Abstract
The objective of this study was to evaluate the expression of estrogen receptors (ER(α) and ER(β)) and androgen receptors (ARs) as prognostic factors for biochemical recurrence, disease progression and survival in patients with pT3N0M0 prostate cancer (PCa) in an urban Greek population. A total of 100 consecutive patients with pT3N0M0 PCa treated with radical prostatectomy participated in the study. The mean age and follow-up were 64.2 and 6 years, respectively. The HSCORE was used for semi-quantitative analysis of the immunoreactivity of the receptors. The prognostic value of the ER(α) and ER(β) and AR was assessed in terms of recurrence, progression, and survival. AR expression was not associated with any of the above parameters; however, both ERs correlated with the prognosis. A univariate Cox regression analysis showed that ER(α) positive staining was significantly associated with a greater hazard for all outcomes. Increased ER(β) staining was significantly associated with a lower hazard for all outcomes in the univariate analysis. When both ER HSCORES were used for the analysis, it was found that patients with high ER(α) or low ER(β) HSCORES compared with patients with negatively stained ER(α) and >1.7 hSCORE ER(β) had 6.03, 10.93, and 10.53 times greater hazard for biochemical disease recurrence, progression of disease and death, respectively. Multiple Cox proportional hazard analyses showed that the age, preoperative prostate specific antigen, Gleason score and ERs were independent predictors of all outcomes. ER expression is an important prognosticator after radical prostatectomy in patients with pT3N0M0 PCa. By contrast, AR expression has limited prognostic value.
Keywords: androgen receptor, estrogen receptor (α), estrogen receptor (β), locally advanced prostate cancer, radical prostatectomy
INTRODUCTION
In Europe, prostate cancer (PCa) is the most common solid neoplasm, representing 22.8% of all newly diagnosed cancer cases among men.1 However, the incidence, although not the mortality, rates vary by more than seven-fold, and the highest rates are observed in Northern and Western European countries, whereas the lowest rates occur in Central and Eastern areas. In Greece, the incidence rate for PCa is one of the lowest, with 34 cases per 100 000. This may be attributed to a variety of factors, such as diet2,3 and opportunistic screening.4 PCa is currently the most frequent cancer and the third most common cause of cancer death in men in the European Union.1 In addition, since 1985, there has been an increase in the incidence of PCa in most countries, even in countries where PCa is not common.1,5 This primarily reflects the widespread availability of prostate specific antigen (PSA) testing, which may result in PCa diagnosis during early stages of the disease.1,2,3,4,5,6 PCa most often affects older men; therefore, there is a greater concern in developed countries, where life expectancy is longer.7
Locally advanced PCa previously accounted for approximately 40% of the newly diagnosed PCas. Today, this figure is lower, and it has been estimated that approximately 20% of emerging PCas are locally advanced.8 This is because of the widespread measurement of serum PSA, which is a diagnostic tool that has led to a remarkable increase in the detection of clinically localized cancers.9,10
Locally advanced PCa stage T3N0M0 is defined as cancer that has either broken the prostatic capsule or infiltrates the seminal vesicles without invasion of the adjacent organs and no evidence of positive lymph nodes or distant metastasis. Many patients presenting with these tumors may be suitable for definitive treatment, whereas others require a more conservative form of treatment. Locally advanced PCa involves a wide range of tumor phenotypes with different prognoses, and more than 50% of these men are at risk for tumor recurrence after local treatment. The management of these patients remains controversial. Therefore, it is important to identify those patients who can receive definitive treatment and those who will not benefit from it.
Surgical treatment in clinical T3 (cT3) PCa has traditionally been discouraged,11 primarily because patients have an increased risk of positive surgical margins (+SMs) and either lymph node or distant metastases.12,13 Although several randomized studies comparing radiation alone with the combination of radiation and hormone therapy (androgen deprivation therapy (ADT)) show a clear advantage for the combination therapy, no study has demonstrated the superiority of combination therapy versus radical prostatectomy.14 Recently, there has been renewed interest in surgical treatment of locally advanced PCa, and several retrospective studies have been published. Although surgical therapy remains controversial, it is increasingly evident that it has a place in the treatment of locally advanced disease, particularly for stage cT3.15,16,17,18,19 Another important factor is the over-staging of cT3 PCa, which is relatively common and occurs in 13%–27% of cases. It appears that patients with pT2 disease and patients with pT3 disease confined within the surgical specimen have similarly good biochemical progression-free survival and progression disease-free survival (PDFS).18,19 Therefore, 56%–78% of patients who initially underwent surgery eventually require adjuvant or salvage therapy, that is, either radiation or hormone or a combination of both. Nevertheless, excellent 5 years, 10 years and 15 years overall survival (OS) and disease-specific survival rates have been published, and these rates are better than those of radiation alone and no different from radiotherapy combined with adjuvant hormonal therapy. Furthermore, patients with pT2 disease and patients with specimen-confined pT3 disease have similarly good biochemical and clinical PFS.15,16,17,18,19
However, it remains to be determined which patients with locally advanced disease cT3 can undergo surgical treatment and benefit from the procedure. The nomograms that include the PSA level, stage and Gleason score can be useful in predicting the pathological stage of disease,20 but there is not enough information to guide the decision-making regarding the treatment modality. Therefore, in addition to the known prognostic parameters used in nomograms, other prognostic parameters, such as distinct histopathological characteristics of the tumor, would improve the decision-making regarding the treatment modality, appropriate patient selection and improved prediction efficacy of the existing nomograms. Useful histopathological parameters that may have important predictive value are estrogen receptor (ER) and androgen receptor (AR) expression in the tumor.
