Abstract
Primary care practitioners play an important role in administering and advocating childhood vaccination to protect our children against infectious diseases and to ensure herd immunity in our population. Primary care practitioners may encounter children who present out-of-schedule, as well as children who come for vaccination with intercurrent illnesses, egg or other allergies, or are on long-term medications. This article describes the approach to these issues and present useful resources and references that primary care practitioners can access.
Keywords: immunisation, vaccination, vaccination schedule
Selvan's mother brings 15-month-old Selvan to your clinic to seek your advice regarding his childhood vaccination. He was born abroad and his medical records show that he has had the following vaccinations: (a) bacillus Calmette-Guérin (BCG) vaccine given at birth; (b) three doses each of diphtheria, pertussis and tetanus (DPT) vaccine and oral polio vaccine (OPV); (c) three doses of hepatitis B, given at birth, and at 1 and 6 months of age; and (d) one dose of measles, mumps and rubella (MMR) vaccine given at 11 months of age. Selvan's mother reports that he is recovering from a ‘flu’ and asks if he is fit for vaccination today.
WHAT IS CHILDHOOD VACCINATION?
The purpose of childhood vaccination is for active immunisation against infectious diseases,(1) as well as to ensure herd immunity(2) in the population. Most children in Singapore are vaccinated according to the National Immunisation Guidelines. In Singapore, the National Childhood Immunisation Programme(3,4) is based on the recommendations of the Expert Committee on Immunisation, which comprises senior officials from Ministry of Health (MOH) Singapore, consultant paediatricians and experts in communicable disease control.
HOW RELEVANT IS THIS TO MY PRACTICE?
Primary care practitioners play an important role in administering and advocating childhood vaccination. Dilemmas faced include children who present out-of-schedule, or with intercurrent illnesses or reports of prior allergic reactions, such as to egg. This article: (a) highlights important information that primary care practitioners should be