Abstract
INTRODUCTION
Necrotising fasciitis (NF) is often found in patients with diabetes mellitus, chronic renal failure, alcoholism, malignancy or liver cirrhosis. However, it remains unknown whether liver cirrhosis is an independent risk factor for the occurrence of NF. This study aimed to determine whether liver cirrhosis is an independent risk factor for the occurrence of NF, and to identify the relationship between severity of liver cirrhosis and occurrence of NF.
METHODS
The National Health Insurance Research Database, maintained by Taiwan’s National Health Insurance programme, was retrospectively analysed, and the hospitalisation data of 40,802 cirrhotic patients and 40,865 randomly selected, age- and gender-matched non-cirrhotic control patients was collected. The medical records of all patients were individually followed for a three-year period from the patients’ first hospitalisation in 2004.
RESULTS
During the three-year follow-up period, there were 299 (0.7%) cirrhotic patients with NF and 160 (0.4%) non-cirrhotic patients with NF. Cox regression analysis showed that liver cirrhosis was a risk factor for the occurrence of NF during the study period (hazard ratio 1.982; p < 0.001). Among cirrhotic patients, those with complicated liver cirrhosis had a higher risk for the occurrence of NF than patients with non-complicated liver cirrhosis (hazard ratio 1.320; p = 0.028).
CONCLUSION
Cirrhotic patients had a higher risk for the occurrence of NF than non-cirrhotic patients, and the risk for NF was especially high among patients with complicated liver cirrhosis.
Keywords: cirrhosis, complicated liver cirrhosis, necrotising fasciitis
INTRODUCTION
Necrotising fasciitis (NF) is a type of soft tissue infection that spreads rapidly and progressively along the deep fascia. It is a life-threatening disease with a course that is often overwhelming in nature. Despite improved medical care, the mortality rate associated with NF remains between 12% and 34%.(1-4) Mortality is especially high among cirrhotic patients with NF, in whom rates have been reported to be nearly ninefold that among non-cirrhotic patients with NF.(1-4)
Liver cirrhosis can weaken the intestinal-portal route barrier, which facilitates the entry of bacteria into the systemic circulation and renders patients prone to various infectious diseases such as spontaneous bacterial peritonitis, respiratory infections or urinary tract infections.(5,6) Several reports have shown that liver cirrhosis is a common underlying disease in patients with NF.(1-4,7,8) However, it remains unknown whether liver cirrhosis can increase the occurrence of NF.
The purpose of this study was twofold, namely to determine whether liver cirrhosis is an independent risk factor for the occurrence of NF, and to identify the relationship between severity of liver cirrhosis and occurrence of NF.
METHODS
This study was approved by the National Health Research Institutes (NHRI) in Taiwan. The National Health Insurance Research Database (NHIRD), established and maintained by the Bureau of National Health Insurance (BNHI), Taiwan, and the NHRI, was used for the purpose of the present study. This database includes all data regarding hospitalised patients in Taiwan. The National Health Insurance (NHI) programme was established in 1995 and encompasses all citizens residing in Taiwan. The BNHI covers over 98% of Taiwan’s population. Although researchers are welcome to use the NHIRD for their studies, the privacy of patients and healthcare providers is protected, as researchers using the database are required to submit their study protocols for evaluation by the NHRI.
In this retrospective study, hospitalised patients diagnosed with liver cirrhosis (in accordance with the International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] code 571.5 or 571.2 in the NHIRD) between 1 January 2004 and 31 December 2004 were included for analysis. Patients aged less than 30 years were excluded from the study, as the aetiologies of liver cirrhosis in such patients are often different from those of other cirrhotic patients. Patients with missing or incomplete medical data were also excluded.
As per Taiwan’s NHI directives, cirrhotic patients with refractory ascites, episodes of oesophageal/gastric variceal bleeding or hepatic encephalopathy can obtain certification to reduce hospitalisation payments. Cirrhotic patients who availed such certification were identified from the database and categorised as those having complicated liver cirrhosis.
