Abstract
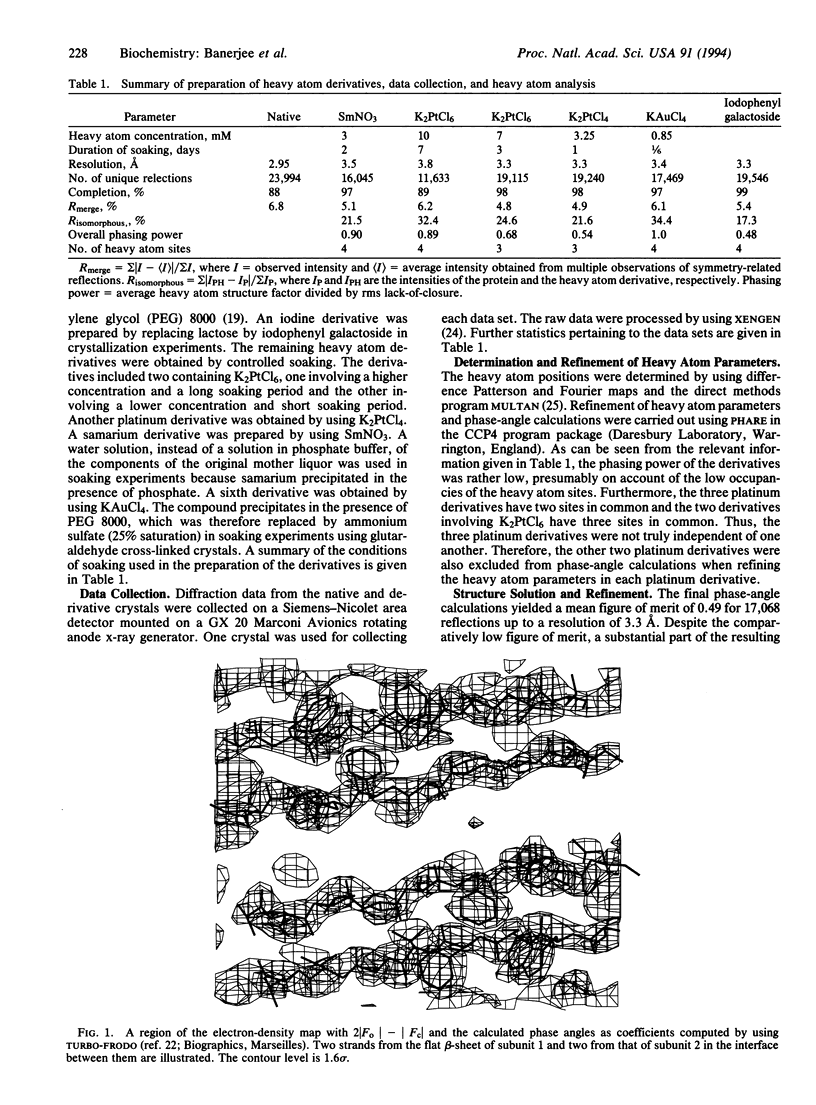
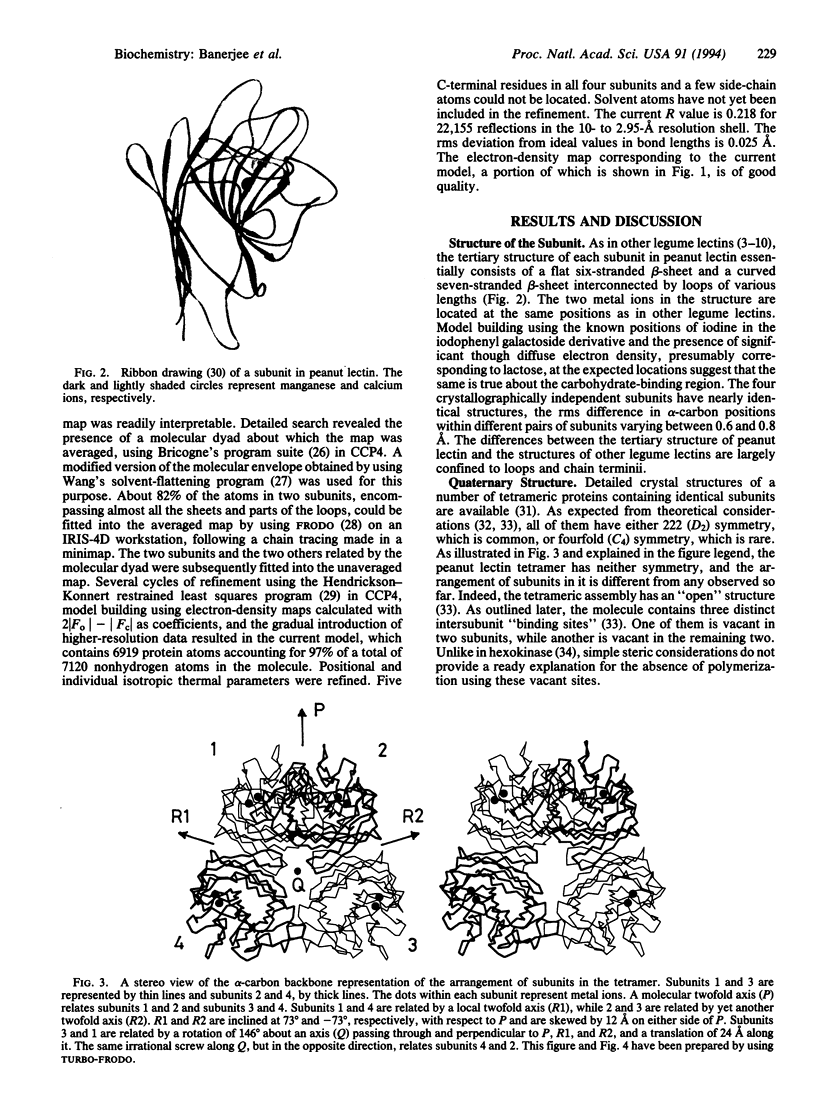
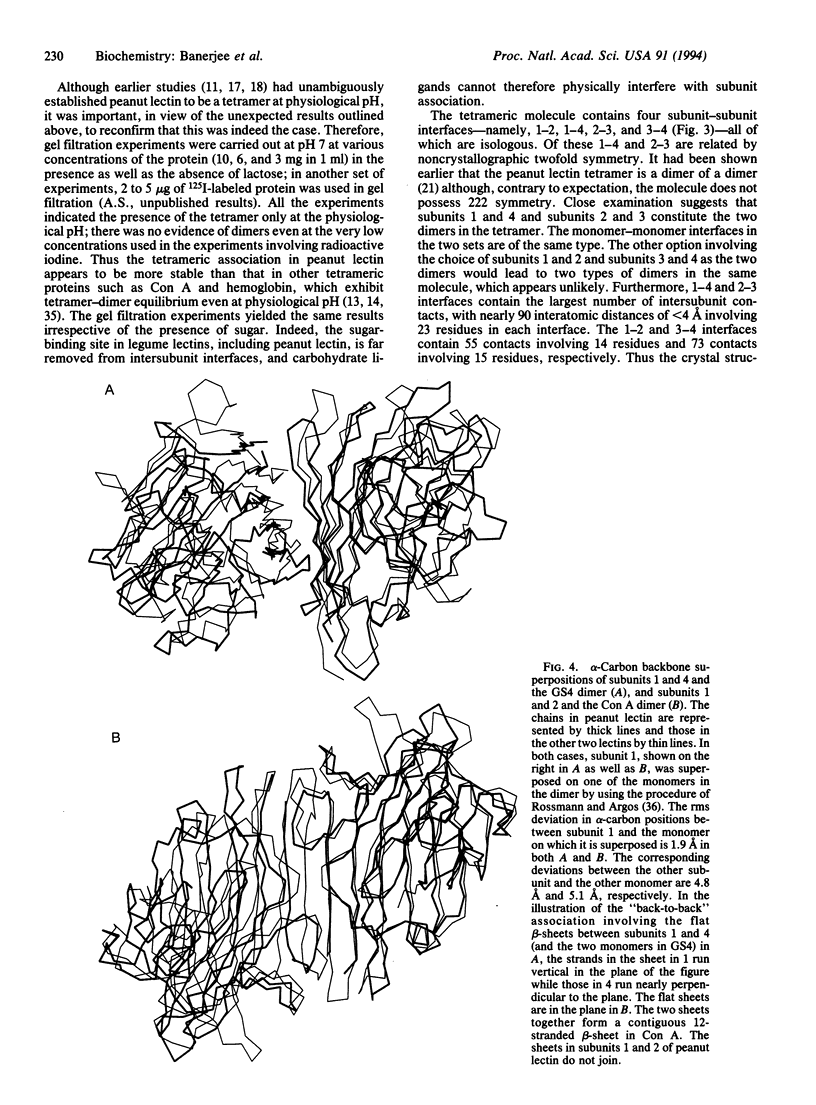
The x-ray crystal structure of the tetrameric T-antigen-binding lectin from peanut, M(r) 110,000, has been determined by using the multiple isomorphous replacement method and refined to an R value of 0.218 for 22,155 reflections within the 10- to 2.95-A resolution range. Each subunit has essentially the same characteristic tertiary fold that is found in other legume lectins. The structure, however, exhibits an unusual quaternary arrangement of subunits. Unlike other well-characterized tetrameric proteins with identical subunits, peanut lectin has neither 222 (D2) nor fourfold (C4) symmetry. A noncrystallographic twofold axis relates two halves of the molecule. The two monomers in each half are related by a local twofold axis. The mutual disposition of the axes is such that they do not lead to a closed point group. Furthermore, the structure of peanut lectin demonstrates that differences in subunit arrangement in legume lectins could be due to factors intrinsic to the protein molecule and, contrary to earlier suggestions, are not necessarily caused by interactions involving covalently linked sugar. The structure provides a useful framework for exploring the structural basis and the functional implications of the variability in the subunit arrangement in legume lectins despite all of them having nearly the same subunit structure, and also for investigating the general problem of "open" quaternary assembly in