aware of, such as vaccines that are legally mandated;(5) (b) discusses some basic principles of vaccine scheduling; (c) addresses issues encountered when vaccinating a child; and (d) presents updates in vaccinations, such as the recent changes in the measles vaccination schedule(6) and the switch from oral to injectable polio vaccine.(7)
COMMON CONSIDERATIONS WHEN ADMINISTERING CHILDHOOD VACCINATION
Ascertain which vaccines to give
Two vaccines are mandatory by law,(5,8) and most of the other vaccines are required by the Ministry of Education for Primary One registration in local schools.(9) The vaccines that are compulsory by law are diphtheria and measles(9) – diphtheria vaccination was made compulsory in 1977,(9) while measles vaccination was introduced in October 1976 and made compulsory in August 1985 for children aged 1–2 years old.(7) Table I shows the immunisation schedule of the two mandatory vaccinations under the Infectious Diseases Act.(8)
Table I.
Vaccines and vaccination schedules mandated by law in Singapore.(8)
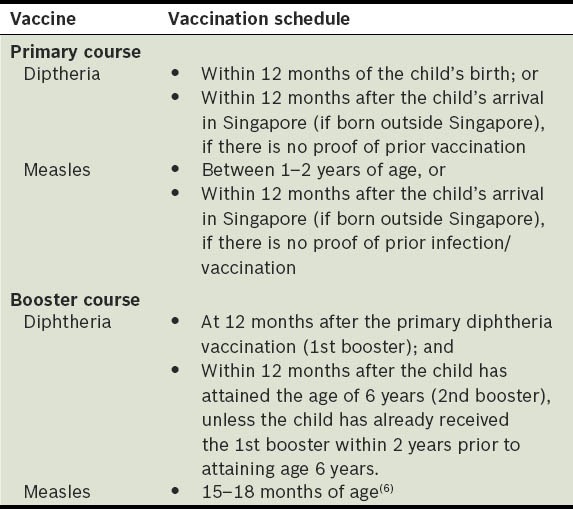
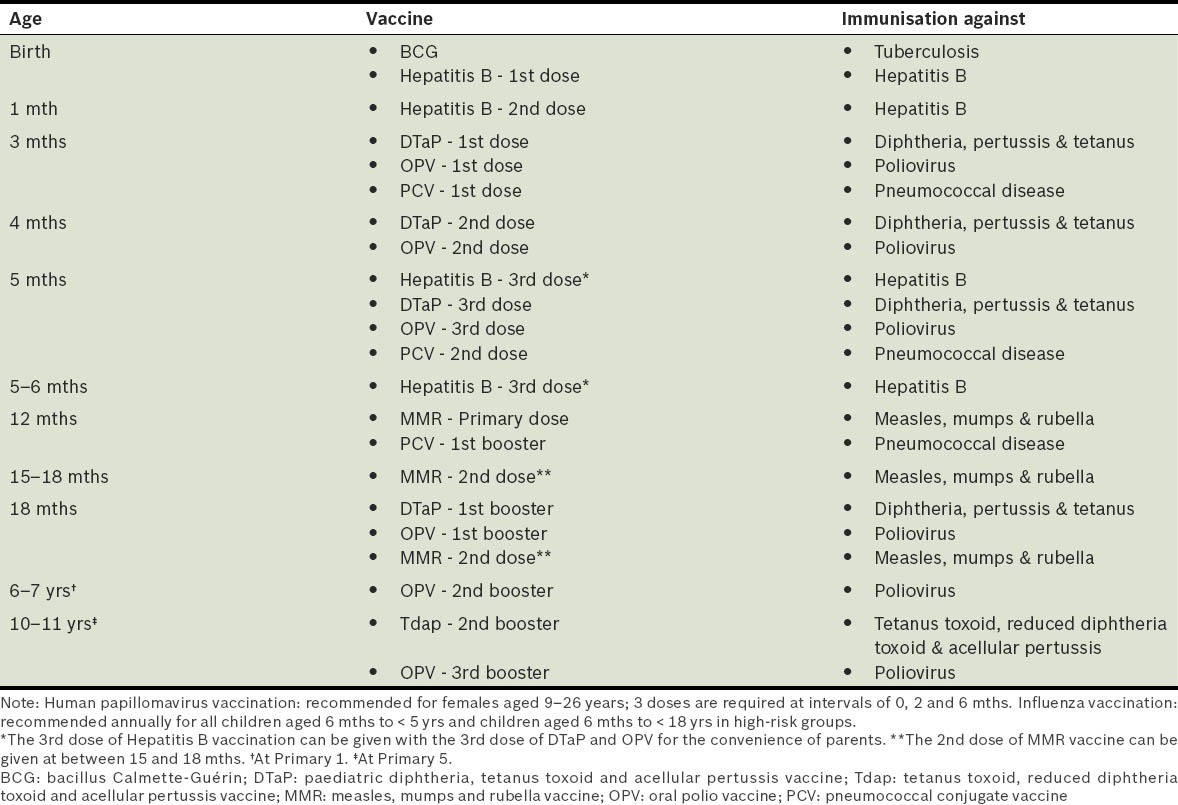
Vaccines that are required for Primary One registration include bacillus Calmette-Guérin (BCG); diphtheria, pertussis and tetanus (DPT); poliomyelitis; measles, mumps and rubella (MMR); and hepatitis B. All children (Singapore and non-Singapore citizens) should have completed the recommended immunisations(9) (Table II) before entry into Primary One. Parents are required to produce documentary evidence of their child's immunisation at the time of registration.(8) Parents of Singapore residents aged 18 years and below can also download their child's immunisation certificates from the National Immunisation Registry website (http://www.nir.hpb.gov.sg/nir/eservices/eservice.jsp).
Table II.
National Childhood Immunisation Schedule (revised in December 2011).(9)
Ascertain the validity of the vaccine doses given
When a child presents to the primary care practitioner after having had a few doses of vaccines, it is important to reconcile the vaccination history, ascertain validity of given doses, and complete the recommended vaccinations according to the national schedule.
