Abstract
Asymmetric septal hypertrophy with systolic anterior motion of the mitral valve is frequently a phenotypic, but not pathognomonic, expression of genetic hypertrophic cardiomyopathy (HCM) with or without obstruction. It can, however, be associated nonspecifically with other forms of increased left ventricular (LV) afterload. We herein report the case of a young man with obesity cardiomyopathy and heart failure who presented with asymmetric septal hypertrophy and marked LV hypertrophy, and endomyocardial biopsy ruled out genetic HCM.
Keywords: asymmetric left ventricular hypertrophy, endomyocardial biopsy, familial hypertrophic cardiomyopathy, morbid obesity
INTRODUCTION
Obesity is associated with increased myocardial wall thickness and varying degrees of diastolic dysfunction.(1,2) The proposed mechanism for the observed myocardial changes is an increase in the total circulating blood volume because of a higher metabolic demand for adipose tissues.(3) Cardiomyopathy in obesity usually presents as dilated cardiomyopathy (DCM) due to eccentric remodelling.(2,4) DCM is an independent predictor of heart failure.(5) On another note, asymmetric septal hypertrophy with systolic anterior motion of the mitral valve is frequently a phenotypic, but not pathognomonic, expression of genetic hypertrophic cardiomyopathy (HCM) with or without obstruction. We herein report the case of a morbidly obese man who presented with congestive heart failure (CHF).
CASE REPORT
Our patient was a morbidly obese 29-year-old man who weighed 149 kg and had a body mass index (BMI) of 40 kg/m2 at presentation. He was previously diagnosed with HCM at 12 years of age, but was subsequently lost to follow-up. In 2010, he developed bilateral deep vein thromboses, where the diagnosis of protein S deficiency was made. He was put on long-term anticoagulation, but lacked compliance to medication. In November 2012, he complained of breathlessness that was made worse on exertion. A clinical diagnosis of CHF was made. Echocardiography showed marked asymmetric septal hypertrophy (septal wall thickness and posterior wall thickness measured 23 mm and 14 mm, respectively; the septal to posterior wall thickness ratio was 1.64), systolic anterior motion of the mitral valve, moderately severe posteriorly-directed mitral regurgitation, and pulmonary artery systolic pressure of 69 mmHg. Left ventricular (LV) systolic function was hyperdynamic, and the maximum pressure gradient across the LV outflow tract was 57 mmHg (Figs. 1 & 2). There was no ‘ground glass’ appearance of the myocardium. Diastolic dysfunction was evident based on the presence of restrictive filling pattern (mitral E/A ratio of 2.15 and short mitral deceleration time of 158 ms) and elevated mitral E/E’ ratio of 15.6 and 18.7 at the septal and lateral mitral annuli, respectively, indicating high filling pressures (Fig. 3). However, these findings are nonspecific and may be widely observed in familial HCM. A diagnosis of diastolic CHF and obstructive HCM was made. Notably, he had a similar presentation at another hospital, ten months before presenting at our institution.
Fig. 1.

Transthoracic echocardiogram of the patient. Parasternal long axis view shows (a) thickened interventricular septum in diastole (IVd) measuring 23 mm and a mild thickened posterior wall in diastole (PWd) measuring 14 mm, and (b) moderately severe posteriorly-directed mitral regurgitation (arrow). (c) Parasternal short axis view shows markedly thickened interventricular septum during diastole (IVd) and a mild thickened posterior wall (PWd), with a ratio of 1.64. (d) Apical four-chamber view shows systolic anterior motion (SAM) of the mitral leaflet during systole (arrow).
Fig. 2.
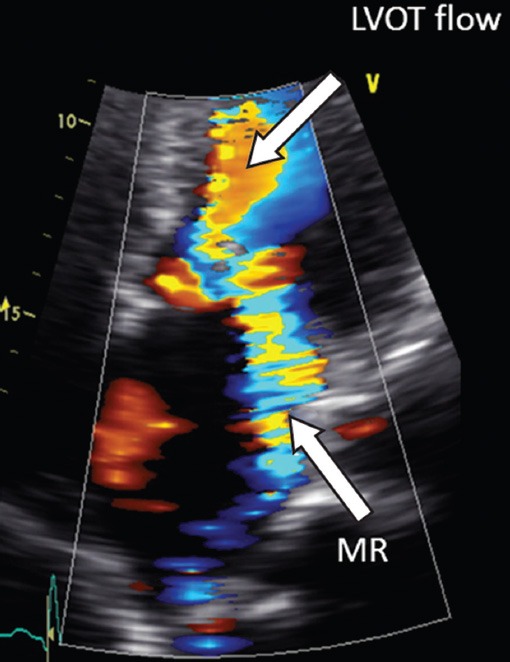
Apical four-chamber echocardiogram view with colour flow imaging shows flow acceleration across the left ventricular outflow tract and eccentric mitral regurgitation directed posterolaterally.