oligomeric proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. W., Reeke G. N., Jr, Wang J. L., Cunningham B. A., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. III. Structure of the monomer and its interactions with metals and saccharides. J Biol Chem. 1975 Feb 25;250(4):1513–1524. [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bourne Y., Abergel C., Cambillau C., Frey M., Rougé P., Fontecilla-Camps J. C. X-ray crystal structure determination and refinement at 1.9 A resolution of isolectin I from the seeds of Lathyrus ochrus. J Mol Biol. 1990 Jul 20;214(2):571–584. doi: 10.1016/0022-2836(90)90199-V. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. J., Koshland D. E., Jr The quaternary structure of proteins composed of identical subunits. J Biol Chem. 1971 May 25;246(10):3092–3102. [PubMed] [Google Scholar]
- Decastel M., De Boeck H., Goussault Y., De Bruyne C. K., Loontiens F. G., Frénoy J. P. Effect of pH on oligomeric equilibrium and saccharide-binding properties of peanut agglutinin. Arch Biochem Biophys. 1985 Aug 1;240(2):811–819. doi: 10.1016/0003-9861(85)90090-6. [DOI] [PubMed] [Google Scholar]
- Einspahr H., Parks E. H., Suguna K., Subramanian E., Suddath F. L. The crystal structure of pea lectin at 3.0-A resolution. J Biol Chem. 1986 Dec 15;261(35):16518–16527. [PubMed] [Google Scholar]
- Fish W. W., Hamlin L. M., Miller R. L. The macromolecular properties of peanut agglutinin. Arch Biochem Biophys. 1978 Oct;190(2):693–698. doi: 10.1016/0003-9861(78)90328-4. [DOI] [PubMed] [Google Scholar]