The general principle behind recommended scheduling of vaccine doses is that the optimal response to a vaccine depends on the age of the child, maturity of the child's immune system and potential interference by passively transferred maternal antibodies. As such, any particular vaccine should be age-appropriate and should not be given before the minimum age stipulated for that vaccine.(10) Moreover, giving doses of vaccines at shorter than recommended intervals may lessen antibody response.(11) Therefore, vaccine doses should be spaced apart and not administered before the minimum interval stipulated. Information on the minimum ages and minimum intervals in between doses can be found in the Advisory Committee on Immunization Practices (ACIP) General Recommendations on Immunization.(12)
In other words, primary care practitioners should take into account the minimum age and minimum interval when ascertaining the validity of the vaccine doses given. In general, vaccine doses administered at ≤ 4 days before the minimum interval or age are considered valid, while doses of any vaccine administered at ≥ 5 days earlier than the minimum interval or age should not be considered valid doses, and should thus be repeated as appropriate. The interval between the repeat and invalid doses should be spaced according to the recommended minimum interval.(11) For example, as the minimum age for MMR vaccination is one year,(12) any MMR vaccine administered ≥ 5 days prior to the age one year should be considered invalid. Another example involves the minimum interval between the first and second doses of the diphtheria, tetanus and acellular pertussis (DTaP) vaccine, which is four weeks;(12) if the second dose is administered at three weeks after the first dose (≥ 5 days before the minimum interval of four weeks), the second dose is considered invalid and should be re-administered four weeks after the invalid one.
Live, attenuated vaccine, if not administered together at the same visit, should be spaced at least four weeks from any other similar vaccines.(13) For instance, if the first primary dose of MMR vaccine is inadvertently administered at age 11½ months, that dose is considered invalid. MMR vaccine should be re-administered when the child is aged 12½ months (spaced at least four weeks from the last received [invalid] dose).
Managing vaccination series that differ from the usual schedule
Ideally, a child residing in Singapore should follow the local immunisation schedule and recommendations, as national schedules take into account local disease epidemiology, as well as programmatic, resource and policy considerations.(14) As mentioned earlier, primary care practitioners will need to ascertain the validity of any given doses in order to decide on the next most appropriate vaccine according to our national schedule. General principles on minimum intervals and minimum ages will apply. Doses administered too early or too close together can lead to suboptimal immune response.
The following questions may be useful:
Which vaccinations are recommended for the child?
What were the previous doses given and are they valid? (The concept of minimum ages and minimum intervals apply.)
If several doses are due, can they be combined?
How do we strike a balance between rapid completion of necessary doses and coadministration of vaccines?
It is important to be mindful of the various sources of data that can be accessed to support your decision. While there are reliable guidelines, such as those from the ACIP and Center for Disease Control and Prevention, Singapore has its own recommendations, and thus the latest MOH circulars will supersede these other guidelines.
CHILD WITH INTERCURRENT ILLNESS
Mild febrile illnesses (e.g. upper respiratory tract infection, otitis media and mild diarrhoea) are not a contraindication to vaccination.(15) Vaccines can also be given if a child is in the recovery phase of any illness. Primary care practitioners should discuss the pros and cons of vaccinating a sick child and alternative options with the parents. If a child has a severe acute illness (e.g. a fever above 38ºC), vaccination with either live or inactivated vaccines should be delayed until the illness has improved. Fever may make the clinical course of any illness less clear (if the fever is due to the vaccine or part of the intercurrent illness) and potentially affect clinical judgement. A sick child may also be fussy and find it more difficult to tolerate any potential vaccine reactions.
