Abstract
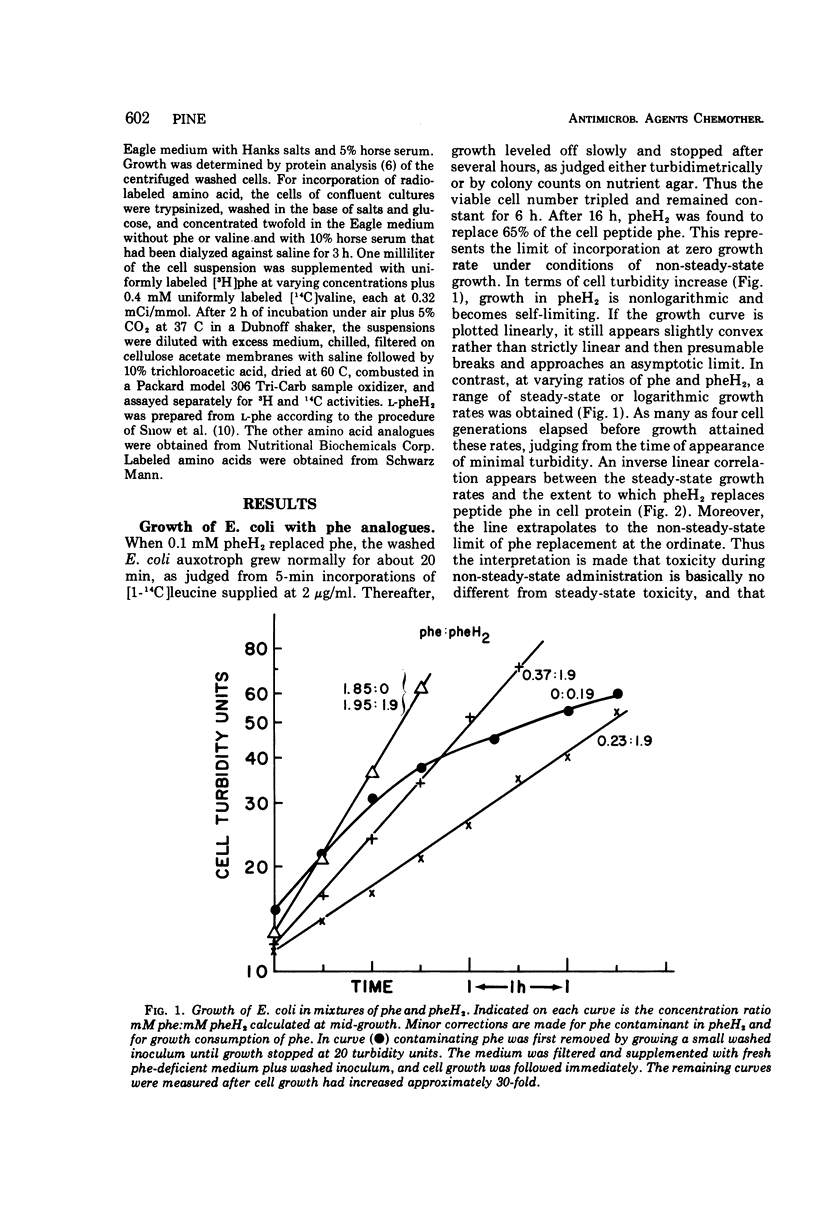
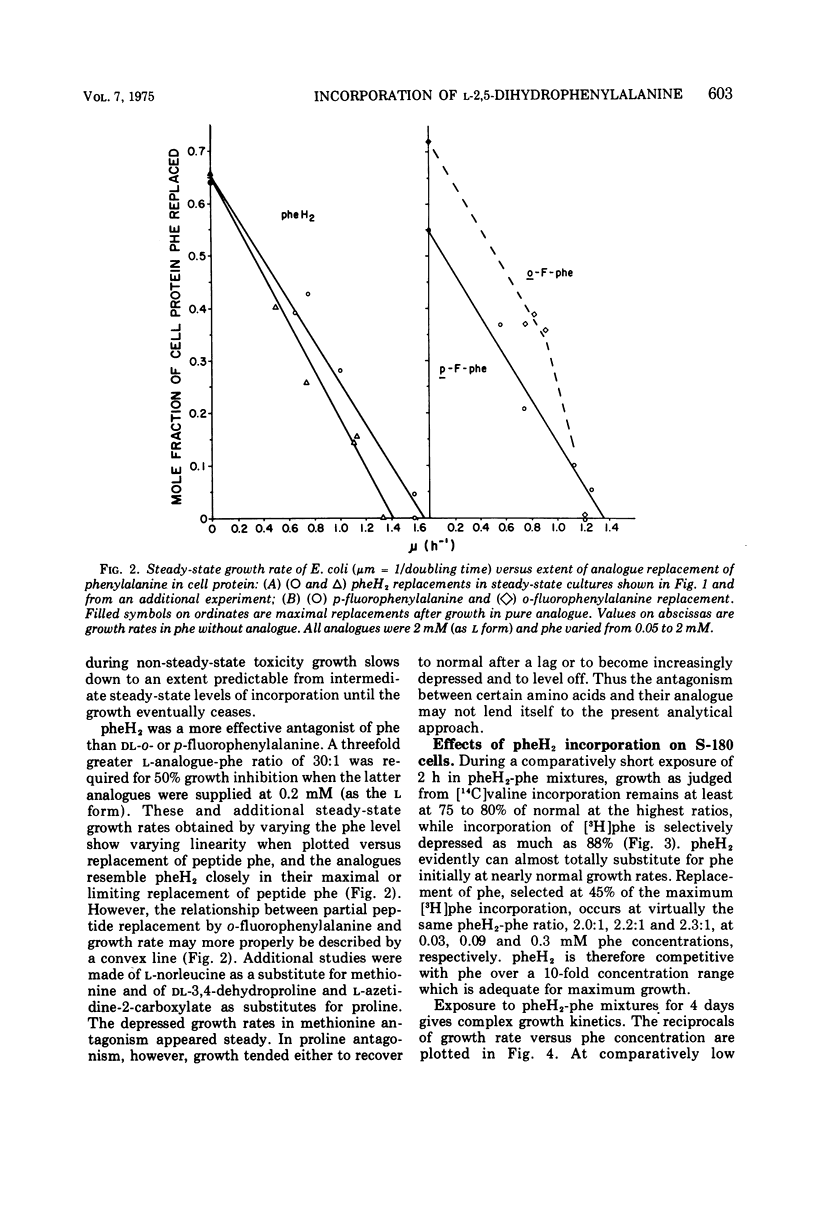
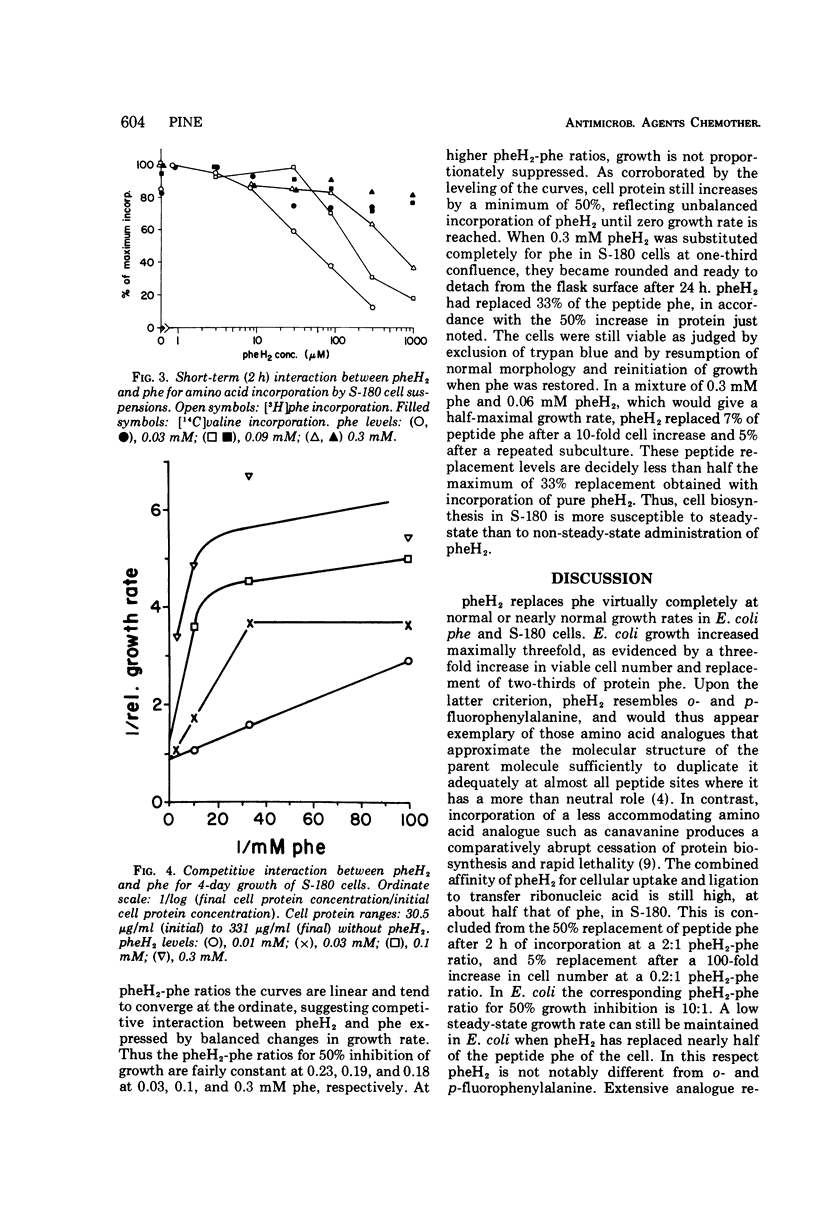
l-2,5-Dihydrophenylalanine is extensively incorporated as a phenylalanine analogue into cell proteins. Phenylalanine-requiring Escherichia coli ATCC 9723f and sarcoma 180 grow at normal rates initially with the analogue and maximally replace 65 and 33%, respectively, of phenylalanine in the peptide residues of their cell protein without death. With the analogue alone growth of E. coli becomes non-steady-state and asymptotically inhibited. In mixtures of the analogue and phenylalanine, growth eventually becomes steady state or logarithmic. The logarithmic rate is inversely proportional to the extent of incorporation of this analogue or of p-fluorophenylalanine, and the projected maximal replacement is the same as that obtained asymptotically with the analogue alone. Thus, the toxicities in steady-state and non-steady-state growth are closely related. Moreover, it