ARs in PCa are now a focus of research efforts for both the treatment and prevention of PCa. Androgens play a fundamental role in the growth, differentiation and maintenance of prostate tissue. Their effects are mediated via a specific AR that belongs to the nuclear receptor family. The AR molecule is a major part of the regulatory androgen-AR complex and is critical in the androgen-AR pathway of PCa.21,22 AR expression may represent a potential prognostic parameter. However, there have been variable results regarding the number of cells expressing ARs in cancer and the ability to predict clinical progression and survival.23 In addition, there is a growing body of evidence to suggest that estrogens and their receptors, primarily ER(α) in the prostatic stroma and ER(β) in the prostatic epithelium, play a significant role in both normal and abnormal development of the prostate gland.24,25,26,27 Estrogens have been shown to be an effective hormonal treatment for advanced PCa for over 60 years and remain a second line of hormonal manipulation.28,29,30 Surprisingly, estrogens may also be associated with the development and progression of PCa.30,31,32 The expression of these receptors and their correlation may be a prognostic factor for determining biochemical relapse and disease progression.
Our objective was to study the expression of the ERs (ER(α) and ER(β)) in relation to the expression of the AR in the prostate tissue of locally advanced PCa and to determine their correlation with biochemical disease-free survival (BDFS), PFS and OS. We also sought to identify the patients with locally advanced PCa stage cT3N0M0 who are appropriate candidates for surgical treatment (radical prostatectomy).
MATERIALS AND METHODS
Inclusion criteria
Enrollment in this study occurred between January 2003 and December 2008. After obtaining approval from the Institutional Research Ethics Board, we performed a prospective study of 214 consecutive patients with non-metastatic locally advanced PCa, pathologically proven stage pT3 and treated with radical prostatectomy and bilateral pelvic lymph node dissection (PLND). No patients with stage pT4 were included. The preoperative inclusion criteria were PSA <20 ng ml−1, Karnofsky status ≥80, negative lymph node status, no documented distant metastasis, no neoadjuvant therapy or drugs that could affect PSA values, such as finasteride and dutasteride, postoperatively − SMs and pathologically negative lymph node metastasis. From a total of 214 patients with pT3 stage PCa (19.5% of all radical prostatectomies during this time), 53 patients (24.7% of the pT3 cases) were excluded from the study due to positive lymph node metastasis after extended PLND, and 39 patients (18.22% of pT3 cases) were also excluded due to +SMs. Another 7 patients with preoperative PSA >20 ng ml−1 and 15 patients who did not complete the scheduled follow-up were also excluded. Finally, 100 patients fulfilled the inclusion criteria.
All patients had been preoperatively staged for metastasis with contrast-enhanced abdominal and pelvic computed tomography (CT), bone scan, and levels of serum alkaline phosphatase. Two dedicated genitourinary pathologists performed the biopsy and pathologic grading, which were assessed based on the Gleason grading system. Extended lymph node dissection was performed in all patients included in the study, following baseline oncologic parameters and the decision of the surgeon.
Postoperative follow-up
The patients were postoperatively followed, and the assessment included a disease-specific history, clinical examination and PSA levels at 3, 6, 9 and 12 months for the 1st year and every 4 months thereafter. Creatine, hemoglobin and liver function monitoring were assessed twice a year. Any single elevated serum PSA level was reconfirmed in a consecutive second valuation. Biochemical recurrence was defined as two consecutive PSA values of 0.2 ng ml−1 or greater. Pelvic and abdominal CT was performed at 12 and 24 months and on demand thereafter. Transrectal ultrasonography and biopsy were used to histologically confirm a diagnosis of local disease recurrence, and bone scintigraphy and chest X-ray were used in patients with elevated PSA levels, positive digital rectal examination and symptoms arising from the skeleton.
Specimen-processing
Radical prostatectomy specimens were processed using the whole-mount technique. Sufficient tissue was available for immunohistochemical analysis in all cases. Two observers determined the number and intensity of immunoreactive nuclei without any knowledge of the clinical data. Paraffin-embedded formalin-fixed (10% neutral formalin) prostate tissue specimens were obtained. The representative sections were held by the positions of the tumor in each case and the positions of the juxtaposed normal tissue, with a thickness of 4 mm.
To identify the human ER(α), we used the lyophilized mouse monoclonal antibody anti-ERA NCL-ER-6F11 (Novocastra, UK). To identify the human ER(β), we used the lyophilized mouse monoclonal antibody anti-ER(β) [14C8] ab288 (Abcam, UK). To identify the human AR, we used the lyophilized mouse monoclonal antibody anti-AR NCL-AR-2F12 (Novocastra, UK). The Dako EnVision™+ system horseradish peroxidase (HRP) (diaminobenzidine (DAB)) protocol was used as the immunohistochemical method. This is a heat-induced epitope retrieval method. We also used an indirect method with a two-step polymer dextran/HRP system for immunostaining (Table 1).
Table 1.
Baseline characteristics of study cohort

Immunostaining process
Primary stage
The sections were incubated at 56°C for 18 h in an incubator. The pretreatment, deparaffinization, rehydration and recovery of the antigenic sites – epitope retrieval were performed with the Dako PT-Link module for tissue specimens (Dako Denmark A/S), which allows for the entire pretreatment process to be combined into 3-in-1 step specimen preparation procedure. For the sample preparation, target retrieval solution pH 9, (10×), (3-in-1) Dako (Dako Denmark A/S) was diluted 1:10 with distilled water, and 1.5 l solution completely covered the slides. Then, the slides were preheated to 65°C, incubated for 15 min at 97°C and cooled in the PT-link until the temperature reached 65°C. The entire procedure required 90 min. The samples were briefly rinsed with running water and then washed with Tris-buffered saline with Tween (TBST) solution (Dakocytomation, Dako Denmark A/S), a Tris-buffered NaCl solution with Tween 20, pH 7.6, twice for 5 min (1:10 dilution in distilled water). To remove the endogenous peroxidase from the tissues, the sections were incubated with 0.3% H2O2 solution in deionized water for 20 min in a dark chamber. A second wash was then performed with TBST solution twice for 5 min. The primary antibody was diluted in Dako Real antibody diluent, which is a Tris-buffer, pH 7.2, with 15 mol l−1 NaN3 and protein. The dilution and incubation conditions for the antibodies are those that combine the best signal strength with the least possible nonspecific staining (background). In our study, this was 1:20 for ER(β) and 1:100 for the ER(α) and AR antibodies. The specific antibody (100 μl) was pipetted onto each section. The sections were mounted on plates and incubated at 4°C for 18 h.