A total of 40,802 cirrhotic patients without baseline NF were enrolled in our study. Cirrhotic patients were stratified according to gender and age (age groups: 30–44 years; 45–59 years; 60–74 years; > 75 years). Age- and gender-matched non-cirrhotic patients without baseline NF were also randomly selected from the database as the control group (n = 40,865). All patients (n = 81,667) were individually followed up for a three-year period, beginning with their first hospitalisation in 2004 until the diagnosis of NF (in accordance with ICD-9-CM codes 728.86), death, loss to follow-up or the end of study.
Time to development of NF was defined as the duration from the date selected for follow-up to the time of hospitalisation for NF. The medical comorbidities selected for the study were alcoholism (ICD-9-CM codes 291, 303, 305.00-305.03 and 571.0-571.3), diabetes mellitus (DM; ICD-9-CM code 250), chronic renal failure (CRF; ICD-9-CM code 585), solid organ transplantation (SOT; ICD-9-CM codes V42.0, V42.1 and V42.7), human immunodeficiency virus (HIV) infection (ICD-9-CM code 042), malignancy (ICD-9-CM code 140-208) and connective tissue disease (CTD; ICD-9-CM codes 710). Comorbidities were identified from the patients’ hospitalisation records available prior to the end of follow-up. The term ‘censored’ was used to indicate patient death, patients lost to follow-up or the end of study.
Data was analysed using the Statistical Package for the Social Sciences for Windows version 13.0 (SPSS Inc, Chicago, IL, USA). Cox regression was used to model all factors associated with time to hospitalisation for NF. Age was entered into the Cox models as a continuous covariable using the following coding: 1 = 30–44 years; 2 = 45–59 years; 3 = 60–74 years; 4 = > 75 years. Potential confounding factors included in the analysis were DM, CRF, alcoholism, malignancy, age groups, SOT, gender, CTD and HIV infection. Hazard ratios (HRs) and 95% confidence intervals (CIs) were presented, with the significance level fixed at p-value ≤ 0.05. The R software for Windows (available at: http://cran.r-project.org/bin/windows/base/) was used to plot the estimated cumulative incidence curves and perform competing risk analysis.(9)
RESULTS
Table I shows the demographic characteristics and comorbidities of cirrhotic and non-cirrhotic patients enrolled in the study. A total of 81,667 patients were enrolled, and the mean age of the cohort was 59.3 ± 14.3 years. Among the 40,802 cirrhotic patients, 8,739 (21.4%) patients had complicated liver cirrhosis and 32,063 (78.6%) patients had non-complicated liver cirrhosis. During the three-year follow-up period, NF occurred in 299 (0.7%) cirrhotic patients and 160 (0.4%) non-cirrhotic patients. The distribution of many of the comorbidities was found to be significantly different between cirrhotic and non-cirrhotic patients.
Table I.
Characteristics of cirrhotic and non-cirrhotic patients.
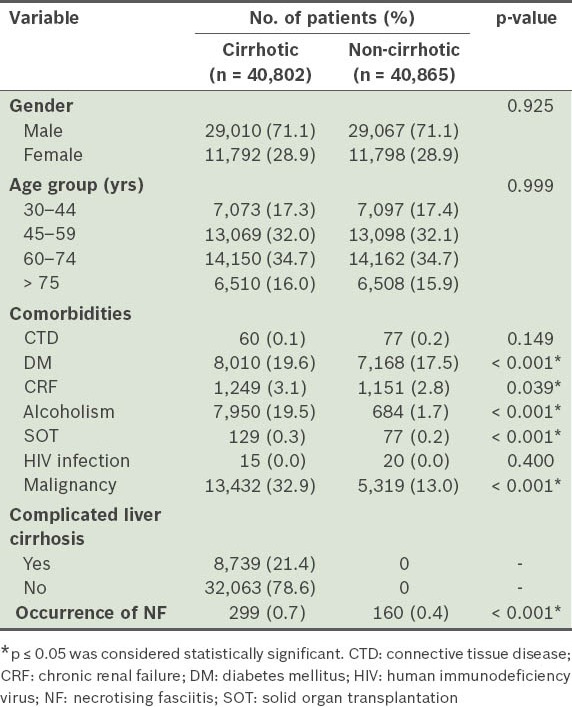
Table II provides the results of Cox regression analysis of the various confounding factors for the occurrence of NF in all patients during the study. Significant positive correlation was found between the occurrence of NF and variables such as liver cirrhosis (HR 1.982; p < 0.001), DM (HR 2.978; p < 0.001), CRF (HR 1.615; p = 0.030) and alcoholism (HR 1.364; p = 0.018). Similarly, significant negative correlation was found between the occurrence of NF and variables such as old age (HR 0.759; p < 0.001) and malignancy (HR 0.462; p < 0.001).
