Abstract
INTRODUCTION
Due to lifelong immunosuppression, renal transplant recipients (RTRs) are at risk of infectious complications such as pneumonia. Severe pneumonia results in respiratory failure and is life-threatening. We aimed to examine the influence of immunosuppressant dose reduction on RTRs with bacterial pneumonia and respiratory failure.
METHODS
From January 2001 to January 2011, 33 of 1,146 RTRs at a single centre developed bacterial pneumonia with respiratory failure. All patients were treated using mechanical ventilation and aggressive therapies in the intensive care unit.
RESULTS
Average time from kidney transplantation to pneumonia with respiratory failure was 6.8 years. In-hospital mortality rate was 45.5% despite intensive care and aggressive therapies. Logistic regression analysis indicated that a high serum creatinine level at the time of admission to the intensive care unit (odds ratio 1.77 per mg/dL, 95% confidence interval 1.01–3.09; p = 0.045) was a mortality determinant. Out of the 33 patients, immunosuppressive agents were reduced in 17 (51.5%). We found that although immunosuppressant dose reduction tended to improve in-hospital mortality, this was not statistically significant. Nevertheless, during a mean follow-up period of two years, none of the survivors (n = 18) developed acute rejection or allograft necrosis.
CONCLUSION
In RTRs with bacterial pneumonia and respiratory failure, higher serum creatinine levels were a mortality determinant. Although temporary immunosuppressant dose reduction might not reduce mortality, it was associated with a minimal risk of acute rejection during the two-year follow-up. Our results suggest that early immunosuppressant reduction in RTRs with severe pneumonia of indeterminate microbiology may be safe even when pathogens are bacterial in nature.
Keywords: immunosuppressant, kidney transplant, mortality, pneumonia, respiratory failure
INTRODUCTION
Kidney transplantation is the treatment of choice for patients with end-stage renal disease (ESRD),(1) and is associated with better survival among all solid organ transplantations, with a reported annual crude mortality rate of 3.4%.(2) However, long-term immunosuppressants that are used to prevent graft rejection may increase the risk of immunocompromise and infection in these patients.(3) Although the incidence of pneumonia is lower for kidney transplantation than all other organ grafts, pneumonia is a common and serious infectious complication in renal transplant recipients (RTRs), possibly leading to respiratory failure, with mortality rates of up to 50%.(4-6)
In part due to their frequent contact with the healthcare system and immunosuppressive status, RTRs may have more resistant organisms, thus leading to the potential development of more fulminant and virulent pneumonia in these patients. While the development of novel immunosuppressive drugs has decreased the risk of acute rejection and improved long-term outcome, a lack of drug management and immune modulation has been reported to result in severe infectious conditions in RTRs.(7) According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines,(8) temporary immunosuppressant reduction may be beneficial in RTRs with opportunistic infection, although data on severe bacterial pneumonia is not known.
Thus, the aim of our study was to evaluate the influence of immunosuppressant dose reduction on patient outcomes in RTRs with respiratory failure due to bacterial pneumonia. We also followed up on pneumonia survivors for two years in order to examine the occurrence of acute allograft rejection in this cohort.
METHODS
All consecutive adult RTRs admitted to Taipei Veterans General Hospital, Taipei, Taiwan, between January 2001 and January 2011 were screened. Among these patients, those admitted for bacterial pneumonia with respiratory failure and those who required mechanical ventilation and aggressive therapies in the intensive care unit (ICU) were included in the study. Trimethoprim-sulfamethoxazole chemoprophylaxis (trimethoprim 80 mg/day; sulfamethoxazole 400 mg/day) against Pneumocystis jirovecii (P. jirovecii) was routinely administered to all patients in the first six months after kidney transplantation.
