Abstract
INTRODUCTION
Clinical practice guidelines recommend dietary sodium restriction in chronic kidney disease (CKD) patients. Compliance with this recommendation in a multiethnic Asian population is not clear. This study assessed the urinary sodium excretion profile of a multiethnic Asian population to estimate the population’s dietary sodium intake.
METHODS
Data on the urinary sodium excretion of 335 participants were obtained from the Asian Kidney Disease Study and Singapore Kidney Function Study. Standard statistical tests and linear regression were used to assess the association between various continuous variables and sodium excretion.
RESULTS
Our study cohort consisted of 335 participants (232 with CKD, 103 healthy) – 51.0% were male; 38.5% were Chinese, 29.6% were Malay, 23.6% were Indian; and 57.3% were hypertensive. The mean age was 53.5 ± 15.1 years and mean urinary sodium excretion was 124.9 ± 68.3 mmol/day. The mean blood pressure of the healthy participants was lower than that of the patients with CKD (p < 0.001). Patients with CKD stages 1–3 excreted an average of > 100 mmol sodium/day. Overall, 40.1% patients with CKD excreted < 100 mmol sodium/day. Indians had higher urinary sodium excretion than the Chinese (p = 0.016) and Malays (p = 0.002). The distribution of urinary sodium excretion in the healthy participants (37.9% excreted < 100 mmol sodium/day) was similar to that seen in the patients with CKD.
CONCLUSION
Although patients with CKD stages 4–5 achieved sodium restriction, healthy persons and patients with early-stage CKD need to increase their efforts in reducing their sodium intake, especially for patients of Indian ethnicity.
Keywords: Asian Continental Ancestry Group, chronic diet, hypertension, kidney failure, sodium
INTRODUCTION
Clinical practice guidelines recommend dietary sodium restriction (< 100 mmol/day) as part of the nutritional management of patients with chronic kidney disease (CKD).(1-3) Excessive sodium intake (i.e. beyond the recommended levels) is associated with hypertension and fluid overload. The proportion of patients with CKD who successfully achieve this target and the effectiveness of the recommendations in a multiethnic Asian population with varied diets and cultural beliefs are unknown.(4,5) Thus, this study aimed to assess the urinary sodium excretion profile of a multiethnic Asian population, as an estimate of the dietary sodium intake of that population. In this cross-sectional study, we compared the dietary sodium intake of patients with CKD with that of healthy participants.
METHODS
The present study is a sub-study of data obtained from the Singapore Kidney Function Study phase 1 (SKFS1) and the Asian Kidney Disease Study (AKDS).(6) This study is approved by the institution review board. Both SKFS1 and AKDS were studies that recruited participants for the primary objective of measuring and estimating glomerular filtration rate (GFR).
In SKFS1, we recruited 103 healthy volunteers presenting to National University Hospital, Singapore. The inclusion criterion was nonpregnant adults aged > 21 years. Volunteers were excluded if they: (a) were unable to consent; (b) had physical conditions that render phlebotomy for blood samples difficult; (c) were unable to collect urine samples successfully; (d) were using regular medications; or (e) had either hypertension, diabetes mellitus, possible kidney dysfunction (determined using urinalysis or renal imaging), or any condition that could potentially interfere with the accuracy of GFR measurement. Volunteers were screened for haematuria, leucocyturia, proteinuria and microalbuminuria using urine dipsticks. The target sample size was three male and three female volunteers per decade from age 21 years per ethnic group (i.e. Chinese, Malay, Indian and others).
In AKDS, we recruited 232 patients with CKD presenting to the outpatient nephrology clinics of the National University Hospital, Singapore. Patients with CKD were eligible for inclusion if they were: (a) not pregnant; (b) adult aged > 21 years; (c) had serum creatinine with an estimated or measured GFR (i.e. using the modification of diet in renal disease formula, Cockroft-Gault(7) formula or creatinine clearance) of 10–90 mL/min; and (d) had stable CKD, defined as two serum creatinine levels that were measured > 60 days apart, with less than 20% difference (the definition of CKD followed that in the clinical practice guidelines).(8) Patients with CKD were excluded if they met any of the following criteria: (a) inability to consent; (b) presence of physical conditions that render phlebotomy for blood samples difficult; (c) inability to collect urine samples successfully; and (d) the presence of acute kidney function deterioration or any condition that could potentially interfere with the accuracy of the GFR measurement. Before recruitment, patients were stratified according to ethnicity (i.e. Chinese, Malay, Indian and others) and gender.