Fig. 3.
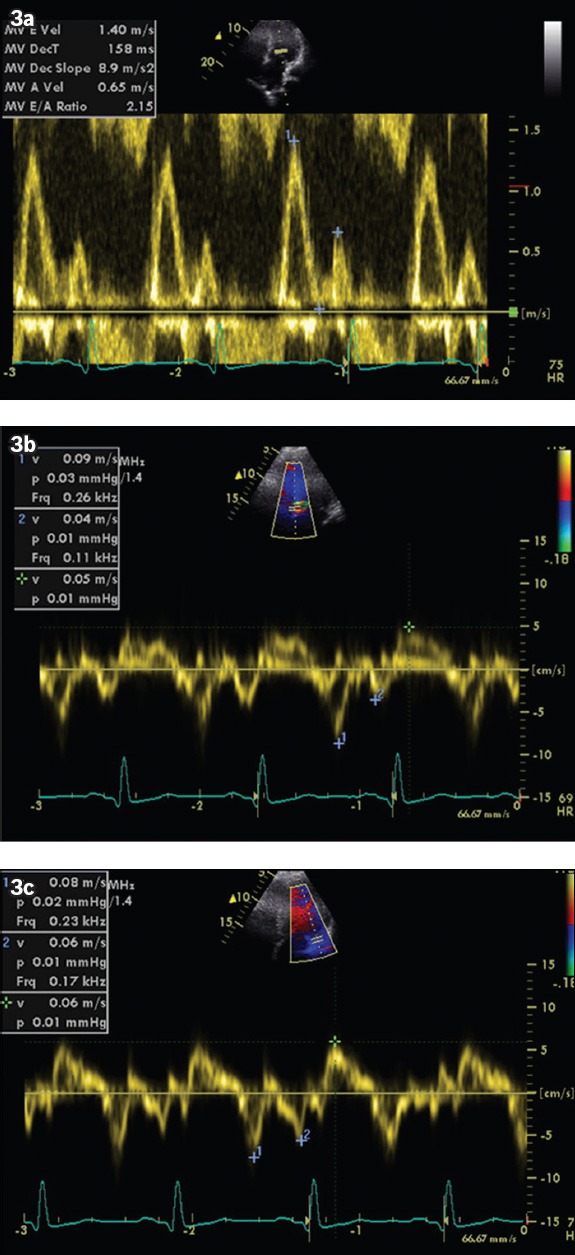
(a) Apical four-chamber view shows mitral inflow velocities. E and A wave velocities measured 140 and 65 millisec, respectively, giving E/A ratio of 2.1. No L wave (mid-diastolic) was observed. (b & c) Tissue Doppler echocardiogram shows E’ velocities measuring 9 cm/s and 7.5 cm/s at the septal and lateral mitral annuli, respectively, giving a E/E’ ratio of 15.6 and 18.7, respectively.
At presentation to our institution, the patient responded to diuretic and noninvasive positive pressure ventilation. He underwent a right heart study that confirmed the diagnosis of severe pulmonary hypertension (the mean pulmonary artery pressure was 61 mmHg), although the pulmonary capillary wedge pressure was elevated at 41 mmHg. Systemic blood pressure was 120/87 mmHg. The ensuing endomyocardial biopsy taken from the right ventricular septum showed nonspecific, mildly hypertrophied myocytes with diameters ranging from 20 µm to 30 µm and containing enlarged nuclei. Neither obvious myofibre disarray nor small intramural coronary arteriole dysplasia was observed (Fig. 4).
Fig. 4.

Photomicrograph of the myocardium shows hypertrophied myofibres with enlarged nuclei (arrowheads), with no evidence of myofibril architectural disarray, and the intramural arterioles appeared normal (arrow) (Haematoxylin & eosin, × 200).
The final diagnosis was diastolic CHF and asymmetric LV hypertrophy contributed to by morbid obesity and possibly obstructive sleep apnoea, where the cardiac morphology mimicked familial hypertrophic obstructive cardiomyopathy (HOCM).
DISCUSSION
Obesity is associated with increased myocardial wall thickness, larger LV mass index by height, and diastolic dysfunction,(1) as evidenced by the impairment of diastolic LV longitudinal function and increased LV diastolic filling pressure.(6) Cardiomyopathy in obesity can present as DCM,(4,7) although this finding has been disputed.(8) In obese patients associated with DCM, myocardial structural changes can be insignificant even at the ultrastructural level.(4) ‘Obesity cardiomyopathy’ has been loosely defined as clinical heart failure directly associated with myostructural changes in a morbidly obese person. Indeed, morbid obesity is an independent predictor of heart failure.(5) There is, thus far, no literature report of obesity cardiomyopathy presenting with asymmetric septal hypertrophy and systolic anterior motion phenotype, especially in the absence of increased afterload such as marked hypertension. Preload reserve is increased in obese patients, as demonstrated by the significant increase in the left atrial dimension.(6)
In our young patient, diabetes mellitus was ruled out based on normal random and fasting blood glucose profile. His recurrent diastolic CHF presentation is highly unusual for a patient with genetic HCM who has neither atrial fibrillation nor a reduced left ventricular ejection fraction < 50% (i.e. end-stage phase of HCM). Histological investigation revealed nonspecific LV hypertrophy, as evidenced by increased myofibre dimensions and connective tissue deposition, albeit the appearance of a classic HOCM cardiac morphology on echocardiography and chest computed tomography (Fig. 5).