Secondary stage
The sections were incubated at room temperature for 30 min and washed with TBST solution twice for 5 min. Next, 100 μl of polymer solution Dako EnVision System/HRP (DAB) was added to each section for 45 min at room temperature, and then the sections were washed again with TBST solution twice for 5 min. The slides were immersed in DAB solution for 5 min in a dark chamber (working solution consists of 18 ml Dako Real substrate buffer, pH 7.5, and 1 ml DAB × 50). The slides were counterstained with hematoxylin (Vector H3404) and dehydrated in graded alcohol, dried and cover slipped (Figure 1a-1j).
Figure 1.
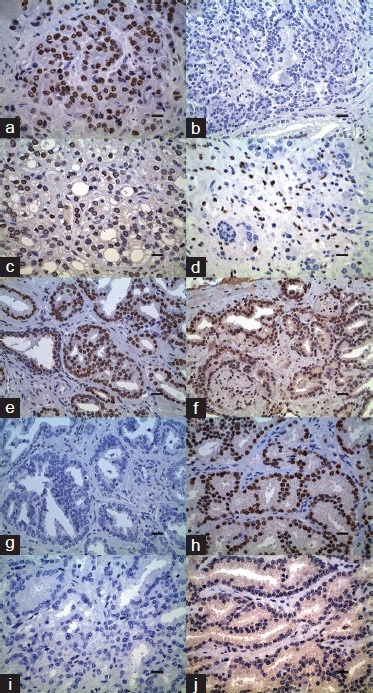
Immunohistochemical analysis in prostate adenocarcinoma. (a) Androgen receptor (AR) expression (diaminobenzidine (DAB)), Gleason (4 + 5) score 9, HSCORE 260, scale bar = 8 µm. (b) Estrogen receptor (ER(α)) expression (DAB), Gleason (4 + 5) score 9, HSCORE 0 with sparse positive cells in cancer, scale bar = 12.5 µm. (c) ER(β) expression (DAB), Gleason (4 + 5) score 9, HSCORE 180, scale bar = 8 µm. (d) ER(α) expression (DAB), Gleason (3 + 4) score 7, HSCORE 25 with positive staining in the stromal cells, scale bar = 8 µm. (e) AR expression (DAB), Gleason (3 + 3) score 6, HSCORE 270, scale bar = 12.5 µm. (f) ER(β) expression (DAB), Gleason (3 + 3) score 6, HSCORE 60, scale bar = 12.5 µm. (g) ΕR(α) expression (DAB), Gleason (3 + 3) score 6, HSCORE 0, scale bar = 12.5 µm. (h) AR expression (DAB), Gleason (3 + 4) score 7, HSCORE 300, scale bar = 8 µm. (i) ER(α) expression (DAB), Gleason (3 + 4) score 7, HSCORE 0 with single cell positivity, scale bar = 8 µm. (j) ER(β) expression (DAB), Gleason (3 + 4) score 7, HSCORE 0, scale bar = 8 μm.
Scoring of immunoreactivity
For the semi-quantitative analysis of immunoreactivity of the steroid receptors, HSCORE was used in this study.33,34 Briefly, at least 1000 tumor cells were counted in each case and the HSCORE was calculated using the following equation: HSCORE= ∑Pi (i + 1). The intensity of staining (i) was evaluated subjectively on a scale of 0–3, such that 0 = No staining, 1 = Weak staining, 2 = Unequivocal moderate staining and 3 = Strong staining. Pi is the percentage of stained epithelial cells for each intensity. In the present study, interobserver differences were <5% and the mean of the two values was obtained. The areas of focal staining with the highest percentage of nuclei positive for AR, ER(α) and ER(β) were used for each Gleason pattern observed in a particular tumor. If more areas from the same pathological category were identified within one prostate, the highest score was used for that category.
Statistical analysis
The quantitative variables are expressed as the mean values (standard deviation (s.d.)) or median values (interquartile range (IQR)). The qualitative variables are expressed as an absolute and relative frequencies. ER(β) was tested for its ability to predict biochemical disease recurrence, progression of disease and death using receiver operating characteristic (ROC) curves. The overall performance of the ROC analysis was quantified by computing the area under the curve (AUC). An area of 1 indicated perfect performance, whereas 0.5 indicated performance that was not different than chance. ROC analysis was used to determine the optimal sensitivity and specificity using various cut-off values for the prediction of outcomes. To test whether the addition of the PSA and Gleason score increases the predictive ability, logistic regression models were used to derive linear predictors and to compare the AUC. The comparison of AUC indicates which model is the best for the discrimination of the outcome measures. To compare the proportions, Fisher's exact tests were used. To compare the mean ER(β) between the patient groups as defined by the PSA and Gleason scores, Student's t-tests and analysis of variance were used, respectively. The prognostic value of ER(α), ER(β), and AR for biochemical disease recurrence, progression of disease and OS was first assessed by univariate Cox regression analysis.35 The variables that showed significant association with the outcomes were included in the multiple Cox proportional-hazard model. The assumption of proportional hazards was evaluated by testing for interactions with continuous time variable. Kaplan–Meier survival estimates for biochemical disease recurrence, progression of disease and OS were graphed for the follow-up period. All reported P values are two-tailed. Statistical significance was set at P < 0.05, and the analyses were conducted using the SPSS statistical software (version 18.0).
