Abstract
INTRODUCTION
Oxidative stress, assessed using 8-hydroxy-2’-deoxyguanosine (8-OHdG), can be associated with arterial stiffness in patients with type 2 diabetes mellitus (T2DM) and/or hypertension (HT). We investigated the correlation between urinary 8-OHdG and pulse wave velocity (PWV) in hypertensive and non-hypertensive T2DM patients with fair glycaemic control to determine the clinical significance of HT as a comorbidity in the diabetic state.
METHODS
Clinical data, including traditional cardiovascular risk factors, diabetic complications, prescribed agents, urinary 8-OHdG level and brachial-ankle PWV, was collected from T2DM patients with and without HT.
RESULTS
There were 76 patients (45 men, 31 women; mean age 61 years; mean haemoglobin A1c level 6.5%) in the study cohort. T2DM patients with HT had significantly higher mean PWV than patients without HT (1,597 cm/s vs 1,442 cm/s; p < 0.05). Patients with HT showed no significant difference in 8-OHdG levels relative to those without HT (median 7.9 ng/mg creatinine vs 8.8 ng/mg creatinine; p > 0.05). Simple linear correlation and stepwise multiple linear regression analyses revealed that 8-OHdG levels correlated independently, significantly and positively with PWV among T2DM patients with HT (r = 0.33, p < 0.05; β = 0.23, p < 0.05). No significant correlation was observed between 8-OHdG levels and PWV among T2DM patients without HT.
CONCLUSION
In the hypertensive state, oxidative stress can be responsible for the development of arterial stiffness, even in patients with fairly well controlled T2DM. Oxidative stress management may be necessary for the prevention of cardiovascular disease in this population.
Keywords: arterial stiffness, atherosclerosis, DNA damage, oxidative stress, pulse wave velocity
INTRODUCTION
The Asia-Pacific region is facing an epidemic of type 2 diabetes mellitus (T2DM), paralleled by high rates of cardiovascular disease (CVD).(1,2) Hypertension (HT) remains a major risk factor for CVD in the region.(3) Further compounding matters, studies report that hyperglycaemia, increased blood pressure and oxidative stress can individually and/or concomitantly cause vascular damage, such as increased arterial stiffness.(4-6) The degree of arterial stiffness is often estimated using modalities such as pulse wave velocity (PWV); in fact, PWV is currently being used as a surrogate index for future CVD outcome.(5)
On DNA oxidation, a hydroxyl group is added to the guanine molecule to form 8-hydroxyguanine, which on oxidative modification yields the product, 8-hydroxy-2’-deoxyguanosine (8-OHdG).(7,8) Hyperglycaemia promotes glucose oxidation and protein glycation, impairs DNA repair with resultant DNA cleavage, and generates reactive oxygen species, thereby leading to increased oxidative stress.(4,7,8) Similarly, HT is accompanied by the formation of advanced glycation end products, and thereby increased oxidative stress and DNA damage.(9,10) For these reasons, the measurement of 8-OHdG has been used by various researchers to evaluate the DNA oxidation associated with the pathophysiology of T2DM and HT.(4,11-16)
A study by Nishikawa et al found that urinary 8-OHdG levels were increased in T2DM patients with poor glycaemic control,(4) while a study by Espinosa et al reported that urinary 8-OHdG levels were increased in patients with HT.(13) However, Roselló-Lletí et al demonstrated that patients with HT and DM do not exhibit any differences with regard to 8-OHdG levels when compared to patients with HT but no DM.(14) Espinosa et al also reported that 8-OHdG levels were reduced following antihypertensive treatment in patients with HT.(13) Studies suggest lowered urinary 8-OHdG levels upon intervention with antidiabetic agents in T2DM patients,(15) or antihypertensive and antidiabetic agents in patients with T2DM and HT.(16) Such changes in 8-OHdG levels are positively correlated with readings taken on the cardio-ankle vascular index, which is also an arterial stiffness index (although it is different from PWV both in principle and clinical feature).(15,16)
While the occurrence of arterial stiffness and CVD have been reported even in non-severe hyperglycaemic states,(17) Barengo and Tuomilehto reported that the presence of HT as a comorbidity in the hyperglycaemic state increased CVD risk in patients with DM.(18) These findings suggest that, aside from glycaemic control, HT and oxidative stress could also contribute to the development of T2DM-related CVD. Although the study by Roselló-Lletí et al found that the co-existence of HT and DM did not always enhance urinary 8-OHdG levels, the arterial stiffness of the patients was not evaluated by these authors.(14) Masugata et al, on the other hand, found a positive correlation between urinary 8-OHdG and the cardio-ankle vascular index in patients with HT.(19) However, this study did not compare findings in patients with HT against patients without HT or patients with DM.