Table II.
Cox regression analysis of confounding factors for the occurrence of necrotising fasciitis in cirrhotic and non-cirrhotic patients.
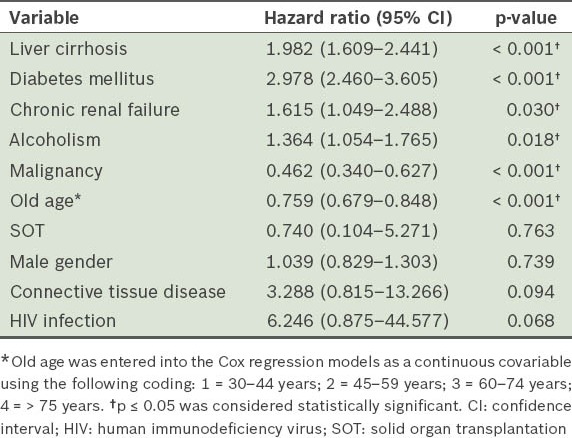
Table III shows the demographic characteristics and comorbidities of patients with complicated and non-complicated cirrhosis. The distribution of many comorbidities and age groups were significantly different between cirrhotic patients with and without complicated liver cirrhosis. During the three-year follow-up period, NF occurred in 95 (1.1%) patients with complicated cirrhosis and 204 (0.6%) patients with non-complicated cirrhosis. As only 15 cirrhotic patients had HIV infection, HIV infection was not entered as a variable into the Cox regression model.
Table III.
Characteristics of all patients with complicated and non-complicated liver cirrhosis (n = 40,802).
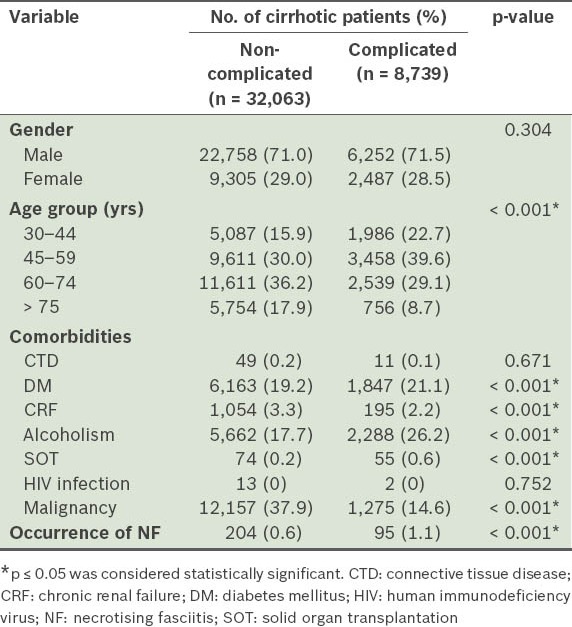
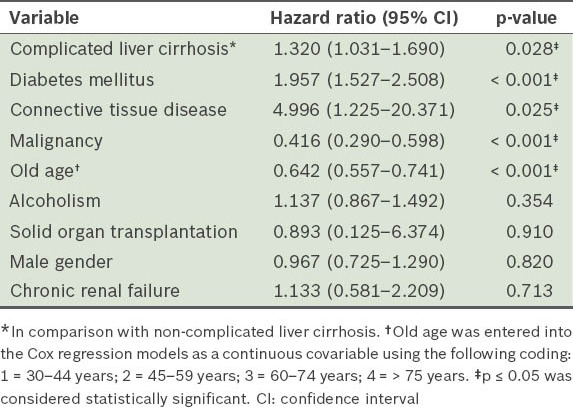
Table IV shows the results of Cox regression analysis of the various confounding factors for the occurrence of NF in cirrhotic patients during the study period. Significant positive correlation was found between the occurrence of NF and variables such as complicated liver cirrhosis (HR 1.320; p = 0.028), DM (HR 1.957; p < 0.001) and CTD (HR 4.996; p = 0.025). However, significant negative correlation was found between the occurrence of NF and variables such as old age (HR 0.642; p < 0.001) and malignancy (HR 0.416; p < 0.001).