RESULTS
A total of 100 patients with a mean age of 64.2 years (range: 57–74, s.d. = 3.0 years) participated in the study. All men were of Greek origin from urban areas. The basic demographics and clinical characteristics of the study cohort are presented in Table 1. The majority of participants (74%) had a Karnofsky score equal to 100, and 22% of the patients had PSA values higher than 10 ng ml−1. Half of the patients had a Gleason score greater than 6%, and 29% of the patients received ADT and RT treatment postsurgically. The mean follow-up period was 6.0 years (s.d. = 2.0), with the median equal to 5.8 years (IQR from 4.6 to 7.5 years). During the follow-up period, biochemical disease recurrence occurred in 62.0% of the patients, progression of disease occurred in 44% of the patients, and 25% of the patients died. The combined outcome of biochemical disease recurrence, progression of disease and death occurred in 62% of the patients and resulted in all patients having biochemical disease recurrence. Fifteen patients (15%) had elevated ER(α), whereas the mean ER(β) HSCORE was 1.6 (0.7) and the mean AR HSCORE was 2.0 (s.d. = 0.5). The mean time interval between patient surgery and biochemical disease recurrence was 2.3 years (s.d. = 1.7 years), whereas the corresponding mean time interval for progression of the disease was 3.1 years (s.d. = 1.5 years) and for death, 4.2 years (s.d. = 1.9 years). Subjects with preoperative PSA >10 ng ml−1 had a mean value for the ER(β) HSCORE equal to 1.2 (s.d. = 0.7), which was significantly lower (P = 0.001) than the corresponding mean value of 1.7 (s.d. = 0.6) for the HSCORE of patients with preoperative PSA <10 ng ml−1. The ER(β) mean values were not different between the groups of patients with Gleason scores <6, 7 or 8–9 (P = 0.845). The proportion of patients with elevated ER(α) was not significantly different according to the preoperative PSA levels (P = 0.091) or the Gleason score (P = 0.804).
The ROC curve analysis (Table 2) showed that the optimal cut-off point of ER(β) HSCORE for the prediction of biochemical disease recurrence was 1.7, with sensitivity equal to 74.2% and specificity equal to 86.8% (Figure 2a). Similarly, an ER(β) value of 1.5 was the optimal cut-off for the prediction of progression of the disease, with a sensitivity of 72.7% and a specificity of 87.5% (Figure 2b). The AUC was 0.83 (95% confidence interval (CI): 0.75–0.91) and 0.84 (95% CI: 0.76–0.92) for biochemical disease recurrence and progression of disease, respectively, which is significantly different from 0.5 (P < 0.001). For the survival, ROC analysis showed a cut-off of 1.5 for ER(β), with a sensitivity of 80.0% and a specificity of 74.7%, with an AUC equal to 0.83 (95% CI: 0.74–0.92) (Figure 2c). The predictive ability of ER(β) for biochemical disease recurrence was not significantly increased by the addition of PSA and/or Gleason score (P > 0.05). For the progression of the disease, the addition of PSA significantly increased the predictive ability of ER(β) (P = 0.046) as indicated by an AUC equal to 0.89. Additionally, for survival outcome, the addition of PSA significantly increased the predictive ability of ER(β) (P = 0.074).
Table 2.
ROC analysis for the prediction of biochemical disease recurrence, progression of disease and death from ER(β)
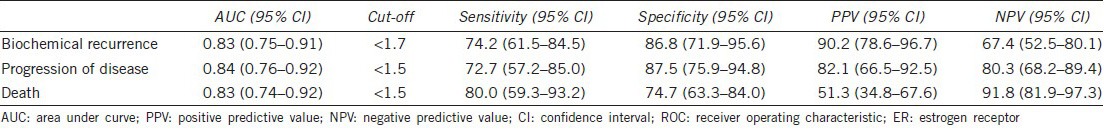
Figure 2.
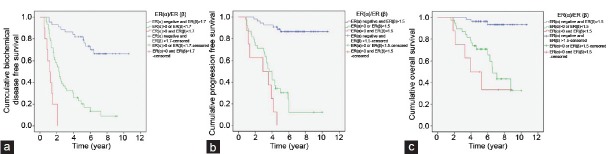
Kaplan–Meier estimates (a) biochemical disease-free survival according to the estrogen receptor ER(α) and ER(β) levels. (b) progression-free survival according to the ER(α) and ER(β) levels. (c) overall survival according to the ER(α) and ER(β) levels.