To the best of our knowledge, the association between urinary 8-OHdG and PWV in T2DM patients with fair glycaemic control in the presence or absence of comorbid HT has not yet been fully documented in the literature. The present study was thus designed to address this lacuna and determine whether a relationship exists between 8-OHdG levels and brachial-ankle PWV readings (which is an often-used modality in the clinical setting(20)) in patients with fairly well controlled T2DM and comorbid HT.
METHODS
The study cohort included 76 patients with T2DM (45 men, 31 women; age range 35–81 years). Patients who had achieved fair glycaemic control (based on haemoglobin A1c [HbA1c] levels of 8.0%, as previously described by Oomichi et al(21)) with diet and/or antidiabetic agent therapy were included. Patients with a history of cardiovascular or cerebrovascular disorders, thyroid disorders, haematological disturbances, and collagen, severe hepatic or renal disease were excluded. The study was approved by the ethics committee of Jichi Medical University, Tochigi, Japan. Informed consent was obtained from all patients enrolled in the study.
Current smoking habits were ascertained via interviews. Information on prescribed agents (e.g. antidiabetic, anti-hypertensive and/or lipid-lowering agents) was obtained from the medical records. Clinical measurements were obtained after overnight fasting. Body mass index (BMI) was calculated based on the weight and height measurements taken when patients were barefoot and wearing light clothing. Blood pressure (BP) was determined in the patient’s right arm using a mercury sphygmomanometer with the patient in a seated position. Patients with systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg and/or those consuming antihypertensive agents were deemed to have HT.(22)
Mean BP (MBP) was calculated using the following equation: MBP = diastolic BP + (systolic BP − diastolic BP)/3. Serum total cholesterol, high-density lipoprotein-cholesterol (HDL-C), triglycerides, creatinine and plasma glucose concentrations were enzymatically measured. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease Study Group formulae;(23) eGFR (men) = 0.741 × 175 × age−0.203 × serum creatinine−1.154 and eGFR (women) = 0.741 × 175 × age−0.203 × serum creatinine−1.154 × 0.742. HbA1c levels were determined using high-performance liquid chromatography. Urinary 8-OHdG level was measured using enzyme-linked immunosorbent assays (Japan Institute for the Control of Aging, Shizuoka, Japan), with interassay and intra-assay coefficients of variation fixed at 2.1% and 4.5%, respectively.(24,25) Urinary samples were collected as spot urine from each patient in the morning before breakfast (7:30–10:00 am; around the same time as blood collection). The collected urinary samples were centrifuged at 800 g for 10 mins and the supernatant was used for assaying. 8-OHdG measurements were expressed in terms of its ratio to creatinine in the same sample (i.e. /mg creatinine). Urinary albumin was measured using the turbidimetric immunoassay method (Wako Pure Chemical Industries Ltd, Osaka, Japan). Diabetic nephropathy was defined in terms of the ratio of albumin to creatinine in the same sample (i.e. /mg creatinine), as albumin/creatinine ratio (ACR) ≥ 30 µg/mg creatinine.(26)
Brachial-ankle PWV measurements were taken using a volume-plethysmographic apparatus (Form/ABI, Colin Medical Technology Co Ltd, Komaki, Japan), with interobserver and intraobserver coefficients of variation fixed at 8.4% and 10.0%, respectively.(20) The cuffs were wrapped around both brachia and ankles, with patients resting in the supine position. Measurements were obtained in a single (one-time) reading following a 5-min rest. The pulse volume waveforms at the brachium and ankle were recorded using a semiconductor pressure sensor. Additionally, the presence of diabetic retinopathy – defined as nonproliferative and/or proliferative retinopathy – was determined by ophthalmoscopic examination.(27)
Data was expressed as mean ± standard deviation or median (interquartile range). Differences in data between groups were examined using the unpaired t-test or chi-square test. A simple linear correlation test (Pearson’s test) and stepwise multiple linear regression analysis were used to observe the correlation between PWV and other measured variables, including 8-OHdG. Stepwise multiple linear regression analysis was used to evaluate PWV with respect to the following variables: age, gender, current smoking habit, duration of DM, BMI, MBP, total cholesterol, HDL-C, triglycerides, HbA1c, creatinine, 8-OHdG, eGFR, nephropathy, retinopathy, and the use of antidiabetic, antihypertensive or lipid-lowering agents. Plasma glucose and ACR were not included as variables for multiple regression due to the colineality and biological nature of HbA1c and nephropathy, respectively. The duration of DM, triglycerides, 8-OHdG and ACR values were log-transformed for multiple linear regression analysis due to their skewed distributions. Statistical significance was defined at a p-value of < 0.05.