CHILD ON MEDICATIONS
Current consumption of antibiotics has no effect on a child's response to inactivated vaccines, toxoids or live, attenuated vaccines, with the exception of live oral typhoid vaccine.(16) Antiviral drugs may affect vaccine replication in some circumstances, but these are not commonly prescribed for children. Antiviral drugs against herpes viruses (e.g. acyclovir) might reduce the efficacy of live, attenuated varicella and zoster vaccines. There is no existing data to suggest commonly used antiviral drugs have an effect on rotavirus vaccine or MMR vaccine.(16) Aerosolised steroids such as asthma preventers are not contraindications to vaccination, nor are short (< 14 days) courses of high-dose steroids given for asthma exacerbations. However, live virus vaccination should be deferred for at least one month after the discontinuation of high-dose systemic steroids given longer than 14 days.(17)
CHILD WITH MISSED OR DELAYED DOSES
It is not necessary to restart immunisation due to an extended interval between doses (e.g. due to missed appointments or defaults); for missed doses, the regime should be continued by simply administering the missed dose.(18)
Vaccination records from other countries may have some issues, such as the lack of a clear patient identifier, documentation in a foreign language, an unfamiliar system of coding or absence of a clear administration date. If there are any doubts about which (if any) immunisations have been given, the primary care practioner should discuss with the child's parents regarding the option to start a complete immunisation programme.
If the child has not had any immunisation and is aged ≥ 12 months, the first priority should be given to the administration of the MMR vaccine, with oral polio vaccine (OPV) and DPT vaccine given at the same time as the MMR vaccine (at separate injection sites) or at an interval of four weeks. Subsequent DPT vaccine and OPV can be administered at eight-week intervals until three doses of DPT vaccine and OPV are given.
Coadministration of vaccines
In most cases, different vaccines can be given simultaneously, but at different sites.(19) For infants and younger children, if more than two vaccines are to be administered in a single limb, the anterolateral thigh is preferred because of the greater muscle mass. The site of the injections should be adequately apart (≥ 1 inch, if possible) so as to differentiate any local reactions; the location of the injections should be clearly documented in electronic medical notes. If live vaccines are not administered during the same visit, they should be separated by an interval of four weeks or more. For a child with missed or delayed vaccination, it may be necessary to discuss with the parents the option to simultaneously administer several vaccines during the same clinic visit.
CHILD WITH ALLERGIES
It is important to ascertain the nature of the allergic reaction. Anaphylactic reactions (e.g. generalised urticaria [hives], angio-oedema [perioral/periorbital], wheezing, hypotension or shock) requiring medical attention typically occur within minutes or hours of receiving a vaccine, and will be listed as a contraindication to a subsequent dose of that vaccine.(20) Other forms of allergy, such as localised contact urticarial and allergic contact dermatitis, are not contraindications to the vaccine.
A child may be allergic to the vaccine antigen or a vaccine component such as animal protein, antibiotic, preservative or stabiliser. The most common animal protein allergen is egg protein (ovalbumin), which is found in some vaccines. Yellow fever and influenza vaccines are prepared using embryonated chicken eggs and should not be given to children with chicken-egg anaphylaxis. Asking parents whether their child has any adverse effects after consuming eggs is a reasonable way to screen for children who might be at risk when receiving yellow fever and influenza vaccines. Those with a history of anaphylactic reactions to egg or egg proteins should not be vaccinated.(21)
In the commercially available MMR vaccine, the measles and mumps component is derived from chick embryo culture, while the rubella component is derived from human diploid cell culture. Egg allergy has been listed as a false contraindication to MMR vaccine(15) in the January 2011 edition of the ACIP General Recommendations for Vaccination. The amount of ovalbumin in MMR vaccine is so small that it is highly unlikely that MMR vaccine would cause a serious allergic reaction in the majority of children.(22) The rare severe allergic reactions after MMR vaccination are not thought to be caused by ovalbumin but by other components of the vaccine (e.g. gelatin).(21) The only subgroups of children with egg allergy who require hospital supervision during MMR vaccination would be those with a history of anaphylactic reaction to eggs, and egg-allergic children who have coexisting asthma.(22) Non-asthmatic children with milder forms of allergy to eggs (e.g. localised urticaria) can be safely vaccinated without additional precautions.(22) Recommendations for MMR vaccination in children with prior history of egg allergy are summarised in Fig. 1. Chickenpox vaccine, derived from human diploid cell culture, does not contain ovalbumin, and can thus be administered to children with previous chicken-egg anaphylaxis.(21)
Fig. 1.