The medical records of patients were retrospectively reviewed. The diagnosis of bacterial pneumonia with respiratory failure was defined in accordance with the criteria set by the Infectious Diseases Society of America/American Thoracic Society.(9,10) Indications for endotracheal mechanical ventilation were determined by the attending physicians and were discretionary. The baseline patient characteristics, including age, gender, comorbidities, causes of ESRD and laboratory data, were recorded. At the point of ICU admission, disease severity was established using APACHE II (Acute Physiology and Chronic Health Evaluation II) classification system and the CURB-65 severity scoring system.(11,12) Absolute lymphocyte count was calculated by multiplying the total white blood cell count by lymphocyte percentage.
Diagnostic investigations undertaken during patients’ ICU stay included sputum microbiology, blood culture, tuberculosis smear and culture, haemogram, biochemistry, chest radiography, and further examinations such as high-resolution computed tomography, fibreoptic bronchoscopy, bronchoalveolar lavage, polymerase chain reaction assay for cytomegalovirus (CMV) and Mycobacterium tuberculosis, and serum Aspergillus galactomannan antigen test.
The immunosuppressive protocol at our hospital generally consisted of maintenance therapy, which included glucocorticoids, cyclosporine, tacrolimus, mycophenolate mofetil and sirolimus in various combinations, usually as sequential triple therapy. The strategy of immunosuppressant reduction, including adjustment of dose, type and timing during ICU admission, was determined according to the discretion of each attending physician.
Binary variables were expressed in counts and percentages. Continuous variables were described as mean ± standard deviation for normally distributed data, and as median (interquartile range [IQR]) for non-normally distributed data. Either chi-square test or Fisher’s exact test was performed for binary variables to detect significant differences in mortality determinants between the hospital survivor and hospital death groups. For continuous variables, Student’s t-test or Mann-Whitney U test was used, as appropriate. Logistic regression analysis was conducted to detect independent risk factors of mortality in RTRs with pneumonia and respiratory failure. Data was analysed using the Statistical Package for the Social Sciences version 19.0 (SPSS Inc, Chicago, IL, USA). All tests were two-tailed and a p-value < 0.05 was considered statistically significant.
RESULTS
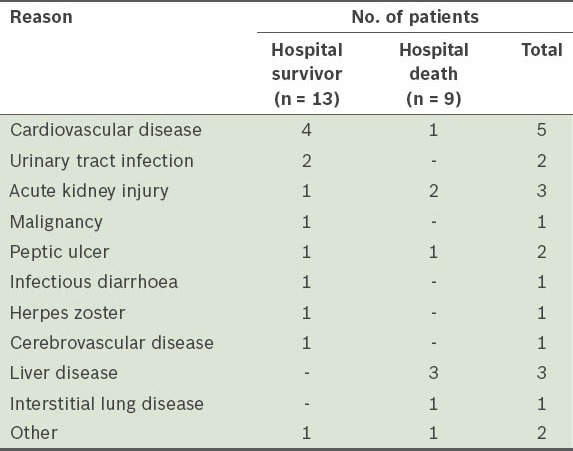
During the study period, 33 out of 1,146 RTRs at our centre developed bacterial pneumonia with respiratory failure. Of these 33 patients, 11 (33.3%) were diagnosed with community-acquired pneumonia (CAP) and the rest (66.7%) with healthcare-associated pneumonia (HCAP).(9) The incidence of bacterial pneumonia with respiratory failure was 2.9% in ten years. In-hospital mortality was found to be 45.5% in our cohort. The reasons for hospitalisation in patients with HCAP are shown in Table I.
Table I.
Reasons for hospitalisation in patients with healthcare-associated pneumonia (n = 22).