RESULTS
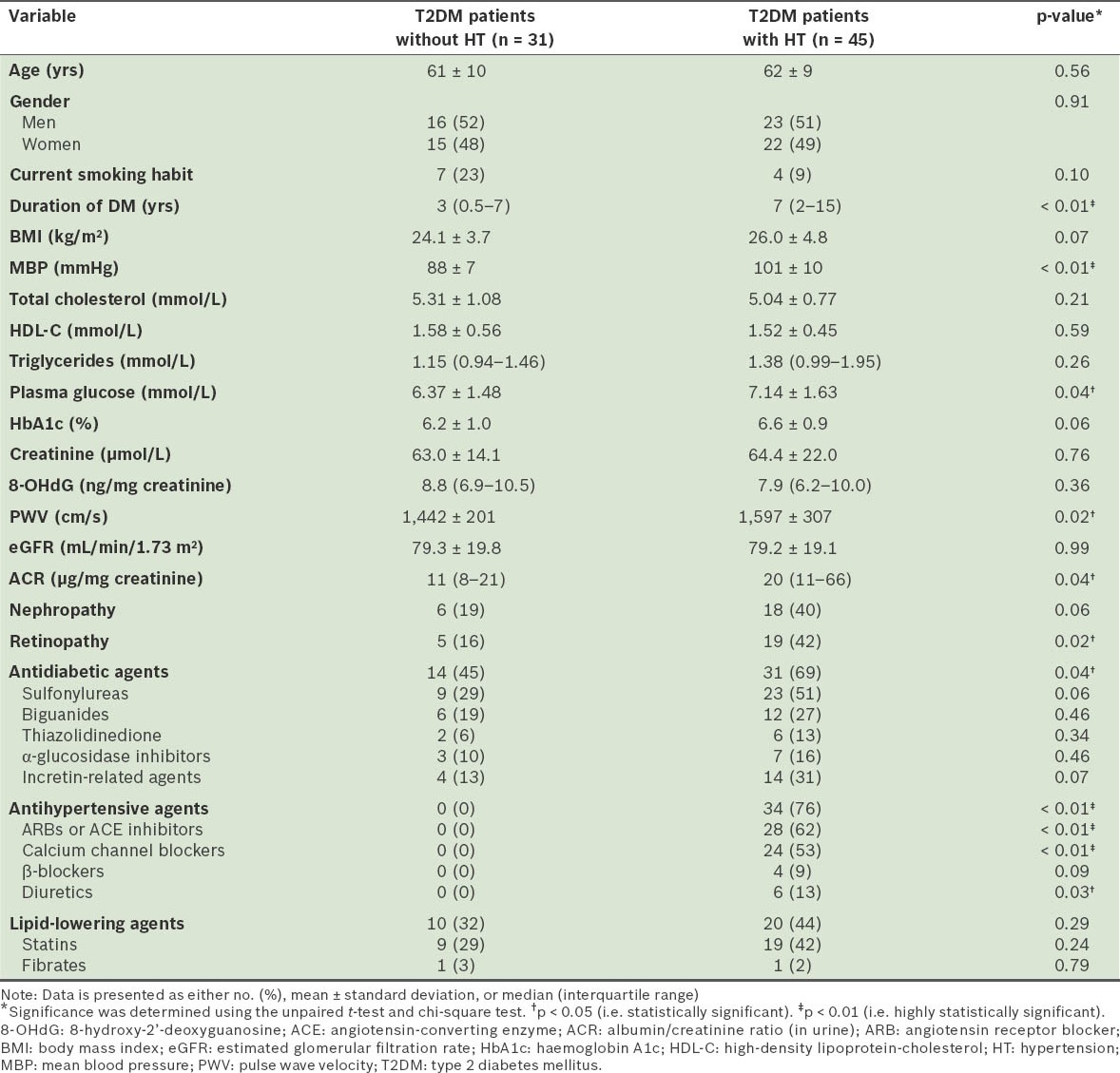
Table I presents the demographic and clinical characteristics of the study population. T2DM patients with HT had a significantly longer duration of DM (p < 0.01); higher levels of MBP (p < 0.01), plasma glucose (p = 0.04), PWV (p = 0.02) and ACR (p = 0.04); a higher prevalence of retinopathy (p = 0.02); and a higher use of antidiabetic (p = 0.04) and antihypertensive agents (all types of agents except β-blockers) (p < 0.01), when compared to T2DM patients without HT. However, there was no significant difference in the 8-OHdG levels of T2DM patients with and without HT.
Table I.
Demographic and clinical characteristics of patients with type 2 diabetes mellitus (T2DM).