- Hardman K. D., Ainsworth C. F. Structure of concanavalin A at 2.4-A resolution. Biochemistry. 1972 Dec 19;11(26):4910–4919. doi: 10.1021/bi00776a006. [DOI] [PubMed] [Google Scholar]
- Ip S. H., Ackers G. K. Thermodynamic studies on subunit assembly in human hemoglobin. Temperature dependence of the dimer-tetramer association constants for oxygenated and unliganded hemoglobins. J Biol Chem. 1977 Jan 10;252(1):82–87. [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Majumdar T., Surolia A. A large scale preparation of peanut agglutinin on a new affinity matrix. Prep Biochem. 1978;8(2-3):119–131. doi: 10.1080/00327487808069054. [DOI] [PubMed] [Google Scholar]
- Mande S. C., Raghunathan S., Salunke D. M., Khan M. I., Swamy M. J., Surolia A., Vijayan M. Structural studies on peanut lectin. Indian J Biochem Biophys. 1988 Feb-Apr;25(1-2):166–171. [PubMed] [Google Scholar]
- ROSSIFANELLI A., ANTONINI E., CAPUTO A. HEMOGLOBIN AND MYOGLOBIN. Adv Protein Chem. 1964;19:73–222. doi: 10.1016/s0065-3233(08)60189-8. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W. Three-dimensional structure of favin: saccharide binding-cyclic permutation in leguminous lectins. Science. 1986 Nov 28;234(4780):1108–1111. doi: 10.1126/science.3775378. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. A comparison of the heme binding pocket in globins and cytochrome b5. J Biol Chem. 1975 Sep 25;250(18):7525–7532. [PubMed] [Google Scholar]
- Salunke D. M., Khan M. I., Surolia A., Vijayan M. Crystallization and preliminary X-ray studies of the anti-T lectin from peanut (Arachis hypogaea). J Mol Biol. 1982 Jan 5;154(1):177–178. doi: 10.1016/0022-2836(82)90425-9. [DOI] [PubMed] [Google Scholar]