Table IV.
Cox regression analysis of confounding factors for the occurrence of necrotising fasciitis in patients with complicated and non-complicated liver cirrhosis.
Estimated cumulative incidence curves were constructed for time to hospitalisation for NF in cirrhotic (n = 40,802) and non-cirrhotic patients (n = 40,865) after the three-year follow-up (Fig. 1). Results of Gray’s test for equality of cumulative incidence functions across groups showed that cumulative incidence curves for cirrhotic and non-cirrhotic patients were statistically different for the occurrence of NF.
Fig. 1.
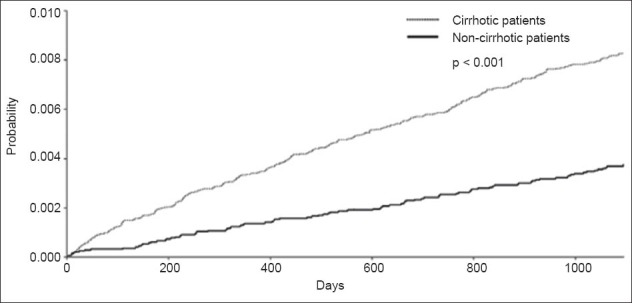
Graph shows esitmated cumulative incidence curves for time to hospitalisation for necrotising fasciitis in cirrhotic and non-cirrhotic patients.
DISCUSSION
The overall incidence of NF has been estimated to range between 0.24–0.4 per 100,000 adults.(10-12) In a large survey of Canadian children, Eneli and Davies found that the incidence of NF caused by Group A streptococci was 0.21 per 100,000 children, while that of NF caused by non-Group A streptococci was 0.08 per 100,000 children.(13) In the present study, we found that, in addition to the incidence of NF in cirrhotic patients (0.7%), the occurrence rate of NF in non-cirrhotic patients (0.4%) was also much higher than reported in the literature. This may partly be due to the fact that those enrolled in our study were all hospitalised patients who often already have comorbidities that contribute to their hospitalisation.
In Taiwan, liver cirrhosis in most adult patients is related to either the hepatitis B or hepatitis C virus.(14,15) In our study, alcoholism accounted for liver cirrhosis in only 19.5% of cirrhotic patients, with liver cirrhosis in the remaining 80.5% of cirrhotic patients being related to viral hepatitis. Our findings suggest that alcoholism was not a key factor affecting the occurrence of NF in cirrhotic populations. We also found that liver cirrhosis itself could increase the risk of occurrence of NF. Other risk factors that were identified for the occurrence of NF in the present study included DM, CRF and alcoholism. Our results were compatible with the findings of earlier studies.(4,8,16-20) However, to our knowledge, no previous cohort study in the literature has proven that liver cirrhosis is a risk factor for the occurrence of NF.
Earlier studies have suggested that NF was slightly more common in patients aged above 50 years(10,20-23) and that malignancy was a contributing factor for NF due to impaired leucocyte function in such patients.(18,24,25) Contrary to these studies, we found that malignancy and age groups were negatively correlated with the occurrence of NF during the three-year follow-up period. These findings may have been influenced by the fact that patient death was indicated as ‘censored’ in our study. Apart from patient deaths, hospitalised patients with malignancy or old age during the three-year follow-up period were also considered as censors. Such categorisation – when few episodes of NF are observed with a large number of censors – may have given rise to some bias and caused an underestimation of the effect of age and malignancy on the occurrence of NF.