In our cohort, T2DM patients with nephropathy showed higher levels of 8-OHdG (8.6 [6.4–10.9] ng/mg creatinine) and PWV (1,549 ± 207 cm/s) when compared to T2DM patients without nephropathy (8-OHdG 8.3 [5.9–10.4] ng/mg creatinine, p = 0.55; PWV 1,527 ± 307 cm/s, p = 0.75), although the difference was not statistically significant. Similarly, the level of 8-OHdG in T2DM patients with retinopathy (7.9 [5.5–11.4] ng/mg creatinine) was not significantly different from that in T2DM patients without retinopathy (8.8 [6.5–10.2] ng/mg creatinine) (p = 0.62). However, T2DM patients with retinopathy showed a significantly higher level of PWV (1,640 ± 305 cm/s) than those without retinopathy (1,485 ± 253 cm/s) (p = 0.03). The 8-OHdG levels of T2DM patients with and without HT were also compared to determine whether there was any difference between patients who were treated with antidiabetic, antihypertensive or lipid-lowering agents and those that were not. No significant differences were found in the 8-OHdG levels of these patients (data not shown).
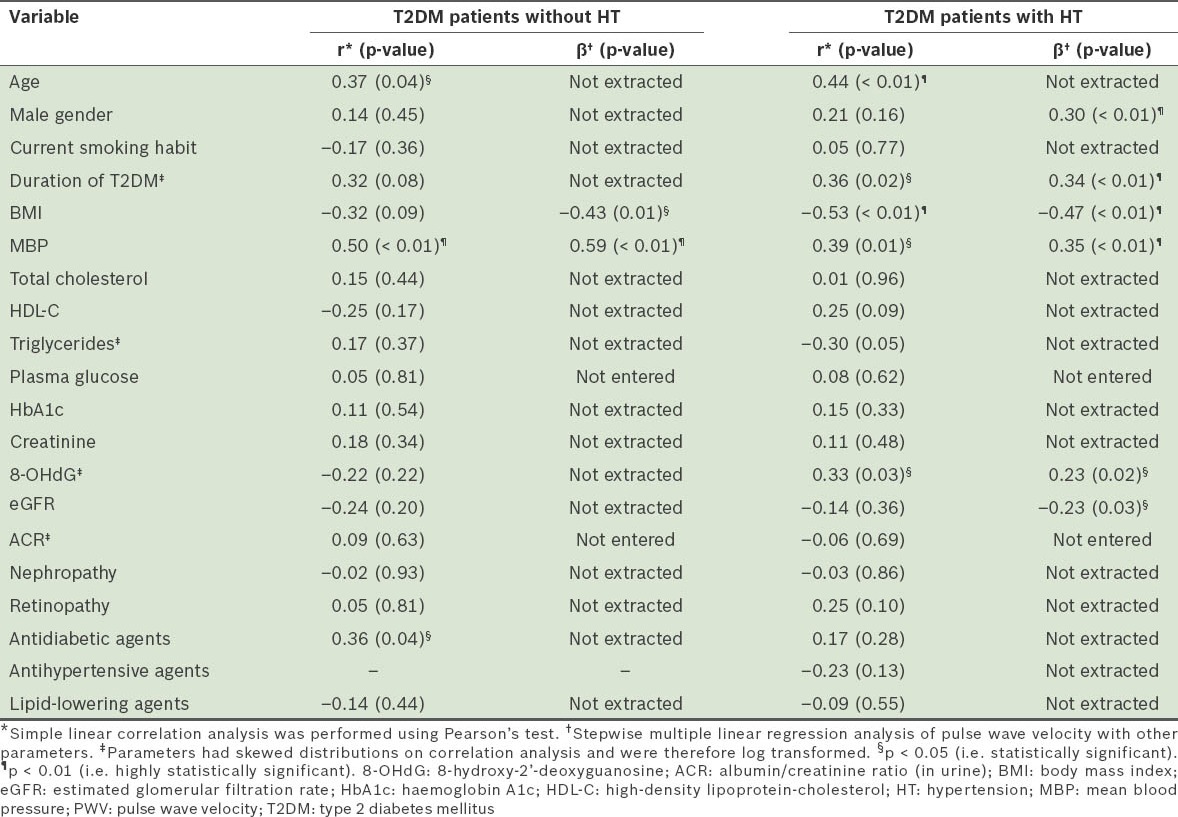
Results of the correlation analysis of PWV with other variables, including 8-OHdG levels, are presented in Table II. Among the T2DM patients without HT, simple linear correlation analysis revealed that there was a significant positive correlation between PWV and age, MBP and the use of antidiabetic agents. Subsequent stepwise multiple linear regression analysis identified BMI (inversely) and MBP (positively) as variables that were independently and significantly correlated with PWV. On the other hand, among the T2DM patients with HT, simple linear correlation analysis revealed a significantly inverse correlation between PWV and BMI, and significantly positive correlations between PWV and age, duration of DM, MBP and 8-OHdG levels. Subsequent stepwise multiple linear regression analysis identified BMI (inversely), eGFR (inversely), male gender (positively), duration of DM (positively), MBP (positively) and 8-OHdG (positively) as variables that were independently and significantly correlated with PWV.
