electron microscopy. A better quality antibody is required to conduct these experiments.
The manchette is a transient structure constituted by microtubular proteins, which forms at early stage of spermatid elongation, and it dissolves with the elongation and condensation processes being completed.39,40 The timing of manchette development is extremely precise. Several findings suggest that some structural proteins can be sorted and transported to the centrosome and the developing sperm flagella by the manchette through the mechanism of intramanchette transport.31,41 Increasing literatures demonstrated that the disruption of manchette components resulted in failure of spermiogenesis; one example is mice lacking MEIG1.10 Our recent studies demonstrated that MEIG1 is present throughout the whole cells of spermatocytes, but is translocated to the manchette of elongating spermatids (unpublished observation). It appears that MEIG1 determines MORN3 manchette localization in the elongating spermatids, as MORN3 is present in the manchette of elongating spermatids of wild-type mice, but in the elongating spermatids of MEIG1-deficient mice, MORN3 is no longer present in the manchette, even if localization of the manchette backbone, α-tubulin remains intact. Given that MORN3 protein expression level was not changed in the MEIG1-deficient mice, MEIG1 might not regulate MORN3 protein expression.
As described previously, two MORN-motif proteins were identified in mouse testis,23,42 and both are essential for spermiogenesis. MSAP (MRON motif-containing sperm-specific axonemal protein) is a male-specific homolog of the meichroacidin gene in carp (Cyprinus carpio). It is expressed during late spermiogenesis and accumulated in basal body and flagellum of spermatids. Another MORN-motif protein, MOPT (MORN2), was identified in mouse testis, transcript of MOPT was highly and specifically expressed in the testis and the skeletal muscle. Furthermore, MOPT transcript and protein were confined mainly to round and elongated spermatids. It first appeared in the proacrosomic vesicles of the early Golgi phase spermatids, and was involved in endosomal trafficking between the plasma membrane and the apex of the acrosome of round spermatids; these suggest that MOPT may play an important role in a dynamic regulation of acrosome biogenesis during late spermiogenesis.5
Although MORN3 and these testis MORN-motif proteins contain MORN motif, their overall homologue is very low, and protein blast using the MORN3 domain for MORN3 antibody production did not identify other proteins, this rules out the possibility that our anti-MORN3 antibody cross-reacted with other MORN-motif proteins; thus, localization reported here is specific for MORN3 protein. Many acrosomal proteins have a short signal peptide in the end; this peptide is usually removed, and the mature protein is usually smaller than the full-length protein. This might explain the reason why MORN3 is slightly smaller than the predicted full-length size in the testis. Interestingly, MORN3 protein expressed in transfected mammalian cells has the predicted molecular mass, indicating that posttranslational of MORN3 occurs only in the testis where specific posttranslational system is present.
In summary, we characterized another MORN-motif protein, MORN3 in mouse, and discovered that the gene is highly expressed in spermiogenic germ cells, where MORN3 is localized in the acrosome and manchette, two male germ cell specific structures that are essential for spermiogenesis, and the manchette localization is dependent on MEIG1. Given that MEIG1 plays an essential role in sperm flagella assembling, MORN3 might form a complex with MEIG1 in the manchette and conducts a similar function as MEIG1, and MORN3/MEIG1 complex might regulate spermiogenesis through a unique pathway in the manchette. Genetic factors for male infertility remain largely unknown, MEIG1/MORN3 genes might be novel genetic factors for male infertility and these genes might be targets for effective treatments for infertile males.
AUTHOR CONTRIBUTIONS
XJS carried out the mammalian expression construct. ZQW carried out cell culture and transient transfection. LZ and YQS carried out RT-PCR test, testicular cell preparation and immunofluorescence. HFL and WL carried out Western blot analysis. MET and GFJ carried out co-immunoprecipitation assay. SZS and ZBZ carried out study design. LZ and ZBZ analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing financial interests.