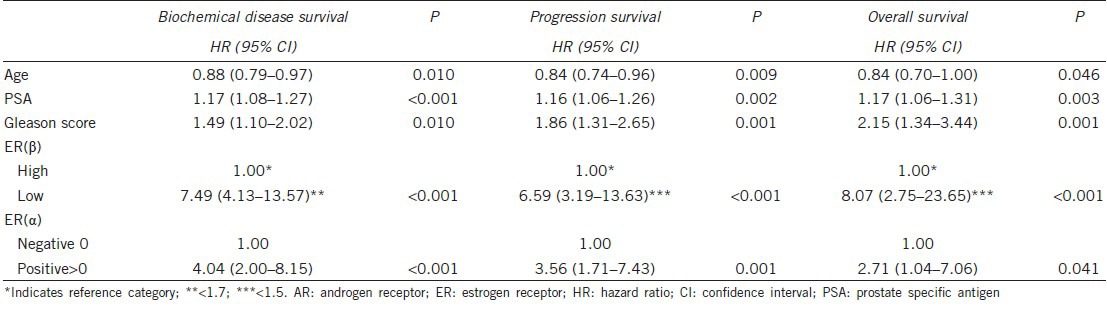
Univariate Cox regression analysis (Table 3) showed that ER(α) >0 (positive staining) was significantly associated with a greater hazard for all outcomes. Increased ER(β) was significantly associated with a lower hazard for all outcomes in the univariate analysis. Using the cut-off provided from the ROC analysis, ER(β) had a significant association with the three study outcomes (P < 0.001), and subjects with low ER(β) values had a greater risk. AR expression was not significantly associated with biochemical disease recurrence (P = 0.873), progression of disease survival (P = 0.443) or OS (P = 0.090). When the combined ER(α) and ER(β) HSCORES were used in the analysis, it was found that patients with high ER(α) or low ER(β) HSCORES compared to patients with both negatively stained ER(α) and >1.7 hSCORE ER(β), had 6.03, 10.93 and 10.53 times greater hazard for biochemical disease recurrence, progression of disease and death, respectively. Similarly, the hazard ratios for patients with high ER(α) and low ER(β) compared to patients with both negatively stained ER(α) and high ER(β) HSCORE >1.7 were 24.87, 22.39 and 17.88 for biochemical disease recurrence, progression of disease and death, respectively. Kaplan–Meier estimates for biochemical disease recurrence, progression of the disease and OS for the combination of ER(α) and ER(β) are presented in Figure 2a–2c. The multiple Cox proportional hazard analysis showed that age, preoperative PSA, Gleason score, ER(α) and ER(β) were independent predictors of all study outcomes (Table 4). Specifically, increased age was found to be associated with a lower risk for all outcomes. In addition, increased preoperative PSA and increased Gleason score were associated with a greater risk for all study outcomes. After adjustment for the other variables, patients with elevated ER(α) had a 4.04 times greater risk for biochemical disease recurrence, 3.56 times greater risk for progression of the disease and 2.71 times greater risk for death. Using the cut-offs provided by the ROC analyses for ER(β), the hazard ratio equal to 7.49 for patients with ER(β) <1.7 was determined for biochemical disease recurrence. In addition, patients with ER(β) <1.5 had 6.59 and 8.07 times greater hazard compared to those with ER(β) >1.5 for progression of disease and OS, respectively.
Table 3.
Association of ER(α), ER(β), AR with biochemical disease survival, progression survival and overall survival (univariate Cox regression models)
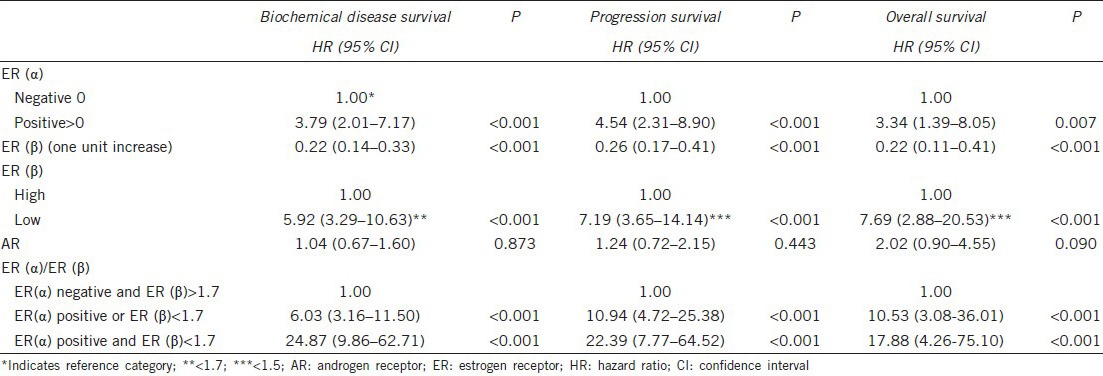
Table 4.
Results from multiple Cox regression analysis for the prediction of biochemical disease survival, progression survival and overall survival
DISCUSSION
We have demonstrated that the degree of expression of the ERs (ER(α) and ER(β)) is directly related to BDFS, PDFS and OS. The two receptor subtypes have distinct roles in several estrogen target cells and tissues. ER(α) and ER(β) have different biological functions as indicated by their distinct expression patterns, and the different phenotypes reported for the two ER isoform knockout animals. Furthermore, ER(α) and ER(β) have overlapping yet unique roles in estrogen signaling.36 This study not only reveals the important role of ERs in PCa growth and progression but also indicates that the role of ER(α) and ER(β) is different and independent of the activity of the ARs. Therefore, we believe that our results are highly significant.
First, the absence of ER(α) expression in locally advanced PCa is associated with a better prognosis of patients who underwent radical prostatectomy. In this regard, patients with positive expression of ER(α) receptors that had a worse prognosis may have started adjuvant external beam radiation therapy early postoperatively, or, in some select cases, could have completely avoided the operation. Our results are consistent with previous studies that showed a significant positive correlation between ER(α) expression and staging or malignant degree.37,38,39,40,41 After adjustment for other variables, patients with positive expression of ER(α) had a 4.04 times greater risk for biochemical disease recurrence, 3.56 times greater risk for progression and 2.71 times greater risk for death.