Table II.
Correlation analysis of pulse wave velocity with other measured variables.
DISCUSSION
We found an independent, significant and positive correlation between the level of urinary 8-OHdG (an oxidative stress marker) and PWV (an arterial stiffness index) in patients with fairly well-controlled T2DM and HT, but not in T2DM patients without HT. Our data indicates that, under the hypertensive state, oxidative stress may be responsible for arterial stiffness even in T2DM patients with fair glycaemic control. Clinicians should therefore be aware that there might be a need for oxidative stress management in this patient population to prevent CVD. The addition of specific anti-hypertensive medications suggested to have antioxidative effects,(16) as well as the inclusion of nonpharmaceutical anti-oxidant therapy (e.g. exercise, vitamin supplementation, and a diet rich in fruits and vegetables)(28-31) may be included in the treatment regimen of these patients.
Several studies have investigated the association between PWV and oxidative stress-related markers.(32-35) For instance, a positive correlation has been reported between 8-iso(epi)-prostaglandin F2α (an F2-isoprostane) and PWV,(32-34) although the markers and patient populations in these earlier studies differed from those used in the present study. Similarly, in a study that evaluated serum 8-OHdG levels in patients with chronic kidney disease, Dalfino et al reported a positive correlation between PWV and 8-OHdG levels.(35) In the present study, although we did not correlate PWV with the same variables as those selected by earlier authors,(32-35) our results were consistent with their inferences. In effect, our findings broaden the current understanding of 8-OHdG and its correlation with PWV to a population of T2DM patients with HT.
We found that 8-OHdG levels were not clearly different between T2DM patients with HT and those without, unlike a previous study by Espinosa et al, which had reported higher 8-OHdG levels in patients with HT than in patients without HT.(13) The 8-OHdG levels of our study cohort were not higher than those reported in healthy people by Kato et al.(36) Also, the 8-OHdG levels observed in our patients were similar to Miyashita et al’s findings on T2DM patients with HT.(16) Nonetheless, further and more extensive studies will be necessary to determine the reason(s) behind the differences in 8-OHdG levels that have been observed by the many studies in the literature.
We found a significant correlation between 8-OHdG levels and PWV in T2DM patients with HT, indicating that the association between oxidative stress and arterial stiffness could be enhanced under the hypertensive state. Our results were partly in agreement with a recent study by Masugata et al, which found a positive correlation between 8-OHdG and the cardio-ankle vascular index in patients with HT,(19) and with other earlier studies that reported that the role of 8-OHdG could be amplified in the hypertensive state when compared to the diabetic state.(14,37)
Various possible biological mechanism(s) may be fundamental to the correlation between urinary 8-OHdG levels and PWV in T2DM patients with HT (Fig. 1). The diabetic and/or hypertensive states are known oxidative stress conditions,(4,6-8) and the association between oxidative stress and vascular damage is thought to be common to the pathophysiologies of HT and DM.(4-6) Circulating blood cells (e.g. superoxide-producing cells, activated-T cells and platelets) and vascular cells (e.g. endothelial cells and smooth muscle cells) can produce oxidative stress.(14,38) Studies have also reported that oxidative stress causes endothelial dysfunction, vascular inflammation, and arterial wall hypertrophy and fibrosis, thus resulting in increased arterial stiffness, with smooth muscle cell proliferation and increased synthesis of the extracellular matrix (i.e. elastin and collagen).(5,6,39) Under the hypertensive state, the processes of vascular damage, which commence with the production of oxidative stress, proceed to the activation of nicotinamide adenine dinucleotide phosphate oxidase and the impaired bioavailability of nitric oxide, with shear stress also bringing into play factors such as the increased activity of vasoconstrictors (e.g. endothelin-1) and the activation of the renin-angiotensin-aldosterone system.(5,6,9,39,40) Oxidative stress, together with factors involved in the hypertensive state, may therefore enhance arterial stiffness, thus accounting for the correlation of 8-OHdG levels with PWV in T2DM patients with HT.
Fig. 1.