- Salunke D. M., Swamy M. J., Khan M. I., Mande S. C., Surolia A., Vijayan M. Arrangement of subunits in peanut lectin. Rotation function and chemical cross-linking studies. J Biol Chem. 1985 Nov 5;260(25):13576–13579. [PubMed] [Google Scholar]
- Senear D. F., Teller D. C. Effects of saccharide and salt binding on dimer-tetramer equilibrium of concanavalin A. Biochemistry. 1981 May 26;20(11):3083–3091. doi: 10.1021/bi00514a015. [DOI] [PubMed] [Google Scholar]
- Senear D. F., Teller D. C. Thermodynamics of concanavalin A dimer-tetramer self-association: sedimentation equilibrium studies. Biochemistry. 1981 May 26;20(11):3076–3083. doi: 10.1021/bi00514a014. [DOI] [PubMed] [Google Scholar]
- Shaanan B., Lis H., Sharon N. Structure of a legume lectin with an ordered N-linked carbohydrate in complex with lactose. Science. 1991 Nov 8;254(5033):862–866. doi: 10.1126/science.1948067. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Fletterick R. J., Anderson W. F., Anderson C. M. High resolution x-ray structure of yeast hexokinase, an allosteric protein exhibiting a non-symmetric arrangement of subunits. J Mol Biol. 1976 Jun 14;104(1):197–122. doi: 10.1016/0022-2836(76)90009-7. [DOI] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Young N. M., Johnston R. A., Watson D. C. The amino acid sequence of peanut agglutinin. Eur J Biochem. 1991 Mar 28;196(3):631–637. doi: 10.1111/j.1432-1033.1991.tb15859.x. [DOI] [PubMed] [Google Scholar]