Second, ER(β) expression in locally advanced PCa, above a certain limit, is highly associated with a better prognosis and increased BDFS, PDFS and OS. These data are supported by other studies that showed that the loss or decrease in ER(β) expression is associated with a higher Gleason grade and PCa with higher metastatic potential.27,38,39,40,41 In our study, we attempted to objectively determine a cut-off point for ER(β) expression that can be used as a predictive factor for a better prognosis, prolonged BDFS, PDFS, and OS, and for determining the treatment modality in patients with locally advanced PCa (radical prostatectomy or not). The ROC analysis showed that the optimal cut-off point of ER(β) to predict biochemical disease recurrence was 1.7 (HSCORE) with a sensitivity of 74.2% and a specificity of 86.8%. Similarly, an ER(β) value of 1.5 (HSCORE) represented the optimal cut-off point for the prediction of disease progression, with a sensitivity of 72.7% and a specificity of 87.5%. If we combine these results with other previous reports that suggest that ER positivity has a prognostic role in PCa in terms of time to progression on hormone therapy42 and other reports concerning the protective effect of phytoestrogens in PCa, the prognostic value of these markers is enhanced.43,44
Third, the combination of low ER(β) expression and positive ER (a) expression rate further predicts biochemical recurrence, disease progression and survival (Figure 2a and 2b). Therefore, it appears that patients with pT3 PCa with high ER(β) expression and no ER(α) expression who undergo radical prostatectomy have better outcomes compared to patients with low ER(β) and positive ER(α) expression. In our study, we used HSCORE to determine the receptor expression levels because it provides a continuous measure of tumor hormone receptor content and has been suggested for universal adoption among pathologists. This semi-quantitative analysis has also been shown to have low intraobserver and interobserver errors.45 In addition, we used a sensitive immunohistochemical method and well characterized specific monoclonal antibody to determine the extent and intensity of ER expression in benign and malignant prostate tissue. We evaluated large tissue sections from radical prostatectomy specimens that contained the greatest amount of high-grade cancer to minimize the heterogeneity of ER expression, which may confound biopsy studies. The potential to use this method for materials from prostate biopsies and the confirmation of cut-off points in future studies using smaller samples of ER expression makes ERs a promising prognosticator for locally advanced PCa. This will improve image-guided prostate biopsy. Finally, radical prostatectomy as a part of a multimodal treatment strategy for patients with cT3 disease offers cancer control, survival rates and complication rates approaching those achieved for cT2 disease. The pathological grade, ploidy, and margins status are significant predictors of outcome after radical prostatectomy.18 ERs are also significant predictors of outcome after radical prostatectomy for these patients. ER expression has the potential for future use as a prognostic factor in locally advanced PCa in conjunction with other known prognostic factors.
Androgen binding to the AR is not the only mechanism by which the AR is activated. Changes in the AR sequence, structure and ligand affinity occur in PCa, and alternative signaling pathways are also activated in hormone-refractory disease.46 AR expression in PCa can be detected throughout progression in both hormone-sensitive and hormone refractory cancers.47,48,49 Immunohistochemical studies have shown that AR expression is heterogeneous in PCa, and some studies correlate AR positivity with a greater degree of differentiation or lower Gleason score, but this is not a universal observation.23,48,49,50,51,52 Other studies correlate a high level of AR expression with aggressive disease and decreased biochemical recurrence-free survival.53 Although there are studies of the mechanism by which androgen-dependent PCa transitions into androgen independence in advanced PCa,54 there is little to no research regarding the expression of ARs in locally advanced PCa and its role as a prognostic factor in clinical outcomes and its correlation with BDFS, PFS and OS. These types of studies may demonstrate which treatment modality should be followed. In our analysis, AR expression was detected in the epithelial nuclei of both cancer tissues and benign counterpart areas with a mean AR HSCORE of 2.0 (s.d. = 0.5). AR expression was not correlated with clinicopathological parameters, such as Gleason score, TNM stage and pretreatment PSA. AR expression was not significantly associated with BDFS (P = 0.873), PFS (P = 0.443) and OS (P = 0.090); therefore, AR expression was not predictive of a higher probability of biochemical disease recurrence, disease progression and death. These results are consistent with Dunsmuir et al.55 who found that AR expression is of limited prognostic value. In our study, patients with locally advanced PCa treated with radical prostatectomy are ideal for identifying the putative role and natural course of AR in PCa because the androgen-AR pathway is most likely undisturbed (no other preoperative treatment). We also used a sensitive immunohistochemical method and a well characterized specific monoclonal antibody to determine the extent and intensity of AR expression. Our results indicate that there may be either limited or no clinical use of AR expression as a prognostic parameter in men with locally advanced PCa.
Our study is the first to suggest a specific cut-off HSCORE for ER(β) (1.7) that is related to biochemical recurrence, disease progression and survival. Moreover, it is the first study that evaluates a certain high-risk PCa patient group (T3N0M0), which is the most controversial group regarding optimal management. The objective is to identify the treatment option that may be more beneficial based on ER expression. Ideally, this information would be of utmost importance at the time of biopsy and diagnosis.
Our study has three limitations that should be discussed. First, this study was conducted with a cohort of Greek men. As discussed in the Introduction, the incidence of PCa in Greece is lower compared to other European areas and the United States. This may be attributed to either dietary factors2,3 (though contradictory data have also been published56) or sporadic screening4 that is used in most urban Greek areas. By contrast, a number of factors in such places, even in Greece, may increase the incidence of PCa.57 In brief, if PCa in Greek individuals has been previously related to parameters that characterize the Greek lifestyle, it is believed that this may play a role in carcinogenesis rather than the expression of special features, such as estrogen and AR expression at a specific pathological stage.
Second, the number of the patients evaluated was rather limited. This is mainly because of the strict inclusion criteria (pathologically T3 PCa, −SMs, negative regional lymph nodes, preoperative PSA <20 ng ml−1, and no previous 5AR inhibitors), which were used to avoid interference from any confounding factors that may affect the results.
Third, the patients who participated were all assessed independently of receiving any adjuvant therapy (radiation, hormones or the combination). Ideally, we should have studied each arm separately. However, the objective was to evaluate estrogen and AR expression with respect to the parameters described above. In this regard, the expression in each cohort would have been affected in the same manner by the postoperative procedures.