An antecedent traumatic wound, which may include trauma, intravenous or hypodermic injection, or surgery, has been suggested as a possible portal of entry for NF.(26,27) However, studies have also found that most cirrhotic patients with NF do not have obvious lesions on the body surface that allow bacterial invasion.(2,7,16) Monomicrobial NF is reported to be more common in cirrhotic patients,(2,16) unlike patients with DM, in whom NF is reported to be mainly polymicrobial.(2,16) Studies have also suggested that Gram-negative bacilli such as Vibrio spp., Klebsiella spp. and Aeromonas spp. are common causes of monomicrobial NF in cirrhotic patients, while Streptococcus pyogenes is commonly seen in patients with DM.(2,7,16) These findings suggest that the clinical setting of NF in cirrhotic patients is different from that in other debilitated individuals. A possible mechanism of NF in cirrhotic patients includes the seeding of bacteria in the oedematous soft tissue via the bloodstream through the intestinal portal route.(2,7) Such aetiology may explain why the simultaneous involvement of two extremities is seen in 28% of cirrhotic patients with NF, with 58% of these patients having concurrent Gram-negative bacteraemia.(2)
In cirrhotic patients, intestinal bacterial overgrowth and increased intestinal permeability contribute to bacterial translocation from the intestines more easily than in normal hosts.(28-32) The concomitant presence of impaired phagocytosis, poor opsonisation capacity, impaired reticuloendothelial system and low complement levels in cirrhotic patients(33-37) makes them all the more prone to bacteraemia.(38-40) Kuo et al reported that the frequencies of bacteraemia were positively correlated to the severity of liver cirrhosis.(41) This might explain our finding that patients with liver cirrhosis had a higher occurrence of NF, and this was especially so among patients with complicated liver cirrhosis.
In our study, CTD was not a risk factor for the occurrence of NF in all patients, although it was a risk factor for the occurrence of NF in cirrhotic patients. This suggests that CTD and liver cirrhosis have a synergistic effect on the occurrence of NF. However, the immunosuppressive status caused by CTD or the immunosuppressive therapies usually prescribed for CTD is not a sufficient cause for bacterial blood stream infections leading to NF. This notwithstanding, the immunosuppressive effects of CTD may further weaken the immune status of cirrhotic patients, thus contributing to higher bacterial bloodstream infection and increased occurrence of NF in this cohort. Further extensive studies will be required to establish this association.
There were some limitations to our study. First, all selected patients were hospitalised patients and non-cirrhotic patients were selected as the control group in accordance with the diagnostic criteria outlined in the ICD-9-CM. This may have led to some bias, as patients who had liver cirrhosis but were not diagnosed during hospitalisation would have been categorised as non-cirrhotic patients in our study. Next, laboratory data regarding bilirubin, albumin and prothrombin time could not be identified based on the ICD-9-CM coding from the database. Similarly, we could not evaluate the severity of liver cirrhosis based on the Child-Pugh score. Then, the database used in our study did not include complete information on individuals, such as the amount of alcohol consumed, or dosage and duration of steroid use. This may have somewhat influenced the results of our study. Furthermore, we could not discount the possibility of miscoding, or assess the validity of NF diagnosis in our database. Finally, the exact aetiology of liver cirrhosis was not identified in our national population-based study. However, despite its limitations, our study is the most complete nationwide population-based study to prove that liver cirrhosis is a risk factor for the occurrence of NF.
To summarise, we found that cirrhotic patients had a higher risk for the occurrence of NF than non-cirrhotic patients, and the risk for NF was especially high among patients with complicated liver cirrhosis. DM, CRF and alcoholism were risk factors for the occurrence of NF in both cirrhotic and non-cirrhotic patients, while CTD was a risk factor for NF in only cirrhotic patients. Old age and malignancy were negatively associated with NF. Future studies should aim to investigate and substantiate the associations noted in this study.