Fig. 5.

Chest CT scan shows four-chamber of the heart. The septal and lateral wall thicknesses are 26 mm and 12.8 mm, respectively, with a resulting septal-to-posterior wall thickness ratio of 2.1.
Kasper et al(7) compared 43 obese with 409 nonobese heart failure patients. While specific aetiologies were found in two-thirds of the nonobese patients, significantly fewer (about a quarter) of the obese cohort had identifiable heart failure risk factors. The most common finding from endomyocardial biopsy in the obese group was mild myocyte hypertrophy (67%). Notably, BMI correlated positively with right heart pressures and cardiac output, pulmonary vascular resistance index and systolic blood pressure. A significantly higher proportion of obese patients were found to have idiopathic DCM, compared with nonobese patients.(7) Using ultrasonic integrated backscatter (IBS) in assessing myocardial reflectivity (index of elevated myocardial collagen content), Di Bello et al(6) showed that obese patients had higher IBS indexes at the septum level than in nonobese patients (i.e. the control group), indicating fibrosis-forming processes. Obese patients also had significantly lower cyclic variation index both at the septum and posterior wall.(6) The study therefore suggested that obese patients exhibit myocardial structural and functional alterations that are physiologically consistent with incipient or subclinical obesity cardiomyopathy.
Additionally, in our young obese patient, it may well be that his diastolic heart failure was precipitated by altered physiological mechanism in the context of obstructive sleep apnoea, which is highly associated with heart failure,(9) chronic circulatory volume overload(10) and systemic inflammation with elevated concentrations of plasma adipokines from excess adipose tissue and cytokines.(11)
In conclusion, this case report highlights the importance of endomyocardial biopsy in patients with HCM with unusual presentation. Aggressive weight loss strategy including bariatric surgery may be effective in reversing LV hypertrophy.(12)
REFERENCES
- 1.Mehlman E, Bright JM, Jeckel K, et al. Echocardiographic evidence of left ventricular hypertrophy in obese dogs. J Vet Intern Med. 2013;27:62–8. doi: 10.1111/jvim.12018. [DOI] [PubMed] [Google Scholar]
- 2.Wong C, Marwick TH. Obesity cardiomyopathy: diagnosis and therapeutic implications. Nat Clin Pract Cardiovasc Med. 2007;4:480–90. doi: 10.1038/ncpcardio0964. [DOI] [PubMed] [Google Scholar]
- 3.Alpert MA, Fraley MA, Birchem JA, Senkottaiyan N. Management of obesity cardiomyopathy. Expert Rev Cardiovasc Ther. 2005;3:225–30. doi: 10.1586/14779072.3.2.225. [DOI] [PubMed] [Google Scholar]
- 4.Saito T, Asai K, Sato S, et al. Myocardial alterations and clinical implications associated with recovery of cardiac function in dilated cardiomyopathy with obesity. Int J Cardiol. 2013;168:144–50. doi: 10.1016/j.ijcard.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 6.Di Bello V, Santini F, Di Cori A, et al. Obesity cardiomyopathy: is it a reality? An ultrasonic tissue characterization study. J Am Soc Echocardiogr. 2006;19:1063–71. doi: 10.1016/j.echo.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Kasper EK, Hruban RH, Baughman KL. Cardiomyopathy of obesity: a clinicopathologic evaluation of 43 obese patients with heart failure. Am J Cardiol. 1992;70:921–4. doi: 10.1016/0002-9149(92)90739-l. [DOI] [PubMed] [Google Scholar]
- 8.Khan MF, Movahed MR. Obesity cardiomyopathy and systolic function: Obesity is not independently associated with dilated cardiomyopathy. Heart Fail Rev. 2013;18:207–17. doi: 10.1007/s10741-012-9320-4. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaltman AJ, Goldring RM. Role of circulatory congestion in the cardiorespiratory failure of obesity. Am J Med. 1976;60:645–53. doi: 10.1016/0002-9343(76)90499-x. [DOI] [PubMed] [Google Scholar]
- 11.Frankel DS, Vasan RS, D’Agostino RB, Sr, et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–62. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198–202. doi: 10.1016/j.cardfail.2007.12.006. [DOI] [PubMed] [Google Scholar]