CONCLUSIONS
Estrogen receptors are implicated in PCa progression. ER expression, as an immunohistochemical marker, has significant prognostic value in terms of biochemical recurrence, progression of disease and OS after radical prostatectomy. Clearly, there is a distinctly different role concerning the prognosis of PCa between the two ER subtypes α and β, confirming the suppressive role of ER(β) and oncogenic role of ER(α). ER expression may be used in the future as a prognostic factor for locally advanced PCa in conjunction with other known prognostic factors and may be used for novel predictive models. It is also possible to standardize and determine the cut-off points in the immunohistochemistry (IHC) method to use ER expression as a prognostic factor routinely. Additional studies are warranted to validate and develop our method, and it should be used not only for radical prostatectomy specimens but also other prostate biopsy materials. By contrast, AR expression has a very limited or no prognostic value in men with locally advanced PCa.
AUTHOR CONTRIBUTIONS
GM was the primary investigator of this study. He conceived of the study, collected the material, designed the study and wrote the main part of the manuscript. MC participated in the study design and performed the statistical analyses. IA participated in the study conception and performed part of the operations. AT and TC were the two pathologists who assessed all specimens for biopsy and IHC. CD performed part of the radical prostatectomies and supervised the entire study. All authors have read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
We thank Kalafati Theodora (histotechnologist), who contributed to the immunostaining and picture generation.
REFERENCES
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Itsiopoulos C, Hodge A, Kaimakamis M. Can the Mediterranean diet prevent prostate cancer? Mol Nutr Food Res. 2009;53:227–39. doi: 10.1002/mnfr.200800207. [DOI] [PubMed] [Google Scholar]
- 3.Tzonou A, Signorello LB, Lagiou P, Wuu J, Trichopoulos D, et al. Diet and cancer of the prostate: a case-control study in Greece. Int J Cancer. 1999;80:704–8. doi: 10.1002/(sici)1097-0215(19990301)80:5<704::aid-ijc13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Stamatiou K, Lardas M, Kostakos E, Koutsonasios V, Lepidas D. Prostate cancer screening in Greece: current facts. Urol J. 2009;6:157–61. [PubMed] [Google Scholar]
- 5.Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–52. doi: 10.1016/j.ejca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int. 2002;90:162–73. doi: 10.1046/j.1464-410x.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- 8.Akre O, Garmo H, Adolfsson J, Lambe M, Bratt O, et al. Mortality among men with locally advanced prostate cancer managed with noncurative intent: a nationwide study in PCBaSe Sweden. Eur Urol. 2011;60:554–63. doi: 10.1016/j.eururo.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 9.Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270:948–54. [PubMed] [Google Scholar]
- 10.Johansson JE, Holmberg L, Johansson S, Bergström R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997;277:467–71. [PubMed] [Google Scholar]
- 11.Hodgson D, Warde P, Gospodarowicz M. The management of locally advanced prostate cancer. Urol Oncol. 1998;4:3–12. doi: 10.1016/s1078-1439(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 12.Fallon B, Williams RD. Current options in the management of clinical stage C prostatic carcinoma. Urol Clin North Am. 1990;17:853–66. [PubMed] [Google Scholar]
- 13.Boccon-Gibod L, Bertaccini A, Bono AV, Dev Sarmah B, Höltl W, et al. Management of locally advanced prostate cancer: a European consensus. Int J Clin Pract. 2003;57:187–94. [PubMed] [Google Scholar]
- 14.Bolla M, Collette L, Blank L, Warde P, Dubois JB, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 15.Yamada AH, Lieskovsky G, Petrovich Z, Chen SC, Groshen S, et al. Results of radical prostatectomy and adjuvant therapy in the management of locally advanced, clinical stage TC, prostate cancer. Am J Clin Oncol. 1994;17:277–85. doi: 10.1097/00000421-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Gerber GS, Thisted RA, Chodak GW, Schroder FH, Frohmuller HG, et al. Results of radical prostatectomy in men with locally advanced prostate cancer: multi-institutional pooled analysis. Eur Urol. 1997;32:385–90. [PubMed] [Google Scholar]
- 17.van den Ouden D, Hop WC, Schröder FH. Progression in and survival of patients with locally advanced prostate cancer (T3) treated with radical prostatectomy as monotherapy. J Urol. 1998;160:1392–7. doi: 10.1097/00005392-199810000-00048. [DOI] [PubMed] [Google Scholar]
- 18.Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005;95:751–6. doi: 10.1111/j.1464-410X.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CY, Joniau S, Oyen R, Roskams T, Van Poppel H. Outcome of surgery for clinical unilateral T3a prostate cancer: a single-institution experience. Eur Urol. 2007;51:121–8. doi: 10.1016/j.eururo.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–8. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Yuan HQ, Kong F, Hu XY, Ren K, et al. Ectopic expression of neurotrophic peptide derived from saposin C increases proliferation and upregulates androgen receptor expression and transcriptional activity in human prostate cancer cells. Asian J Androl. 2007;9:601–9. doi: 10.1111/j.1745-7262.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 22.Altuwaijri S, Wu CC, Niu YJ, Mizokami A, Chang HC, et al. Expression of human AR cDNA driven by its own promoter results in mild promotion, but not suppression, of growth in human prostate cancer PC-3 cells. Asian J Androl. 2007;9:181–8. doi: 10.1111/j.1745-7262.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 23.Qiu YQ, Leuschner I, Braun PM. Androgen receptor expression in clinically localized prostate cancer: immunohistochemistry study and literature review. Asian J Androl. 2008;10:855–63. doi: 10.1111/j.1745-7262.2008.00428.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang WY, Prins GS. Estrogen receptor-beta: implications for the prostate gland. Prostate. 1999;40:115–24. doi: 10.1002/(sici)1097-0045(19990701)40:2<115::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Ho SM. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem. 2004;91:491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 26.Risbridger GP, Ellem SJ, McPherson SJ. Estrogen action on the prostate gland: a critical mix of endocrine and paracrine signaling. J Mol Endocrinol. 2007;39:183–8. doi: 10.1677/JME-07-0053. [DOI] [PubMed] [Google Scholar]