The study coordinator ensured that the participants only initiated the 24-hour urine collections and GFR measurements if they were not titrating or starting on new medications. All participants performed a 24-hour urine collection, and presented the following day for GFR measurement and a blood test, and to hand in the collected urine samples. GFR was determined by three-sample plasma clearance of an intravenous bolus of 99mtechnetium-diethylene triamine pentaacetic acid (99mTc-DTPA),(9) and body surface area was calculated using the du Bois equation.(10) Urinary sodium excretion (mmol/day) was measured, using the 24-hour urine collections, to estimate dietary sodium intake. In the present study, the 24-hour urine collections for total sodium excretion were taken to be reflective of the daily sodium intake of patients with CKD, even those on diuretics or renin-angiotensin-aldosterone system blockers, based on the assumption that the patients had: (a) a relatively stable dietary sodium intake; (b) stable kidney function; and (c) no changes in the medications taken.(11-13)
The data obtained was presented as mean ± standard deviation or median (interquartile range). Analysis of variance and t-test were used to assess whether urinary sodium excretion was significantly associated with gender, ethnicity or CKD stages, whereas linear regression was used to assess whether urinary sodium excretion was significantly associated with age, height, weight, body mass index or GFR. All statistical analyses were performed using JMP 10 (SAS Institute, Cary, NC, USA). A p-value < 0.05 was considered statistically significant.
RESULTS
Our study cohort had a total of 335 participants. When a body mass index of 22.99 kg/m2 (i.e. the normal upper limit for Asians) was used to calculate ideal body weight, we found that the healthy participants (i.e. volunteers recruited for SKFS1) were 5.0 ± 10.4 kg overweight, while the participants with CKD (i.e. patients recruited for AKDS) were 11.8 ± 13.6 kg overweight (Table I).
Table I.
Characteristics of the study participants (n = 335).
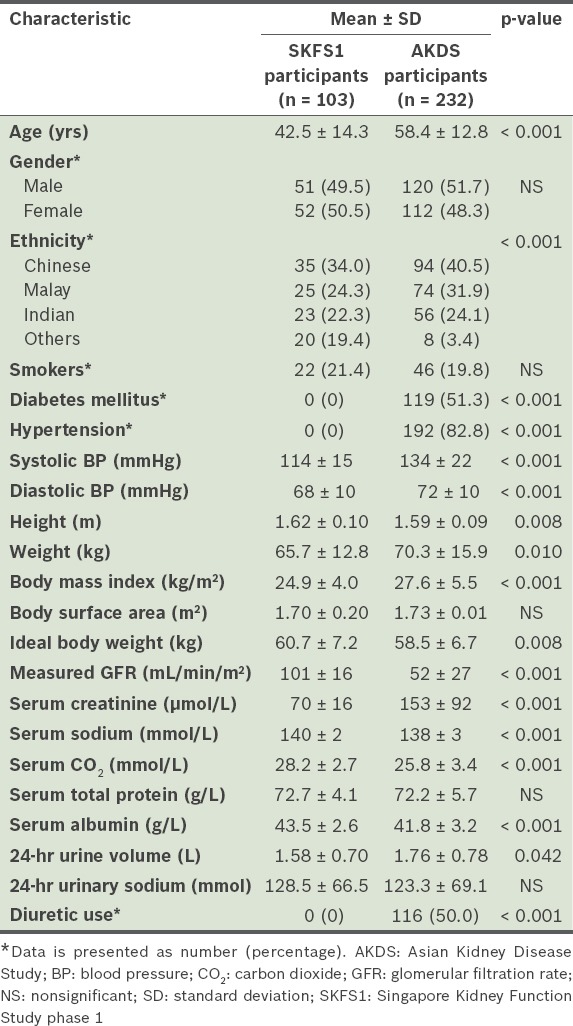
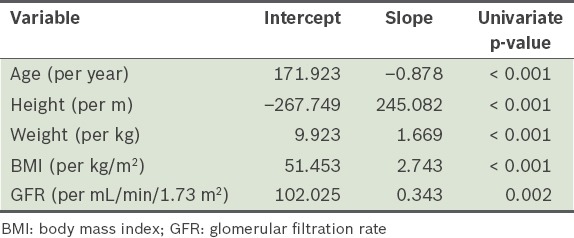
In the present study, urinary sodium excretion was significantly higher among men than women (p < 0.001) (Table II). We also found that Indian participants excreted significantly more sodium than the Chinese (p = 0.016) and Malay (p = 0.002) participants. Analysis using logistic regression showed that urinary sodium excretion (in mmol) was negatively associated with age (p < 0.001), and positively associated with height (p < 0.001), weight (p < 0.001), body mass index (p < 0.001) and GFR (p = 0.002) (Table III). Mean