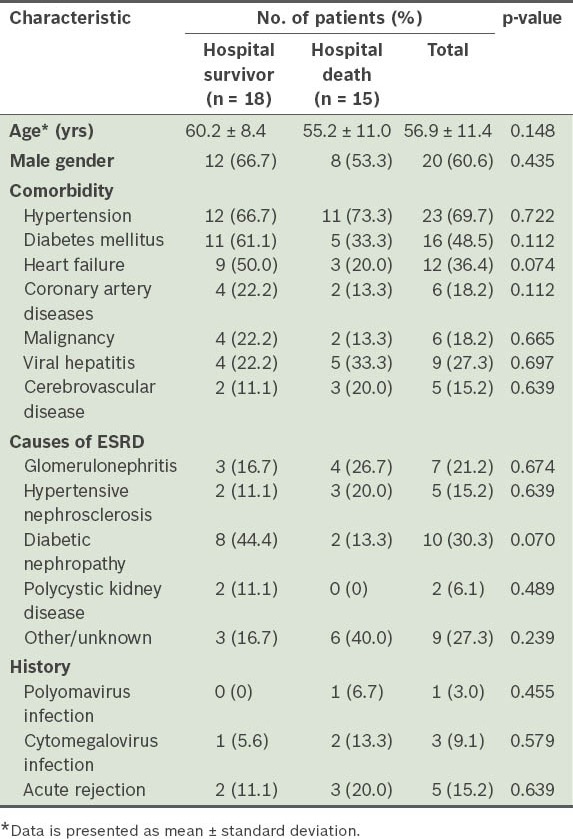
Patient demographics, comorbidities and causes of ESRD are compiled in Table II. The comorbidities observed in our cohort included hypertension (69.7%), heart failure (36.4%), coronary artery disease (18.2%), diabetes mellitus (48.5%), malignancy (18.2%), viral hepatitis (27.3%) and cerebrovascular disease (15.2%). Major causes of ESRD were diabetes mellitus (30.3%), glomerulonephritis (21.2%) and hypertensive nephrosclerosis (15.2%); the rest had unknown reasons (27.3%). Five patients had a history of acute rejection, with a median interval of 13.5 (IQR 2.4–29.2) mths before ICU admission.
Table II.
Characteristics of patients with end-stage renal disease (ESRD).
As shown in Table III, the average time from kidney transplantation to the development of pneumonia was 6.8 years in our cohort. The median time between the onset of respiratory symptoms and ICU admission was 1.0 (IQR 0.0–3.3) day in the hospital survivor group and 4.0 (IQR 1.0–6.0) days in the hospital death group. There were no significant differences in APACHE II and CURB-65 scores between the survivor and death groups at ICU admission.
Table III.
Characteristics of patients with bacterial pneumonia and respiratory failure.
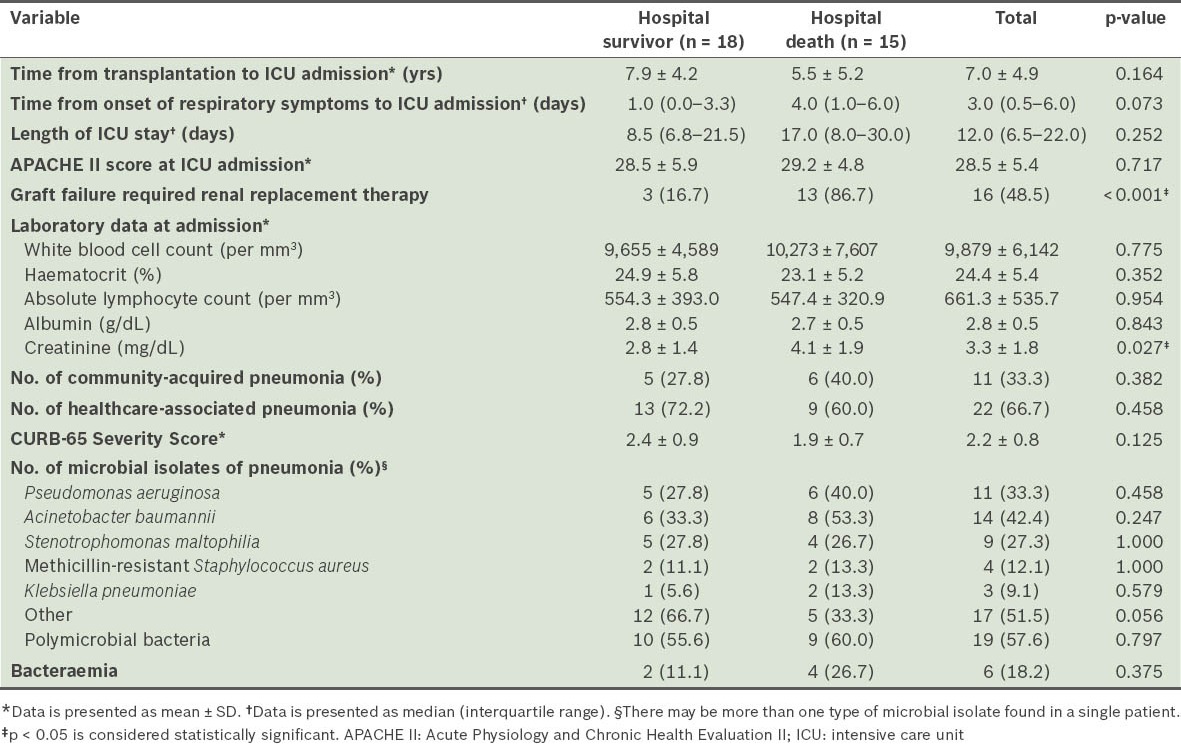
The microbial isolates from the tracheal aspirate were Acinetobacter baumannii (A. baumannii; 42.4%), Pseudomonas aeruginosa (P. aeruginosa; 33.3%), Stenotrophomonas maltophilia (27.3%), methicillin-resistant Staphylococcus aureus (12.1%), Klebsiella pneumoniae (9.1%) and others (51.5%). Polymicrobial bacteria were noted in 19 (57.6%) patients – two different