ACKNOWLEDGEMENTS
This study is based in part on data from the NHIRD provided by the BNHI, Department of Health, and managed by the NHRI. The interpretation and conclusions contained herein do not represent those of BNHI or NHRI.
REFERENCES
- 1.Anaya DA, McMahon K, Nathens AB, et al. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg. 2005;140:151–7. doi: 10.1001/archsurg.140.2.151. [DOI] [PubMed] [Google Scholar]
- 2.Lee CC, Chi CH, Lee NY, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial Gram-negative bacillary infections. Diagn Microbiol Infect Dis. 2008;62:219–25. doi: 10.1016/j.diagmicrobio.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Huang KF, Hung MH, Lin YS, et al. Independent predictors of mortality for necrotizing fasciitis: a retrospective analysis in a single institution. J Trauma. 2011;71:467–73. doi: 10.1097/TA.0b013e318220d7fa. [DOI] [PubMed] [Google Scholar]
- 4.Bair MJ, Chi H, Wang WS, et al. Necrotizing fasciitis in southeast Taiwan: clinical features, microbiology, and prognosis. Int J Infect Dis. 2009;13:255–60. doi: 10.1016/j.ijid.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–7. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 6.Fernández J, Navasa M, Gómez J, et al. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–8. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 7.Cheng NC, Tai HC, Tang YB, et al. Necrotising fasciitis: clinical features in patients with liver cirrhosis. Br J Plast Surg. 2005;58:702–7. doi: 10.1016/j.bjps.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Hung CC, Chang SC, Lin SF, et al. Clinical manifestations, microbiology and prognosis of 42 patients with necrotizing fasciitis. J Formos Med Assoc. 1996;95:917–22. [PubMed] [Google Scholar]
- 9.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–7. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien KL, Beall B, Barrett NL, et al. Epidemiology of invasive group a streptococcus disease in the United States, 1995-1999. Clin Infect Dis. 2002;35:268–76. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
- 11.Kaul R, McGeer A, Low DE, et al. Population-based surveillance for group A streptococcal necrotizing fasciitis: Clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med. 1997;103:18–24. doi: 10.1016/s0002-9343(97)00160-5. [DOI] [PubMed] [Google Scholar]
- 12.Baxter F, McChesney J. Severe group A streptococcal infection and streptococcal toxic shock syndrome. Can J Anaesth. 2000;47:1129–40. doi: 10.1007/BF03027968. [DOI] [PubMed] [Google Scholar]
- 13.Eneli I, Davies HD. Epidemiology and outcome of necrotizing fasciitis in children: an active surveillance study of the Canadian Paediatric Surveillance Program. J Pediatr. 2007;151:79–84. doi: 10.1016/j.jpeds.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Hsu HC, Lin WS, Tsai MJ. Hepatitis-B surface antigen and hepatocellular carcinoma in Taiwan. With special reference to types and localization of HBsAg in the tumor cells. Cancer. 1983;52:1825–32. doi: 10.1002/1097-0142(19831115)52:10<1825::aid-cncr2820521011>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Tsai JF, Chang WY, Jeng JE, et al. Hepatitis C virus infection as a risk factor for non-alcoholic liver cirrhosis in Taiwan. J Med Virol. 1993;41:296–300. doi: 10.1002/jmv.1890410407. [DOI] [PubMed] [Google Scholar]
- 16.Wong CH, Chang HC, Pasupathy S, et al. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85-A:1454–60. [PubMed] [Google Scholar]
- 17.Lin C, Yeh FL, Lin JT, et al. Necrotizing fasciitis of the head and neck: an analysis of 47 cases. Plast Reconstr Surg. 2001;107:1684–93. doi: 10.1097/00006534-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Green RJ, Dafoe DC, Raffin TA. Necrotizing fasciitis. Chest. 1996;110:219–29. doi: 10.1378/chest.110.1.219. [DOI] [PubMed] [Google Scholar]