is proposed that single salient protein defects may determine the extent of growth rate reduction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fickenscher U., Keller-Schierlein W., Zähner H. Stoffwechselprodukte von Mikroorganismen. 87. L-2,5-Dihydrophenylalanin. Arch Mikrobiol. 1971;75(4):346–352. [PubMed] [Google Scholar]
- Fickenscher U., Zähner H. Stoffwechselprodukte von Mikroorganismen. 88. Zur Wirkungsweise des L-2,5-Dihydrophenylalanins--eines Phenylalaninantagonisten. Arch Mikrobiol. 1971;76(1):28–46. [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- Genghof D. S. 2,5-dihydrophenylalanine as an inhibitor of microbial growth. Can J Microbiol. 1970 Jun;16(6):545–547. doi: 10.1139/m70-091. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Stringent control of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Dec;116(3):1253–1257. doi: 10.1128/jb.116.3.1253-1257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell J. P., Pruess D. L., Demny T. C., Williams T., Stempel A. L-3-(2,5-dihydrophenyl)alanine, an antimetabolite of L-phenylalanine produced by a streptomycete. Jpn J Antibiot. 1970 Dec;23(6):618–619. doi: 10.7164/antibiotics.23.618. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Anderson D. L., Rogers P. Mechanism of canavanine death in Escherichia coli. II. Membranes-bound canavanyl-protein and nuclear disruption. J Mol Biol. 1968 May 14;33(3):861–872. doi: 10.1016/0022-2836(68)90324-0. [DOI] [PubMed] [Google Scholar]
- Snow M. L., Lauinger C., Ressler C. 1,4-cyclohexadiene-1-alanine (2,5-dihydrophenylalanine) a new inhibitor of phenylalanine for the rat and Leuconostoc dextranicum 8086. J Org Chem. 1968 May;33(5):1774–1780. doi: 10.1021/jo01269a016. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Miyairi N., Kunugita K., Shimizu K., Sakai H. L-1,4-cyclohexadiene-1-alanine, an antagonist of phenylalanine from Streptomyces. J Antibiot (Tokyo) 1970 Nov;23(11):537–541. doi: 10.7164/antibiotics.23.537. [DOI] [PubMed] [Google Scholar]