isolates were identified in 13 patients, while three were identified in 6 patients. In all, six patients had bacteraemia. Of these six, one and two bacteraemic patients in the survivor and death groups, respectively, shared the same pathogens in each microbial isolate from the tracheal aspirate. Blood culture of the remaining three patients yielded mainly Gram-positive pathogens, Enterococcus faecalis and methicillin-sensitive Staphylococcus aureus. No significant difference in the microbial isolates between the hospital survivor and death groups was noted.
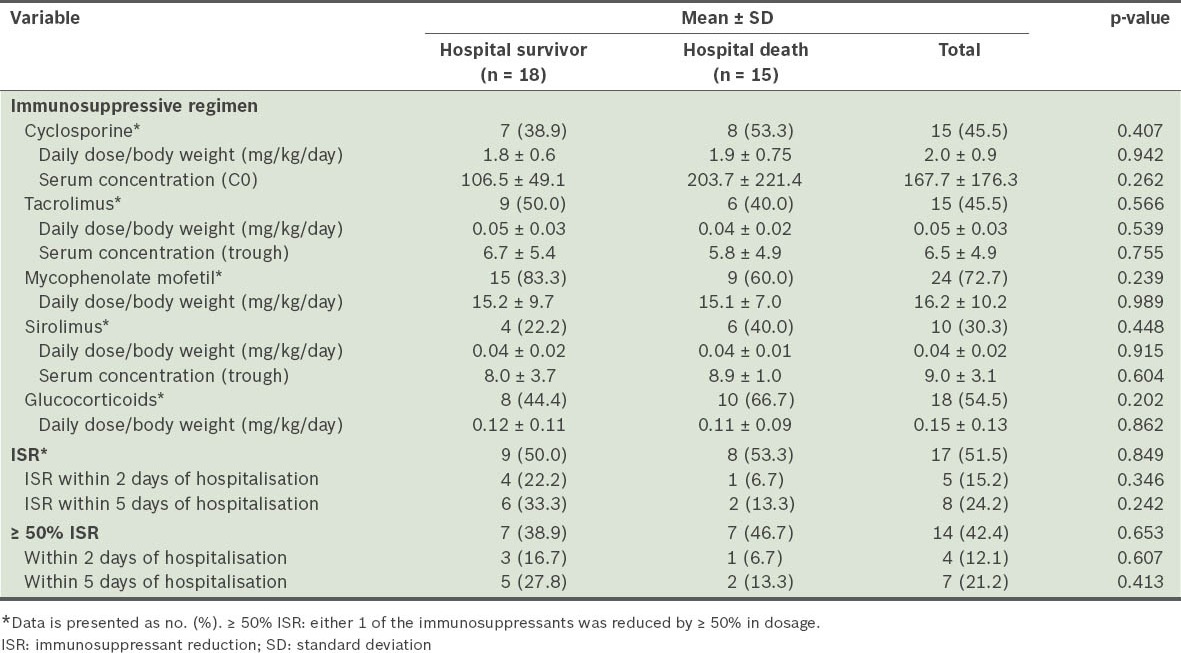
Table IV indicates that the most frequently prescribed immunosuppressant regimens were calcineurin inhibitors (45.5%) or sirolimus (45.5%) in combination with mycophenolate mofetil (72.7%) and glucocorticoids (54.5%). There was no significant difference between the hospital survivor and death groups with regard to daily dose per body weight and serum concentrations of immunosuppressants. Immunosuppressants were reduced in 17 (51.5%) patients.
Table IV.
Regimens and immunosuppressant dose reduction agents.
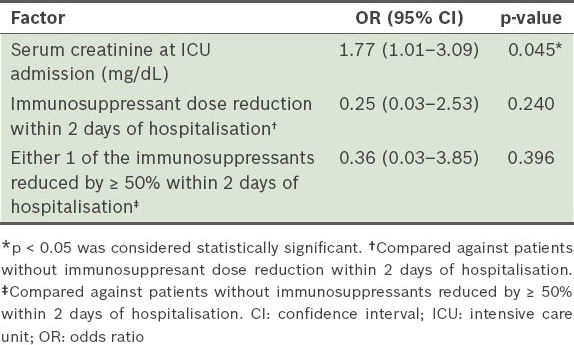
As shown in Table V, higher serum creatinine levels at the time of ICU admission (odds ratio [OR] 1.77, 95% confidence interval [CI] 1.01–3.09; p = 0.045) were a determinant of in-hospital mortality. Although immunosuppressant dose reduction tended to improve in-hospital mortality, this did not reach statistical significance. Goodness of fit for the logistic regression model was reassured (p = 0.346). Renal replacement therapy was required in three patients in the survivor group during ICU stay; two of these patients were dialysis-free at discharge. During a mean follow-up period of two years, none of the survivors (n = 18) developed acute rejection or allograft necrosis.
Table V.
Logistic regression analysis of the determinants of in-hospital mortality.
DISCUSSION
Our findings indicate that a higher serum creatinine level at the time of ICU admission was a determinant of mortality for RTRs with respiratory failure due to bacterial pneumonia. In our study, patients in the hospital death group had worse renal function at admission than those in the hospital survivor group (serum creatinine 4.1 ± 1.9 mg/dL vs 2.8 ± 1.4 mg/dL). In a multicentre study, Canet et al reported that renal function was a significant predictor of graft survival and dialysis-free survival for RTRs with acute respiratory failure.(13) According to James et al, impaired renal function in normal populations in Canada was associated with a significantly higher risk of hospitalisation and death due to pneumonia.(14) Viasus et al found that mortality due to pneumonia was higher in patients with chronic kidney disease (CKD) than in non-CKD patients (15.8% vs 8.3%).(15) According to Kasiske et al, more advanced CKD stages one year post-transplant were associated with increased risks of long-term mortality and graft failure.(16) Eitner et al have suggested that RTRs with renal insufficiency were associated with high risks of opportunistic infection, especially P. jirovecii pneumonia.(17) Our study, which also focused on severe bacterial pneumonia in RTRs, showed that renal function was a significant short-term mortality determinant in such patients.