sodium excretion was not found to be significantly associated with diuretic use or diabetic status, and was similar between the healthy participants and the patients with CKD. On average, 67.3% (115/171) of the patients with CKD stages 1–3 excreted > 100 mmol sodium/day. Overall, 40.1% (93/232) of the patients with CKD excreted < 100 mmol sodium/day and 10.3% (24/232) excreted < 50 mmol sodium/day. The distribution of sodium excretion was similar among the healthy participants – 37.9% (39/103) excreted < 100 mmol sodium/day and 11.7% (12/103) excreted < 50 mmol sodium/day. Although three patients with CKD were on sodium bicarbonate, this did not impact their mean urinary sodium excretion.
Table II.
Results of analysis of variance and t-test performed to test for factors significantly associated with urinary sodium excretion.
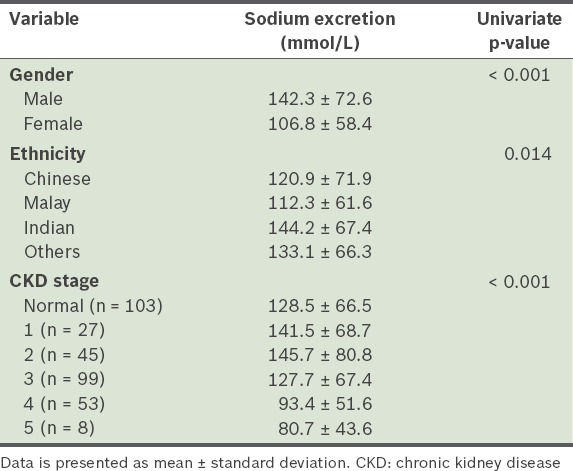
Table III.
Results of the logistic regression analysis performed to test for factors significantly associated with urinary sodium excretion.
DISCUSSION
To achieve good blood pressure control, patients with CKD need to adhere to dietary sodium restrictions. Good blood pressure control is crucial in retarding the progression of CKD. According to the current guidelines, patients with CKD are recommended a dietary sodium intake of less than 100 mmol/day.(11) In the present study, we found that dietary sodium intake (based on urinary sodium excretion) was high in a large proportion of patients with CKD. This finding is similar to the results of other CKD retardation studies.(14) While patients with more advanced CKD appeared to achieve the sodium restriction targets in the present study, those with CKD stages 1–3 did not. Anecdotally, dietetic assessment and counselling is a fee-for-service programme; therefore, many of the participants declined dietitian consultations in primary care and nephrology specialty clinics.
Even when angiotensin receptor blockers are administered, high urinary sodium excretion is associated with faster CKD progression and worse cardiovascular outcomes.(14) Arguably, efforts to retard CKD should be applied more vigorously during the earlier stages of CKD; it is then that the barriers to adequate dietetics services should be identified and removed.(15) However, it appears that patients who have more advanced CKD and more frequent follow-ups (and thus, a greater awareness) are more receptive toward dietary counselling. This results in a greater proportion of them achieving the sodium restriction targets. Moreover, as patients with advanced CKD often exhibit symptoms such as with oedema and fluid overload, they are more likely to comply with a salt-restricted diet.