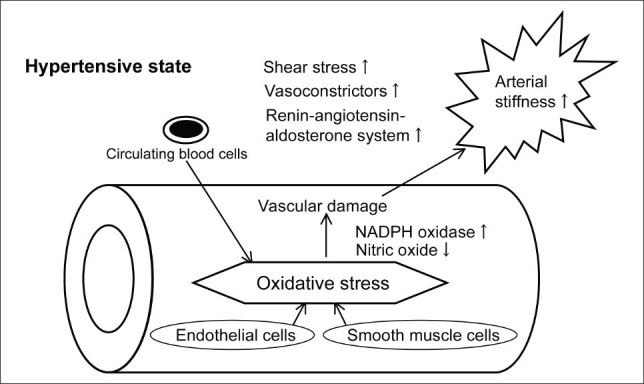
Possible mechanism(s) accounting for the association between oxidative stress and arterial stiffness in hypertensive patients. NADPH: nicotinamide adenine dinucleotide phosphate
We found that, in T2DM patients, male gender (in T2DM patients with HT), duration of DM (in T2DM patients with HT) and MBP (in T2DM patients with and without HT) were independently, significantly and positively correlated with PWV, while BMI (in T2DM patients with and without HT) and eGFR (in T2DM patients with HT) were independently, significantly and inversely correlated with PWV. The correlation between these variables and arterial stiffness, as indicated by our results, were also seen in previous studies – male gender,(41,42) BP,(43,44) duration of DM,(45) eGFR(46) and BMI(47,48) – although the characteristics of the various study populations were different. Although the inverse correlation between BMI and arterial stiffness in our study was unexpected, we found that similar results had also been reported previously.(47,48)
There were some limitations to the present study. The sample size was relatively small. Similarly, the inclusion of hypertensive patients without DM as control patients might have been better for comparison purposes. Our study had a cross-sectional design, and therefore could not entirely define the cause-and-effect relationship between 8-OHdG levels and PWV. While 8-OHdG levels are generally acknowledged to be an indicator of oxidative damage and atherogenesis, contradictory associations have also been reported in the literature, with a recent study by Huh et al suggesting that 8-OHdG might have an inhibitory role on atherosclerosis.(49) Only patients with a specific level of glycaemic control were enrolled in our study. We found no significant differences between the 8-OHdG levels of T2DM patients with and without HT, weak correlations between 8-OHdG levels and the use of prescribed agents, and weak correlations between diabetic complications and PWV. However, the small sample size and the predetermined level of glycaemic control in our study might have partly accounted for our results. In contrast to our study, Aso et al demonstrated a significant association between PWV and diabetic complications.(50) As various measures other than PWV measurement can be used for the evaluation of arterial stiffness, comparative studies that include the assessment of these variables may be useful for confirming our findings.(15,16,19) The evaluation of markers associated with endothelial dysfunction would also be useful in this context. Therefore, future prospective studies, with larger and varied populations, that evaluate the many markers involved with oxidative stress are warranted.
In summary, we report an independent, significant and positive correlation between urinary 8-OHdG levels and PWV in T2DM patients with HT, but not in those without HT. Our findings suggest that oxidative stress could be associated with the development of arterial stiffness in patients with fairly well controlled T2DM under the hypertensive state. Further studies will be necessary to clarify the biological mechanism(s) responsible, as well as the clinical relevance of the aforementioned relationship.
REFERENCES
- 1.Sheu WH, Rosman A, Mithal A, et al. Addressing the burden of type 2 diabetes and cardiovascular disease through the management of postprandial hyperglycaemia: an Asian-Pacific perspective and expert recommendations. Diabetes Res Clin Pract. 2011;92:312–21. doi: 10.1016/j.diabres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–57. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung N, Baek S, Chen MF, et al. Expert recommendations on the challenges of hypertension in Asia. Int J Clin Pract. 2008;62:1306–12. doi: 10.1111/j.1742-1241.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa T, Sasahara T, Kiritoshi S, et al. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care. 2003;26:1507–12. doi: 10.2337/diacare.26.5.1507. [DOI] [PubMed] [Google Scholar]
- 5.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–39. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 6.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med. 2012;44(Suppl 1):S2–16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 7.Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41:1144–9. doi: 10.1515/CCLM.2003.177. [DOI] [PubMed] [Google Scholar]
- 8.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Negishi H, Njelekela M, Ikeda K, et al. Assessment of in vivo oxidative stress in hypertensive rats and hypertensive subjects in Tanzania, Africa. Hypertens Res. 2000;23:285–9. doi: 10.1291/hypres.23.285. [DOI] [PubMed] [Google Scholar]