RTRs have an increased risk of infection due to their immunosuppressive status. Indeed, infection has been shown to be the leading cause of ICU hospitalisation and death in RTRs, whether with or without graft failure.(18,19) Adequate adjustment of exogenous immunity in the face of infection and risk of rejection in RTRs may be difficult, remaining controversial to this day. Stamm proposed that, in general, reduction in intensity of immune suppression may be considered in life-threatening situations.(20) Other studies suggest that approaching the immunosuppressive regimen in stable RTRs with a minimalisation strategy may reduce the risk of graft dysfunction and complication such as cardiovascular disease or malignancy.(21-24) However, there is little recommendation or consensus on immunosuppressant dose reduction in RTRs under severe infectious conditions.
Reduction of immunosuppressants in RTRs according to the KDIGO guidelines may be indicated in opportunistic infections such as those due to Epstein-Barr virus, herpes simplex virus types 1 and 2, varicella-zoster virus, Herpes zoster virus, life-threatening CMV disease, polyomavirus and P. jirovecii.(8) In our study, although immunosuppressant dose reduction in RTRs with severe bacterial pneumonia tended to improve short-term survival, this did not reach statistical significance. This finding could probably be due to the relatively small sample size of our study, as evidenced by ORs that were wide and over 1.0 (Table V). Another study that included viral and fungal infections in addition to bacterial infections in RTRs reported that aggressive reduction of immunosuppressive therapy under highly fatal circumstances was associated with lower mortality rates.(25) We opted to focus on a more homogeneous patient population (i.e. RTRs with only bacterial pneumonia) in our study, as the survival benefit of immunosuppressant reduction under opportunistic infections might have been a significant confounding factor.
Although important, adequate immune modulation in RTRs is difficult, as immune function must not be so low that it allows worse infections to occur, and yet not so high as to cause acute rejection.(26,27) Hospital survivors in our study were followed up for two years, and we found that there was no acute rejection or allograft necrosis in patients with temporary and drastic immunosuppressant reduction. In routine practice, immediate identification of the pathogen responsible for pneumonia in RTRs may not be possible. Empirically, our results suggest that early immunosuppressant reduction for RTRs with severe pneumonia of indeterminate microbiology was likely safe, as no further increases in acute rejection were noted in our patients even when pathogens were bacterial in nature.
Patients with CKD may develop more severe pneumonia, although only obscure symptoms and signs may be noted at presentation.(15) This may partly be due to the presence of multiple comorbidities and alterations of the immune system in the CKD patient population. In our study cohort, in-hospital mortality was 45.5%, which was close to a predicted mortality of 60.5%, as derived from the APACHE II scores. However, the mortality rate in our study is higher than that in previous studies, which ranged from 22.5% to 55.8%.(5,13,28,29) The higher mortality rate found in our study may be due to the greater disease severity observed in our patients (as evidenced by their higher APACHE II scores). However, APACHE II scores were not significantly predictive of in-hospital mortality in our study, which is likely due to our small sample size. We also found that patients who needed renal replacement therapy during their stay in ICU had poor prognosis, similar to the results of a study by Aldawood.(18) Of the survivors in the present study, 3 (16.7%) developed graft failure and required dialysis during ICU stay. This is consistent with the findings of Canet et al who, in a multicentre study, showed that the prevalence of graft failure requiring dialysis in patients with acute respiratory failure was 23.5%.(13) Our findings were in agreement with a previous study by Kaplan and Meier-Kriesche, which showed that impaired renal function may lead to a loss of the protective effect of the transplant kidney, and this may be associated with an increased risk of death following graft failure.(30)
In our patients, A. baumannii and P. aeruginosa were the leading microbial isolates. Most of our patients were diagnosed as having HCAP. Unlike CAP, HCAP is associated with a high rate of multidrug-resistant pathogens.(31) A single-centre study by Hoyo et al also reported that P. aeruginosa was the most common isolated microorganism in RTRs with nosocomial pneumonia.(6) Studies suggest that causative microorganisms are more virulent and drug-resistant in patients with nosocomial pneumonia than in those with CAP.(6,31) According to a previous study, attributable mortality of ventilator-associated pneumonia was between 33% and 50% in ICU patients, similar to that in our study.(9) This may explain why the survival benefit of immunosuppressant reduction was offset by the high mortality rates associated with multiple drug-resistant or highly virulent pathogens in our patients. These results suggest that multidrug-resistant bacterial pathogens should be considered in RTRs with severe infection.(6) Timely broad-spectrum empirical antibiotics and early recognition of pathogens should therefore be implemented to achieve optimal treatment of RTRs with respiratory failure due to bacterial pneumonia.
