Abstract
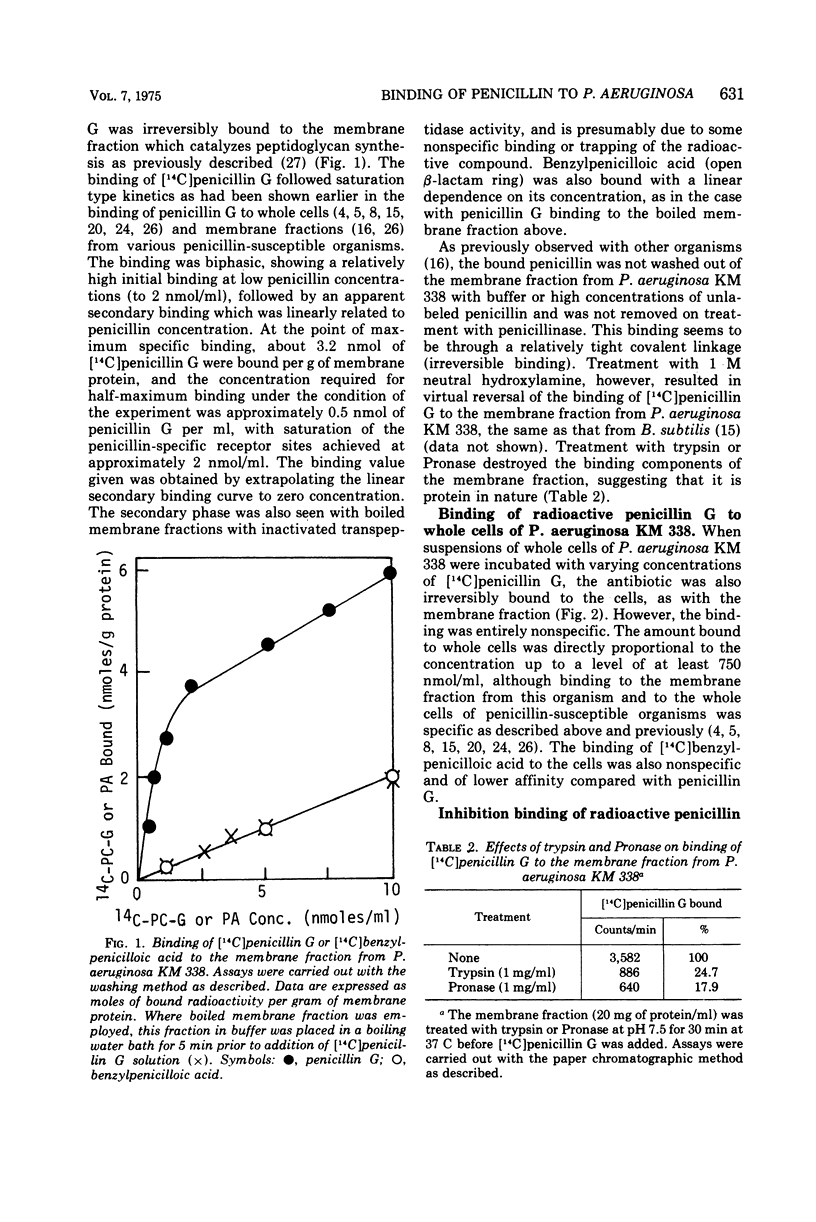
A comparison of the binding of radioactive penicillin G to whole cells and the membrane fraction derived from Pseudomonas aeruginosa KM 338 was made. This organism has intrinsic resistance to penicillin. The binding to the membrane fraction which catalyzed peptidoglycan synthesis followed saturation type kinetics and saturation was achieved at approximately 2 nmol of penicillin G per ml, whereas binding to the whole cells was entirely of the nonsaturation type. The binding of carbenicillin to the membrane fraction was determined by competition between radioactive penicillin G and unlabeled carbenicillin for the binding sites. It was bound at the same sites in almost the same manner. When whole cells were pretreated with high concentration of unlabeled penicillin G or carbenicillin, the subsequent binding of radioactive penicillin G to the membrane fraction from carbenicillin-treated cells was entirely nonspecific, but with penicillin G-pretreated cells it was still specific. There was apparently specific binding of radioactive penicillin G to ethylenediaminetetraacetate-treated cells. P. aeruginosa KM 338 had an extremely low activity of β-lactamase compared with other enzyme-producing organisms. This enzyme from P. aeruginosa KM 338 was of the cephalosporinase type. These data indicate that penicillin resistance of P. aeruginosa KM 338 may be a consequence of the development of a permeability barrier which prevents the antibiotic from reaching its sites of action in the cytoplasmic membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E., Asscher A. W. Action of ethylenediaminetetra-acetic acid (EDTA) on carbenicillin-resistant strains of Pseudomonas aeruginosa. J Med Microbiol. 1972 Aug;5(3):355–359. doi: 10.1099/00222615-5-3-355. [DOI] [PubMed] [Google Scholar]
- COOPER P. D. Site of action of radiopenicillin. Bacteriol Rev. 1956 Mar;20(1):28–48. doi: 10.1128/br.20.1.28-48.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N., Pollock M. R. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol. 1966;28:237–323. doi: 10.1002/9780470122730.ch4. [DOI] [PubMed] [Google Scholar]
- DUERKSEN J. D. LOCALIZATION OF THE SITE OF FIXATION OF THE INDUCER, PENICILLIN, IN BACILLUS CEREUS. Biochim Biophys Acta. 1964 May 18;87:123–140. doi: 10.1016/0926-6550(64)90053-2. [DOI] [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- Eagon R. G. Cell wall-associated inorganic substances from Pseudomonas aeruginosa. Can J Microbiol. 1969 Feb;15(2):235–236. doi: 10.1139/m69-039. [DOI] [PubMed] [Google Scholar]
- Edwards J. R., Park J. T. Correlation between growth inhibition and the binding of various penicillins and cephalosporins to Staphylococcus aureus. J Bacteriol. 1969 Aug;99(2):459–462. doi: 10.1128/jb.99.2.459-462.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullbrook P. D., Elson S. W., Slocombe B. R-factor mediated beta-lactamase in Pseudomonas aeruginosa. Nature. 1970 Jun 13;226(5250):1054–1056. doi: 10.1038/2261054a0. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., LIGHTBOWN J. W. A general theory for plate assay of antibiotics with some practical applications. J Gen Microbiol. 1952 Aug;7(1-2):129–143. doi: 10.1099/00221287-7-1-2-129. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Effect of EDTA upon bacterial permeability to benzylpenicillin. Biochem Biophys Res Commun. 1965 Sep 22;20(6):688–691. doi: 10.1016/0006-291x(65)90070-7. [DOI] [PubMed] [Google Scholar]