- 27.Singh PB, Matanhelia SS, Martin FL. A potential paradox in prostate adenocarcinoma progression: oestrogen as the initiating driver. Eur J Cancer. 2008;44:928–36. doi: 10.1016/j.ejca.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 28.Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102:899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- 29.Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98–106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Bosland MC. The role of estrogens in prostate carcinogenesis: a rationale for chemoprevention. Rev Urol. 2005;7(Suppl 3):S4–10. [PMC free article] [PubMed] [Google Scholar]
- 31.Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, et al. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008;22:1512–20. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- 32.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goulding H, Pinder S, Cannon P, Pearson D, Nicholson R, et al. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Hum Pathol. 1995;26:291–4. doi: 10.1016/0046-8177(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 34.Thike AA, Chng MJ, Fook-Chong S, Tan PH. Immunohistochemical expression of hormone receptors in invasive breast carcinoma: correlation of results of H-score with pathological parameters. Pathology. 2001;33:21–5. [PubMed] [Google Scholar]
- 35.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–202. [Google Scholar]
- 36.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, et al. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–81. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 37.Yang GS, Wang Y, Wang P, Chen ZD. Expression of oestrogen receptor-alpha and oestrogen receptor-beta in prostate cancer. Chin Med J (Engl) 2007;120:1611–5. [PubMed] [Google Scholar]
- 38.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, et al. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5. [PubMed] [Google Scholar]
- 40.Latil A, Bièche I, Vidaud D, Lidereau R, Berthon P, et al. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61:1919–26. [PubMed] [Google Scholar]
- 41.Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate. 2003;54:79–87. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 42.Emtage LA, Dunn PJ, Rowse AD. Androgen and oestrogen receptor status in benign and neoplastic prostate disease. Study of prevalence and influence on time to progression and survival in prostate cancer treated by hormone manipulation. Br J Urol. 1989;63:627–33. doi: 10.1111/j.1464-410x.1989.tb05259.x. [DOI] [PubMed] [Google Scholar]
- 43.Klein EA. Opportunities for prevention of prostate cancer: genetics, chemoprevention, and dietary intervention. Rev Urol. 2002;4(Suppl 5):S18–28. [PMC free article] [PubMed] [Google Scholar]
- 44.Hempstock J, Kavanagh JP, George NJ. Growth inhibition of prostate cell lines in vitro by phyto-oestrogens. Br J Urol. 1998;82:560–3. doi: 10.1046/j.1464-410x.1998.00769.x. [DOI] [PubMed] [Google Scholar]
- 45.Cohen DA, Dabbs DJ, Cooper KL, Amin M, Jones TE, et al. Interobserver agreement among pathologists for semiquantitative hormone receptor scoring in breast carcinoma. Am J Clin Pathol. 2012;138:796–802. doi: 10.1309/AJCP6DKRND5CKVDD. [DOI] [PubMed] [Google Scholar]
- 46.Gnanapragasam VJ, Robson CN, Leung HY, Neal DE. Androgen receptor signalling in the prostate. BJU Int. 2000;86:1001–13. doi: 10.1046/j.1464-410x.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 47.Sadi MV, Walsh PC, Barrack ER. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer. 1991;67:3057–64. doi: 10.1002/1097-0142(19910615)67:12<3057::aid-cncr2820671221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 48.Chodak GW, Kranc DM, Puy LA, Takeda H, Johnson K, et al. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J Urol. 1992;147:798–803. doi: 10.1016/s0022-5347(17)37389-5. [DOI] [PubMed] [Google Scholar]
- 49.Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, Verleun-Mooijman MC, Trapman J, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol. 1994;144:735–46. [PMC free article] [PubMed] [Google Scholar]
- 50.Lee DK, Chang C. Endocrine mechanisms of disease: expression and degradation of androgen receptor: mechanism and clinical implication. J Clin Endocrinol Metab. 2003;88:4043–54. doi: 10.1210/jc.2003-030261. [DOI] [PubMed] [Google Scholar]
- 51.Takeda H, Akakura K, Masai M, Akimoto S, Yatani R, et al. Androgen receptor content of prostate carcinoma cells estimated by immunohistochemistry is related to prognosis of patients with stage D2 prostate carcinoma. Cancer. 1996;77:934–40. [PubMed] [Google Scholar]
- 52.Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, et al. Androgen receptor status of lymph node metastases from prostate cancer. Prostate. 1996;28:129–35. doi: 10.1002/(SICI)1097-0045(199602)28:2<129::AID-PROS9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 53.Li R, Wheeler T, Dai H, Frolov A, Thompson T, et al. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–34. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 55.Dunsmuir WD, Gillett CE, Meyer LC, Young MP, Corbishley C, et al. Molecular markers for predicting prostate cancer stage and survival. BJU Int. 2000;86:869–78. doi: 10.1046/j.1464-410x.2000.00916.x. [DOI] [PubMed] [Google Scholar]
- 56.Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K, et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;87:1405–13. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh CC, Thanos A, Mitropoulos D, Deliveliotis C, Mantzoros CS, et al. Risk factors for prostate cancer: a case-control study in Greece. Int J Cancer. 1999;80:699–703. doi: 10.1002/(sici)1097-0215(19990301)80:5<699::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