There were some limitations to our study. First, this was a single-centre study with a retrospective design and a relatively small sample size. Second, the benefit of immunosuppression reduction for RTRs with clinically irrelevant pneumonia was unknown. Third, it is challenging to distinguish between pathogens and colonisers in clinical practice. For this reason, in our study, microbial cultures with positive results on Gram staining and compatible clinical presentation were considered as positive for infection.
In conclusion, 2.9% of RTRs in the present study developed severe bacterial pneumonia requiring mechanical ventilation, with a 45.5% mortality rate during an average post-transplant period of 6.8 years. Higher chronicity of the graft kidney was associated with higher mortality in patients with severe bacterial pneumonia. However, while temporary immunosuppressant dose reduction under such highly fatal circumstances might not reduce mortality, it was found to be associated with a minimal risk of acute allograft rejection. Our findings suggest that early immunosuppressant reduction before microbiological confirmation should be reasonable and safe in RTRs with severe pneumonia caused by either opportunistic or bacterial pathogens.
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917–22. doi: 10.1681/ASN.V115917. [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–14. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 4.Washer GF, Schroter GP, Starzl TE, Weil R., 3rd Causes of death after kidney transplantation. JAMA. 1983;250:49–54. [PMC free article] [PubMed] [Google Scholar]
- 5.Candan S, Pirat A, Varol G, et al. Respiratory problems in renal transplant recipients admitted to intensive care during long-term follow-up. Transplant Proc. 2006;38:1354–6. doi: 10.1016/j.transproceed.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 6.Hoyo I, Linares L, Cervera C, et al. Epidemiology of pneumonia in kidney transplantation. Transplant Proc. 2010;42:2938–40. doi: 10.1016/j.transproceed.2010.07.082. [DOI] [PubMed] [Google Scholar]
- 7.Fortun J, Martin-Davila P, Pascual J, et al. Immunosuppressive therapy and infection after kidney transplantation. Transpl Infect Dis. 2010;12:397–405. doi: 10.1111/j.1399-3062.2010.00526.x. [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 10.Mandell LA, Wunderink RG, Anzueto A, Bar, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 12.British Thoracic Society Standards of Care C. BTS Guidelines for the Management of Community Acquired Pneumonia in Adults. Thorax. 2001;56(Suppl 4):IV1–64. doi: 10.1136/thorax.56.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canet E, Osman D, Lambert J, et al. Acute respiratory failure in kidney transplant recipients: a multicenter study. Crit Care. 2011;15:R91. doi: 10.1186/cc10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James MT, Quan H, Tonelli M, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis. 2009;54:24–32. doi: 10.1053/j.ajkd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Viasus D, Garcia-Vidal C, Cruzado JM, et al. Epidemiology, clinical features and outcomes of pneumonia in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:2899–906. doi: 10.1093/ndt/gfq798. [DOI] [PubMed] [Google Scholar]