ACKNOWLEDGMENTS
This research was supported by NIH grant HD076257, Virginia Commonwealth University Presidential Research Incentive Program and Massey Cancer Award (to Zhi-Bing Zhang), Natural Science Foundation of China (81172462, 81300536, 81270739, 81370750), Natural Science Funds of Hubei Province of China (2013CFB331, 2012FFB04904), and the Youth Talents of Science and Technology Projects of Health Department of Hubei Province of China (QJX2012-22). Tissue processing and staining were performed in Histology Core Facility of the Wuhan University of Science and Technology and Virginia Commonwealth University. Confocal microscopy was performed in South-Central University for Nationalities and the Imaging Core of Virginia Commonwealth University (5P30NS047463).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Pudney J. Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech. 1995;32:459–97. doi: 10.1002/jemt.1070320602. [DOI] [PubMed] [Google Scholar]
- 2.Keber R, Rozman D, Horvat S. Sterols in spermatogenesis and sperm maturation. J Lipid Res. 2013;54:20–33. doi: 10.1194/jlr.R032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidaran MA, Kistler WS. Transcriptional and translational control of the message for transition protein 1, a major chromosomal protein of mammalian spermatids. J Biol Chem. 1987;262:13309–15. [PubMed] [Google Scholar]
- 4.Berruti G. Signaling events during male germ cell differentiation: bases and perspectives. Front Biosci. 1998;3:D1097–108. doi: 10.2741/a347. [DOI] [PubMed] [Google Scholar]
- 5.Choi YJ, Hwang KC, Park JY, Park KK, Kim JH, et al. Identification and characterization of a novel mouse and human MOPT gene containing MORN-motif protein in testis. Theriogenology. 2010;73:273–81. doi: 10.1016/j.theriogenology.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Hong S, Choi I, Woo JM, Oh J, Kim T, et al. Identification and integrative analysis of 28 novel genes specifically expressed and developmentally regulated in murine spermatogenic cells. J Biol Chem. 2005;280:7685–93. doi: 10.1074/jbc.M412444200. [DOI] [PubMed] [Google Scholar]
- 7.Don J, Winer MA, Wolgemuth DJ. Developmentally regulated expression during gametogenesis of the murine gene meg1 suggests a role in meiosis. Mol Reprod Dev. 1994;38:16–23. doi: 10.1002/mrd.1080380104. [DOI] [PubMed] [Google Scholar]
- 8.Ever L, Steiner R, Shalom S, Don J. Two alternatively spliced Meig1 messenger RNA species are differentially expressed in the somatic and in the germ-cell compartments of the testis. Cell Growth Differ. 1999;10:19–26. [PubMed] [Google Scholar]
- 9.McClintock TS, Glasser CE, Bose SC, Bergman DA. Tissue expression patterns identify mouse cilia genes. Physiol Genomics. 2008;32:198–206. doi: 10.1152/physiolgenomics.00128.2007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Shen X, Gude DR, Wilkinson BM, Justice MJ, et al. MEIG1 is essential for spermiogenesis in mice. Proc Natl Acad Sci U S A. 2009;106:17055–60. doi: 10.1073/pnas.0906414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Zhang L, Song S, Teves ME, Li H, et al. The mouse transcription factor-like 5 gene encodes a protein localized in the manchette and centriole of the elongating spermatid. Andrology. 2013;1:431–9. doi: 10.1111/j.2047-2927.2013.00069.x. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Komazaki S, Sasamoto K, Yoshida M, Nishi M, et al. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J Cell Biol. 2001;154:1059–67. doi: 10.1083/jcb.200105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Sugimoto A, Ohshima Y, Takeshima H. Important role of junctophilin in nematode motor function. Biochem Biophys Res Commun. 2001;289:234–9. doi: 10.1006/bbrc.2001.5951. [DOI] [PubMed] [Google Scholar]
- 15.Shimada H, Koizumi M, Kuroki K, Mochizuki M, Fujimoto H, et al. ARC3, a chloroplast division factor, is a chimera of prokaryotic FtsZ and part of eukaryotic phosphatidylinositol-4-phosphate 5-kinase. Plant Cell Physiol. 2004;45:960–7. doi: 10.1093/pcp/pch130. [DOI] [PubMed] [Google Scholar]
- 16.Gubbels MJ, Vaishnava S, Boot N, Dubremetz JF, Striepen B. A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J Cell Sci. 2006;119:2236–45. doi: 10.1242/jcs.02949. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson DJ, Sahoo N, Pinches RA, Bumstead JM, Tomley FM, et al. MORN1 has a conserved role in asexual and sexual development across the Apicomplexa. Eukaryot Cell. 2008;7:698–711. doi: 10.1128/EC.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorestani A, Sheiner L, Yang K, Robertson SD, Sahoo N, et al. A Toxoplasma MORN1 null mutant undergoes repeated divisions but is defective in basal assembly, apicoplast division and cytokinesis. PLoS One. 2010;5:e12302. doi: 10.1371/journal.pone.0012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorestani A, Ivey FD, Thirugnanam S, Busby MA, Marth GT, et al. Targeted proteomic dissection of Toxoplasma cytoskeleton sub-compartments using MORN1. Cytoskeleton (Hoboken) 2012;69:069–85. doi: 10.1002/cm.