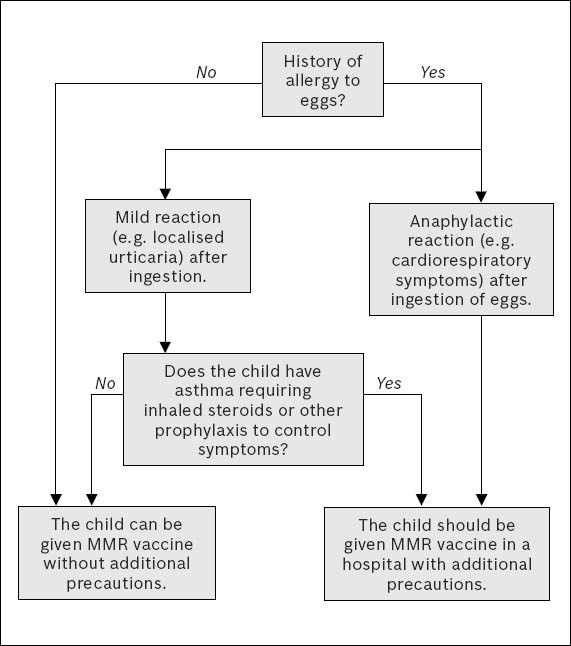
Algorithm for administering MMR vaccine in children who are allergic to eggs [adapted from Khakoo and Lack(22)].
Antibiotic allergy
Certain vaccines contain trace amounts of neomycin. Individuals who have experienced an anaphylactic reaction to neomycin should not receive these vaccines. A localised allergy reaction (e.g. contact dermatitis) experienced after the use of topical neomycin is not a contraindication to the administration of vaccines that contain neomycin. Penicillin allergy is not a contraindication to vaccination, as none of the currently licensed vaccines contain penicillin or any of its derivatives.(21)
Latex allergy
The most common type of latex sensitivity is contact dermatitis resulting from wearing latex-containing gloves. If a person reports a severe (anaphylactic) allergy to latex, vaccines supplied in vials or syringes that contain natural rubber should not be administered unless the benefit of vaccination clearly outweighs the risk of an allergic reaction to the vaccine. For latex allergies other than anaphylactic allergies (e.g. a history of contact allergy to latex gloves), vaccines supplied in vials or syringes that contain dry natural rubber or natural rubber latex can be administered.(23)
You check the documentation on the types of vaccines Selvan had received, and the dates on which he had received them, against the recommended minimum ages and intervals published by the ACIP. The dose of MMR vaccine received before the minimum age of 12 months is not valid and should be repeated. You update the child's National Immunisation Records accordingly.
Based on the clinical examination, you conclude that the child has a mild upper respiratory tract infection, but is otherwise healthy and thriving. You have identified the following vaccinations that need to be administered: a repeat dose of MMR vaccine, booster DPT, OPV, pneumococcal conjugate (optional) and varicella vaccines (optional). After your discussion with the child's mother regarding the pros and cons of coadministration of vaccines and intercurrent illness, it is agreed that only the repeat dose of MMR vaccine will be administered for this visit.
TAKE HOME MESSAGES
The local immunisation schedule is based on local disease epidemiology and may differ from other national immunisation schedules.
Diphtheria and measles vaccinations are compulsory by law under the Infectious Diseases Act in Singapore.
A vaccine is considered valid provided it has not been given before the minimum age and interval from the preceding dose (if applicable) recommended for that particular vaccine.
Live, attenuated vaccine, if not administered together at the same visit, should be spaced at least four weeks from any other live, attenuated vaccines.
Mild febrile illnesses are not contraindications to vaccination. The pros and cons of proceeding with vaccination should be discussed with and agreed upon by the parents.
Antibiotics and aerosolised steroids are not contraindications to vaccination. Live virus vaccination should be deferred for at least one month after the discontinuation of high-dose systemic steroids that have been consumed for more than 14 days.