A large proportion of patients with CKD also have metabolic acidosis, which if treated with sodium bicarbonate (for acid neutralisation), would make sodium targets harder to achieve.(16) However, in the present study, it was interesting that the urinary sodium excretion of the three patients who were on sodium bicarbonate supplementation was not significantly higher. As ethnic differences in food choices may also impact sodium intake, more rigorous counselling may be required to reduce the sodium intake of patients with CKD who are of Indian ethnicity.(4) There is a need for research that explores the presence of cultural and linguistic barriers in the dietary practices and counselling of patients with CKD in a multiethnic population.
The sodium intake of our study cohort, which consisted of healthy participants and participants with CKD, appeared to be lower than that of the general population (according to the Report of the National Nutrition Survey 2010 published by the Health Promotion Board, Singapore).(17) The healthy participants in the present study may have been healthier than the general population because individuals with diabetes mellitus and hypertension were excluded. Also, the participants with CKD in the present study may have consumed less sodium as a result of disease awareness and dietary management. However, it is difficult to quantify the effect of dietary intervention on urinary sodium excretion. Future studies to address this are needed.
The strengths of our prospective study include a large sample size of patients with reference GFR measurements, and the use of systematic urine collections for objective sodium intake measurements. The urine collections were consistently performed by the same research personnel. Recruitment of participants for the present study was also strategised to ensure that: (a) there was a range of GFRs among all the participants; and (b) the proportion of participants were representative of the ethnicity and gender ratios of the general population. One limitation of the present study was that there was a lack of information on previous formal dietetic assessments and interventions by a dietitian (most patients would have received dietary advice during follow-up sessions).
To conclude, the patients with CKD in the present study were either overweight or obese, and had a higher salt intake than that recommended in the guidelines. There is a need to address the cultural, linguistic and socioeconomic barriers to dietary compliance in a multiethnic population.
ACKNOWLEDGEMENTS
We acknowledge members of the Asian Kidney Disease Study and Singapore Kidney Function Study, which include Prof Sunil Sethi, Dr Arvind Kumar Sinha and Dr Borys Shuter. This study was primarily funded by the National Kidney Foundation of Singapore (NKFRC2007/08). Dr Teo, the first author of the present study, was also supported by the National Medical Research Council and the Ministry of Education Academic Research Fund (Yong Loo Lin School of Medicine Faculty Research Committee, National University of Singapore).
REFERENCES
- 1.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35:S1–140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 2.Pollock C, Voss D, Hodson E, Crompton C. The CARI guidelines. Nutrition and growth in kidney disease. Nephrology (Carlton) 2005;10(Suppl 5):S177–230. doi: 10.1111/j.1440-1797.2005.00506_1.x. [DOI] [PubMed] [Google Scholar]
- 3.Chronic Kidney Disease Evidence-Based Nutrition Practice Guideline. American Dietetic Association [online] [Accessed August 16 2012]. Available at: http://www.adaevidencelibrary.com/topic.cfm?cat=3927 .
- 4.Cultural sensitivity: definition, application, and recommendations for diabetes educators. Diabetes Educ. 2002;28:922–7. doi: 10.1177/014572170202800607. [DOI] [PubMed] [Google Scholar]
- 5.Brown TL. Meal-Planning Strategies: Ethnic Populations. Diabetes Spectrum. 2003;16:190–2. [Google Scholar]
- 6.Teo BW, Xu H, Wang D, et al. GFR Estimating equations in a multiethnic Asian population. Am J Kidney Dis. 2011;58:56–63. doi: 10.1053/j.ajkd.2011.02.393. [DOI] [PubMed] [Google Scholar]
- 7.Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 9.Fleming JS, Zivanovic MA, Blake GM, Burniston M, Cosgriff PS. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun. 2004;25:759–69. doi: 10.1097/01.mnm.0000136715.71820.4a. [DOI] [PubMed] [Google Scholar]
- 10.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known 1916. Nutrition. 1989;5:303–11. [PubMed] [Google Scholar]
- 11.Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–290. [PubMed] [Google Scholar]
- 12.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 13.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319–28. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambers Heerspink HJ, Holtkamp FA, Parving HH, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–7. doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]
- 15.Lim YP. Sharing Singapore's experience in dietetic practice and school nutrition programmes. Asia Pac J Clin Nutr. 2008;17(suppl 1):361–4. [PubMed] [Google Scholar]
- 16.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–84. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singapore: Health Promotion Board; 2010. Report of the National Nutrition Survery 2010. [Google Scholar]