- 10.Xu S, Touyz RM. Reactive oxygen species and vascular remodelling in hypertension: still alive. Can J Cardiol. 2006;22:947–51. doi: 10.1016/s0828-282x(06)70314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leinonen J, Lehtimäki T, Toyokuni S, et al. New biomarker evidence of oxidative DNA damage in patients with non-insulin-dependent DM. FEBS Lett. 1997;417:150–2. doi: 10.1016/s0014-5793(97)01273-8. [DOI] [PubMed] [Google Scholar]
- 12.Kanauchi M, Nishioka H, Hashimoto T. Oxidative DNA damage and tubulointerstitial injury in diabetic nephropathy. Nephron. 2002;91:327–9. doi: 10.1159/000058412. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa O, Jiménez-Almazán J, Chaves FJ, et al. Urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxo-dG), a reliable oxidative stress marker in hypertension. Free Radic Res. 2007;41:546–54. doi: 10.1080/10715760601164050. [DOI] [PubMed] [Google Scholar]
- 14.Roselló-Lletí E, de Burgos FG, Morillas P, et al. Impact of cardiovascular risk factors and inflammatory status on urinary 8-OHdG in essential hypertension. Am J Hypertens. 2012;25:236–42. doi: 10.1038/ajh.2011.202. [DOI] [PubMed] [Google Scholar]
- 15.Nagayama D, Saiki A, Endo K, et al. Improvement of cardio-ankle vascular index by glimepiride in type 2 diabetic patients. Int J Clin Pract. 2010;64:1796–801. doi: 10.1111/j.1742-1241.2010.02399.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyashita Y, Saiki A, Endo K, et al. Effects of olmesartan, an angiotensin II receptor blocker, and amlodipine, a calcium channel blocker, on Cardio-Ankle Vascular Index (CAVI) in type 2 diabetic patients with hypertension. J Atheroscler Thromb. 2009;16:621–6. doi: 10.5551/jat.497. [DOI] [PubMed] [Google Scholar]
- 17.Li CH, Wu JS, Yang YC, et al. Increased arterial stiffness in subjects with impaired glucose tolerance and newly diagnosed diabetes but not isolated impaired fasting glucose. J Clin Endocrinol Metab. 2012;97:pE658–62. doi: 10.1210/jc.2011-2595. [DOI] [PubMed] [Google Scholar]
- 18.Barengo NC, Tuomilehto JO. Blood pressure treatment target in patients with diabetes mellitus – current evidence. Ann Med. 2012;44(Suppl 1):S36–42. doi: 10.3109/07853890.2012.679961. [DOI] [PubMed] [Google Scholar]
- 19.Masugata H, Senda S, Murao K, et al. Association between urinary 8-hydroxydeoxyguanosine, an indicator of oxidative stress, and the cardio-ankle vascular index in hypertensive patients. J Atheroscler Thromb. 2012;19:747–55. [PubMed] [Google Scholar]
- 20.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–64. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 21.Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29:1496–500. doi: 10.2337/dc05-1887. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Imai E, Horio M, Nitta K, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41–50. doi: 10.1007/s10157-006-0453-4. [DOI] [PubMed] [Google Scholar]
- 24.Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2’-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–74. [PubMed] [Google Scholar]
- 25.Yoshida R, Ogawa Y, Kasai H. Urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine values measured by an ELISA correlated well with measurements by high-performance liquid chromatography with electrochemical detection. Cancer Epidemiol Biomarkers Prev. 2002;11:1076–81. [PubMed] [Google Scholar]
- 26.Rossi MC, Nicolucci A, Pellegrini F, et al. Identifying patients with type 2 diabetes at high risk of microalbuminuria: results of the DEMAND (Developing Education on Microalbuminuria for Awareness of reNal and cardiovascular risk in Diabetes) Study. Nephrol Dial Transplant. 2008;23:1278–84. doi: 10.1093/ndt/gfm798. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 28.Thompson HJ, Heimendinger J, Haegele A, et al. Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis. 1999;20:2261–6. doi: 10.1093/carcin/20.12.2261. [DOI] [PubMed] [Google Scholar]
- 29.Miquel J. Can antioxidant diet supplementation protect against age-related mitochondrial damage? Ann N Y Acad Sci. 2002;959:508–16. doi: 10.1111/j.1749-6632.2002.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 30.Nojima H, Watanabe H, Yamane K, et al. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2008;57:170–6. doi: 10.1016/j.metabol.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Ozkanlar S, Akcay F. Antioxidant vitamins in atherosclerosis - animal experiments and clinical studies. Adv Clin Exp Med. 2012;21:115–23. [PubMed] [Google Scholar]