- 16.Kasiske BL, Israni AK, Snyder JJ, Skeans MA. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. 2011;57:466–75. doi: 10.1053/j.ajkd.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 17.Eitner F, Hauser IA, Rettkowski O, et al. Risk factors for Pneumocystis jiroveci pneumonia (PcP) in renal transplant recipients. Nephrol Dial Transplant. 2011;26:2013–7. doi: 10.1093/ndt/gfq689. [DOI] [PubMed] [Google Scholar]
- 18.Aldawood A. The course and outcome of renal transplant recipients admitted to the intensive care unit at a tertiary hospital in Saudi Arabia. Saudi J Kidney Dis Transpl. 2007;18:536–40. [PubMed] [Google Scholar]
- 19.Ojo AO, Hanson JA, Wolfe RA, et al. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307–13. doi: 10.1046/j.1523-1755.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 20.Stamm AM. Infection in organ-transplant recipients. N Engl J Med. 1998;339:1246. doi: 10.1056/NEJM199810223391714. [DOI] [PubMed] [Google Scholar]
- 21.Lindemann M, Heinemann FM, Horn PA, Witzke O. Immunity to pneumococcal antigens in kidney transplant recipients. Transplantation. 2010;90:1463–7. doi: 10.1097/TP.0b013e3181f5d878. [DOI] [PubMed] [Google Scholar]
- 22.Etienne I, Toupance O, Benichou J, et al. A 50% reduction in cyclosporine exposure in stable renal transplant recipients: renal function benefits. Nephrol Dial Transplant. 2010;25:3096–106. doi: 10.1093/ndt/gfq135. [DOI] [PubMed] [Google Scholar]
- 23.Watorek E, Szymczak M, Boratynska M, Patrzalek D, Klinger M. Cardiovascular risk in kidney transplant recipients receiving Mammalian target of rapamycin inhibitors. Transplant Proc. 2011;43:2967–9. doi: 10.1016/j.transproceed.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Luan FL, Steffick DE, Ojo AO. Steroid-free maintenance immunosuppression in kidney transplantation: is it time to consider it as a standard therapy? Kidney Int. 2009;76:825–30. doi: 10.1038/ki.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sileri P, Pursell KJ, Coady NT, et al. A standardized protocol for the treatment of severe pneumonia in kidney transplant recipients. Clin Transplant. 2002;16:450–4. doi: 10.1034/j.1399-0012.2002.02079.x. [DOI] [PubMed] [Google Scholar]
- 26.van de Wetering J, van der Mast BJ, de Kuiper P, et al. Reduction of immunosuppressive load in renal transplant recipients with a low donor-specific cytotoxic T-lymphocyte precursor frequency is safe. Transplant Proc. 2005;37:779–81. doi: 10.1016/j.transproceed.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 27.Li SH, Wang W, Hu XP, et al. Monitoring immune function after rapid corticosteroid reduction in kidney transplant recipients. Chin Med J (Engl) 2011;124:679–82. [PubMed] [Google Scholar]
- 28.Klouche K, Amigues L, Massanet P, et al. Outcome of renal transplant recipients admitted to an intensive care unit: a 10-year cohort study. Transplantation. 2009;87:889–95. doi: 10.1097/TP.0b013e31819a688a. [DOI] [PubMed] [Google Scholar]
- 29.Kirilov D, Cohen J, Shapiro M, Grozovski E, Singer P. The course and outcome of renal transplant recipients admitted to a general intensive care unit. Transplant Proc. 2003;35:606. doi: 10.1016/s0041-1345(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant. 2002;2:970–4. doi: 10.1034/j.1600-6143.2002.21015.x. [DOI] [PubMed] [Google Scholar]
- 31.Polverino E, Torres A. Current perspective of the HCAP problem: is it CAP or is it HAP? Semin Respir Crit Care Med. 2009;30:239–48. doi: 10.1055/s-0029-1202940. [DOI] [PubMed] [Google Scholar]