Yellow fever and influenza vaccines are prepared using embryonated chicken eggs and should not be given to children with chicken-egg anaphylaxis.
REFERENCES
- 1.Atkinson W, Wolfe S, Hamborsky J, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th ed. Washington: Public Health Foundation; 2012. [Accessed January 6, 2014]. Centers for Disease Control and Prevention. Principles of vaccination. online. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/prinvac.html. [Google Scholar]
- 2.Fine PEM, Mulholland K. Community immunity. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Philadelphia: Elsevier; 2012. pp. 1395–412. [Google Scholar]
- 3.Health Promotion Board Singapore. National Childhood Immunization Programme. [online] [Accessed January 6, 2014]. Available at: http://www.hpb.gov.sg/HOPPortal/programmes-article/630.
- 4.Ministry of Health, Singapore. National Childhood Immunisation Schedule [online] [Accessed January 6, 2014]. Available at: http://www.moh.gov.sg/content/moh_web/home/diseases_and_conditions/i.html.
- 5.Ministry of Health Singapore. Infectious Diseases Act [online] [Accessed January 6, 2014]. Available at: http://www.moh.gov.sg/content/moh_web/home/legislation/legislation_and_guidelines/infectious_diseasesact.html .
- 6.Ministry of Health, Singapore. Changes in National Immunisation Schedule. MOH Circular September 2011 MH 34:55/2 [Google Scholar]
- 7.Ministry of Health, Singapore. Changes in National Immunisation Schedule. MOH Circular May 2013 MH 34:09/1 V16 [Google Scholar]
- 8.Attorney General's Chambers. Infectious Diseases (Diphtheria and Measles Vaccinations) Regulations. Infectious Diseases Act. [Accessed January 6, 2014]. online. Available at: http://statutes.agc.gov.sg/aol/search/display/view.w3p;page=0;query=Id%3A%22d4b595c6-c1d1-4112-95d6-4e0ce2660f1f%22%20Status%3Ainforce;rec=0;whole=yes .
- 9.Ministry of Education Singapore. Required Documents for Primary One Registration Exercise [online] [Accessed January 6, 2014]. Available at: http://www.moe.gov.sg/education/admissions/primary-one-registration/required-documents/#immunisation-cert.
- 10.Health Promotion Board Singapore. Immunisation Chart Based on Age [online] [Accessed January 6, 2014]. Available at: http://www.hpb.gov.sg/HOPPortal/gamesandtools-article/3216.
- 11.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Timing and spacing of immunobiologics. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No. 2):4–6. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) Table 1. Recommended Minimum ages and Intervals between vaccine doses. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No. 2):36–7. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) Table 3. Guidelines for spacing of live and inactivated antigens. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No.2):38. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf. [Google Scholar]
- 14.World Health Organization. Footnote, Table 2 - Summary of WHO Position Papers – Recommended routine immunization for children. (updated 1 August 2013) WHO Recommendations for Routine Immunization [online] [Accessed January 6, 2014]. Available at: http://www.who.int/immunization/policy/Immunization_routine_table2.pdf .
- 15.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Table 7. Conditions commonly misperceived as contraindications to vaccination. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No.2):42–3. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Concurrent administration of antimicrobial agents and vaccines. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No.2):23. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf . [Google Scholar]
- 17.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Conditions or drugs that might cause immunodeficiencies:corticosteroids. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No. 2):22. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf. [Google Scholar]
- 18.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Lapsed vaccination schedule. [Accessed January 6 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No. 2):10. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Simultaneous administration. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No. 2):6. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf . [Google Scholar]
- 20.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Managing acute vaccine reactions. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No. 2):12. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Severe allergy to vaccine components. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No. 2):24. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf . [Google Scholar]
- 22.Khakoo GA, Lack G. Recommendations for using MMR vaccine in children allergic to eggs. BMJ. 2000;320:929–32. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Latex allergy. [Accessed January 6, 2014];General Recommendations on Immunization. Morbidity and Mortality Weekly Report. 2011 Jan 28;60(No. 2):25. online. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf . [Google Scholar]