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mecklenburg KL. Drosophila retinophilin contains MORN repeats and is conserved in humans. Mol Genet Genomics. 2007;277:481–9. doi: 10.1007/s00438-007-0211-7. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya MR, Gerdts J, Naylor SA, Royse EX, Ebstein SY, et al. A model of toxic neuropathy in Drosophila reveals a role for MORN4 in promoting axonal degeneration. J Neurosci. 2012;32:5054–61. doi: 10.1523/JNEUROSCI.4951-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphray SJ, Oliver K, Hunt AR, Plumb RW, Loveland JE, et al. DNA sequence and analysis of human chromosome 9. Nature. 2004;429:369–74. doi: 10.1038/nature02465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokuhiro K, Hirose M, Miyagawa Y, Tsujimura A, Irie S, et al. Meichroacidin containing the membrane occupation and recognition nexus motif is essential for spermatozoa morphogenesis. J Biol Chem. 2008;283:19039–48. doi: 10.1074/jbc.M708590200. [DOI] [PubMed] [Google Scholar]
- 24.Shetty J, Klotz KL, Wolkowicz MJ, Flickinger CJ, Herr JC. Radial spoke protein 44 (human meichroacidin) is an axonemal alloantigen of sperm and cilia. Gene. 2007;396:93–107. doi: 10.1016/j.gene.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romrell LJ, Bellvé AR, Fawcett DW. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976;49:119–31. doi: 10.1016/0012-1606(76)90262-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Jones BH, Tang W, Moss SB, Wei Z, et al. Dissecting the axoneme interactome: the mammalian orthologue of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Proteomics. 2005;4:914–23. doi: 10.1074/mcp.M400177-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Tsuneoka M, Nishimune Y, Ohta K, Teye K, Tanaka H, et al. Expression of Mina53, a product of a Myc target gene in mouse testis. Int J Androl. 2006;29:323–30. doi: 10.1111/j.1365-2605.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- 28.Mortimer D, Curtis EF, Camenzind AR. Combined use of fluorescent peanut agglutinin lectin and Hoechst 33258 to monitor the acrosomal status and vitality of human spermatozoa. Hum Reprod. 1990;5:99–103. doi: 10.1093/oxfordjournals.humrep.a137050. [DOI] [PubMed] [Google Scholar]
- 29.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–13. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat Cell Biol. 2012;14:140–7. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kierszenbaum AL. Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Dev. 2002;63:1–4. doi: 10.1002/mrd.10179. [DOI] [PubMed] [Google Scholar]
- 32.Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol. 2004;67:271–84. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- 33.Berruti G, Paiardi C. Acrosome biogenesis: revisiting old questions to yield new insights. Spermatogenesis. 2011;1:95–98. doi: 10.4161/spmg.1.2.16820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wouters-Tyrou D, Martinage A, Chevaillier P, Sautière P. Nuclear basic proteins in spermiogenesis. Biochimie. 1998;80:117–28. doi: 10.1016/s0300-9084(98)80018-7. [DOI] [PubMed] [Google Scholar]
- 35.Hu ZZ, Valencia JC, Huang H, Chi A, Shabanowitz J, et al. Comparative bioinformatics analyses and profiling of lysosome-related organelle proteomes. Int J Mass Spectrom. 2007;259:147–60. doi: 10.1016/j.ijms.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet. 2008;9:359–86. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Teves ME, Shen X, Nagarkatti-Gude DR, Hess RA, et al. Mouse RC/BTB2, a member of the RCC1 superfamily, localizes to spermatid acrosomal vesicles. PLoS One. 2012;7:e39846. doi: 10.1371/journal.pone.0039846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clermont Y, Oko R, Hermo L. Cell biology of mammalian spermatogenesis. In: Desjardins C, Ewing L, editors. Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993. p. 332. [Google Scholar]
- 40.Meistrich M. Nuclear morphogenesis during spermiogenesis. In: de Kretser D, editor. Molecular Biology of the Male Reproductive System. New York: Academic Press; 1993. p. 67. [Google Scholar]
- 41.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 42.Ju TK, Huang FL. MSAP, the meichroacidin homolog of carp (Cyprinus carpio), differs from the rodent counterpart in germline expression and involves flagellar differentiation. Biol Reprod. 2004;71:1419–29. doi: 10.1095/biolreprod.104.030346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MORN3 associates with MEIG1 in vivo
Examination of anti-MORN3 antibody by Western blot analysis and immunofluorescence
Immunofluorescence on mouse mixed germ cells using preimmune serum
MORN3 protein expression level was not changed in the testis of Meig1-deficient mice