- 19.McHenry CR, Piotrowski JJ, Petrinic D, et al. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–63. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010;49:1051–7. doi: 10.2169/internalmedicine.49.2964. [DOI] [PubMed] [Google Scholar]
- 21.Mulla ZD. Streptococcal myositis. Br J Plast Surg. 2003;56:424. doi: 10.1016/s0007-1226(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 22.Childers BJ, Potyondy LD, Nachreiner R, et al. Necrotizing fasciitis: a fourteen-year retrospective study of 163 consecutive patients. Am Surg. 2002;68:109–16. [PubMed] [Google Scholar]
- 23.Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg. 1996;224:672–83. doi: 10.1097/00000658-199611000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamagni TL, Darenberg J, Luca-Harari B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46:2359–67. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy JJ, Granger R, Blair GK, et al. Necrotizing fasciitis in childhood. J Pediatr Surg. 1995;30:1131–4. doi: 10.1016/0022-3468(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 26.Nuwayhid ZB, Aronoff DM, Mulla ZD. Blunt trauma as a risk factor for group A streptococcal necrotizing fasciitis. Ann Epidemiol. 2007;17:878–81. doi: 10.1016/j.annepidem.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Headley AJ. Necrotizing soft tissue infections: a primary care review. Am Fam Physician. 2003;68:323–8. [PubMed] [Google Scholar]
- 28.Garcia-Tsao G, Wiest R. Gut microflora in the pathogenesis of the complications of cirrhosis. Best Pract Res Clin Gastroenterol. 2004;18:353–72. doi: 10.1016/j.bpg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Galati JS, Holdeman KP, Bottjen PL, et al. Gastric emptying and orocecal transit in portal hypertension and end-stage chronic liver disease. Liver Transpl Surg. 1997;3:34–8. doi: 10.1002/lt.500030105. [DOI] [PubMed] [Google Scholar]
- 30.Gunnarsdottir SA, Sadik R, Shev S, et al. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98:1362–70. doi: 10.1111/j.1572-0241.2003.07475.x. [DOI] [PubMed] [Google Scholar]
- 31.Riordan SM, Williams R. The intestinal flora and bacterial infection in cirrhosis. J Hepatol. 2006;45:744–57. doi: 10.1016/j.jhep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–33. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 33.Fierer J, Finley F. Deficient serum bactericidal activity against Escherichia coli in patients with cirrhosis of the liver. J Clin Invest. 1979;63:912–21. doi: 10.1172/JCI109391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-González M, Boixeda D, Herrero D, et al. Effect of granulocyte-macrophage colony-stimulating factor on leukocyte function in cirrhosis. Gastroenterology. 1993;105:527–31. doi: 10.1016/0016-5085(93)90730-z. [DOI] [PubMed] [Google Scholar]
- 35.Mellencamp MA, Preheim LC. Pneumococcal pneumonia in a rat model of cirrhosis: effects of cirrhosis on pulmonary defense mechanisms against Streptococcus pneumoniae. J Infect Dis. 1991;163:102–8. doi: 10.1093/infdis/163.1.102. [DOI] [PubMed] [Google Scholar]
- 36.Homann C, Varming K, Høgåsen K, et al. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut. 1997;40:544–9. doi: 10.1136/gut.40.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rimola A, Soto R, Bory F, et al. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4:53–8. doi: 10.1002/hep.1840040109. [DOI] [PubMed] [Google Scholar]
- 38.Borzio M, Salerno F, Piantoni L, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41–8. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 39.Thulstrup AM, Sørensen HT, Schønheyder HC, et al. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin Infect Dis. 2000;31:1357–61. doi: 10.1086/317494. [DOI] [PubMed] [Google Scholar]
- 40.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016–20. doi: 10.1378/chest.124.3.1016. [DOI] [PubMed] [Google Scholar]
- 41.Kuo CH, Changchien CS, Yang CY, et al. Bacteremia in patients with cirrhosis of the liver. Liver. 1991;11:334–9. doi: 10.1111/j.1600-0676.1991.tb00539.x. [DOI] [PubMed] [Google Scholar]