- Heilmann H. D. On the peptidoglycan of the cell walls of Pseudomonas aeruginosa. Eur J Biochem. 1972 Dec 18;31(3):456–463. doi: 10.1111/j.1432-1033.1972.tb02552.x. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Lawrence P. J., Rogolsky M., Hanh V. T. Binding of radioactive benzylpenicillin to sporulating Bacillus cultures: chemistry and fluctuations in specific binding capacity. J Bacteriol. 1971 Nov;108(2):662–667. doi: 10.1128/jb.108.2.662-667.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XV. The binding of radioactive penicillin to the particulate enzyme preparation of Bacillus subtilis and its reversal with hydroxylamine or thiols. J Biol Chem. 1970 Jul 25;245(14):3653–3659. [PubMed] [Google Scholar]
- Lawrence P. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XVI. The reversible fixation of radioactive penicillin G to the D-alanine carboxypeptidase of Bacillus subtilis. J Biol Chem. 1970 Jul 25;245(14):3660–3666. [PubMed] [Google Scholar]
- Martin H. H., Heilmann H. D., Preusser H. J. State of the rigid-layer in celll walls of some gram-negative Bacteria. Arch Mikrobiol. 1972;83(4):332–346. doi: 10.1007/BF00425246. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. The inhibition of mucopeptide synthesis by benzylpenicillin in relation to irreversible fixation of the antibiotic by staphylococci. Biochem J. 1967 Apr;103(1):90–102. doi: 10.1042/bj1030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R., Plapp R. Binding of 14 C-penicillin G to Proteus mirabilis. Arch Mikrobiol. 1972;83(3):246–260. doi: 10.1007/BF00645125. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Blumberg P. M., Suginaka H., Umbreit J., Wickus G. G. How penicillin kills bacteria: progress and problems. Proc R Soc Lond B Biol Sci. 1971 Dec 31;179(1057):369–383. doi: 10.1098/rspb.1971.0103. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Blumberg P. M., Strominger J. L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5279–5288. [PubMed] [Google Scholar]
- Suginaka H., Ichikawa A., Kotani S. Penicillin-resistant mechanisms in Pseudomonas aeruginosa: effects of penicillin G and carbenicillin on transpeptidase and C -alanine carboxypeptidase activities. Antimicrob Agents Chemother. 1974 Dec;6(6):672–675. doi: 10.1128/aac.6.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Richmond M. H. R factors, beta-lactamase, and carbenicillin-resistant Pseudomonas aeruginosa. Lancet. 1971 Aug 14;2(7720):342–344. doi: 10.1016/s0140-6736(71)90060-2. [DOI] [PubMed] [Google Scholar]
- Weiser R., Asscher A. W., Wimpenny J. In vitro reversal of antibiotic resistance by ethylenediamine tetraacetic acid. Nature. 1968 Sep 28;219(5161):1365–1366. doi: 10.1038/2191365a0. [DOI] [PubMed] [Google Scholar]