- 32.Kals J, Kampus P, Kals M, et al. Impact of oxidative stress on arterial elasticity in patients with atherosclerosis. Am J Hypertens. 2006;19:902–8. doi: 10.1016/j.amjhyper.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Kals J, Kampus P, Kals M, et al. Inflammation and oxidative stress are associated differently with endothelial function and arterial stiffness in healthy subjects and in patients with atherosclerosis. Scand J Clin Lab Invest. 2008;68:594–601. doi: 10.1080/00365510801930626. [DOI] [PubMed] [Google Scholar]
- 34.Ha CY, Kim JY, Paik JK, et al. The association of specific metabolites of lipid metabolism with markers of oxidative stress, inflammation and arterial stiffness in men with newly diagnosed type 2 Diabetes. Clin Endocrinol (Oxf) 2012;76:674–82. doi: 10.1111/j.1365-2265.2011.04244.x. [DOI] [PubMed] [Google Scholar]
- 35.Dalfino G, Simone S, Porreca S, et al. Bone morphogenetic protein-2 may represent the molecular link between oxidative stress and vascular stiffness in chronic kidney disease. Atherosclerosis. 2010;211:418–23. doi: 10.1016/j.atherosclerosis.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Kato M, Iida M, Goto Y, Kondo T, Yajima I. Sunlight exposure-mediated DNA damage in young adults. Cancer Epidemiol Biomarkers Prev. 2011;20:1622–8. doi: 10.1158/1055-9965.EPI-11-0228. [DOI] [PubMed] [Google Scholar]
- 37.Abdilla N, Tormo MC, Fabia MJ, et al. Impact of the components of metabolic syndrome on oxidative stress and enzymatic antioxidant activity in essential hypertension. J Hum Hypertens. 2007;21:68–75. doi: 10.1038/sj.jhh.1002105. [DOI] [PubMed] [Google Scholar]
- 38.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–32. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 39.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–9. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa S, Mori T, Nako K, Ito S. Combination therapy with renin-angiotensin system inhibitors and the calcium channel blocker azelnidipine decreases plasma inflammatory markers and urinary oxidative stress markers in patients with diabetic nephropathy. Hypertens Res. 2008;31:1147–55. doi: 10.1291/hypres.31.1147. [DOI] [PubMed] [Google Scholar]
- 41.Nordstrand N, Gjevestad E, Dinh KN, et al. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc Disord. 2011;11:7. doi: 10.1186/1471-2261-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim S, Choi HJ, Shin H, et al. Subclinical atherosclerosis in a community-based elderly cohort: the Korean Longitudinal Study on Health and Aging. Int J Cardiol. 2012;155:126–33. doi: 10.1016/j.ijcard.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 43.Tomita H, Kawamoto R, Tabara Y, Miki T, Kohara K. Blood pressure is the main determinant of the reflection wave in patients with type 2 diabetes. Hypertens Res. 2008;31:493–9. doi: 10.1291/hypres.31.493. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Jiang CQ, Lam TH, et al. Brachial-ankle pulse wave velocity and cardiovascular risk factors in the non-diabetic and newly diagnosed diabetic Chinese: Guangzhou Biobank Cohort Study-CVD. Diabetes Metab Res Rev. 2010;26:133–9. doi: 10.1002/dmrr.1059. [DOI] [PubMed] [Google Scholar]
- 45.Kim WJ, Park CY, Park SE, et al. The association between regional arterial stiffness and diabetic retinopathy in type 2 diabetes. Atherosclerosis. 2012;225:237–41. doi: 10.1016/j.atherosclerosis.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa N, Takahashi F, Chinda J, et al. A newly estimated glomerular filtration rate is independently associated with arterial stiffness in Japanese patients. Hypertens Res. 2008;31:193–201. doi: 10.1291/hypres.31.193. [DOI] [PubMed] [Google Scholar]
- 47.Maple-Brown LJ, Piers LS, O’Rourke MF, Celermajer DS, O’Dea K. Central obesity is associated with reduced peripheral wave reflection in Indigenous Australians irrespective of diabetes status. J Hypertens. 2005;23:1403–7. doi: 10.1097/01.hjh.0000173524.80802.5a. [DOI] [PubMed] [Google Scholar]
- 48.Budimir D, Jeroncic A, Gunjaca G, et al. Sex-specific association of anthropometric measures of body composition with arterial stiffness in a healthy population. Med Sci Monit. 2012;18:CR65–71. doi: 10.12659/MSM.882457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huh JY, Son DJ, Lee Y, et al. 8-Hydroxy-2-deoxyguanosine prevents plaque formation and inhibits vascular smooth muscle cell activation through Rac1 inactivation. Free Radic Biol Med. 2012;53:109–21. doi: 10.1016/j.freeradbiomed.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aso K, Miyata M, Kubo T, et al. Brachial-ankle pulse wave velocity is useful for evaluation of complications in type 2 diabetic patients. Hypertens Res. 2003;26:807–13. doi: 10.1291/hypres.26.807. [DOI] [PubMed] [Google Scholar]


