Abstract
INTRODUCTION
Preoperative staging is essential for the optimal treatment and surgical planning of colorectal cancers. This study was aimed to evaluate the accuracy of colorectal cancer staging done using contrast-enhanced multidetector computed tomographic colonography (CEMDCTC).
METHODS
We recruited 25 patients with 28 proven colorectal cancers. A 16-slice multidetector computed tomography scanner was used to generate two-dimensional multiplanar reformatted sagittal, coronal and oblique coronal images, and three-dimensional virtual colonography (endoluminal) images. Axial and reformatted views were analysed, and TNM staging was done. Patients underwent surgery and conventional colonoscopy, and surgical histopathological correlation was obtained.
RESULTS
The diagnostic accuracies for TNM colorectal cancer staging were 92.3% for T staging, 42.3% for N staging and 96.1% for M staging using CEMDCTC. There was excellent positive correlation for T staging between CEMDCTC and both surgery (κ-value = 0.686) and histopathology (κ-value = 0.838) (p < 0.0001), and moderate positive correlation for N staging between CEMDCTC and surgery (κ-value = 0.424; p < 0.0001). The correlation between CEMDCTC and histopathology for N staging was poor (κ-value = 0.186; p < 0.05); the negative predictive value was 100% for lymph node detection. Moderate positive correlation was seen for M staging between CEMDCTC and both surgery (κ-value = 0.462) and histopathology (κ-value = 0.649). No false negatives were identified in any of the M0 cases.
CONCLUSION
CEMDCTC correlated well with pathologic T and M stages, but poorly with pathologic N stage. It is an extremely accurate tool for T staging, but cannot reliably distinguish between malignant lymph nodes and enlarged reactive lymph nodes.
Keywords: colonography, colorectal cancer, contrast-enhanced multidetector computed tomographic colonography
INTRODUCTION
Colorectal cancer is the third most common cancer in both men and women.(1) The pathological stage of the cancer is the most important predictive factor of overall survival in patients with colorectal cancer.(2,3) Between 1.5% and 9.0% of patients with colorectal carcinoma have a second synchronous cancer, and 27%–55% have multiple coexistent adenomatous polyps.(4) Evaluation of the entire colon and accurate preoperative staging are essential for the optimal treatment and surgical planning of colorectal cancers. This also helps to identify patients who may benefit from chemoradiation.(5) In patients with synchronous liver metastases, studies have shown that simultaneous hepatic resection is suitable for patients with 0–3 colorectal lymph node metastases, whereas neoadjuvant chemotherapy prior to resection may be more suitable for patients with ≥ 4 colorectal lymph node metastases.(6)
As the low spatial and contrast resolution of conventional computed tomography (CT) protocols does not allow detailed evaluation, CT is not recommended for colorectal cancer staging.(7,8) However, multidetector computed tomography (MDCT), an advancement of CT technology, can acquire multiple simultaneous slices in a single breath-hold. Its advantages include faster scanning time, better spatial resolution, lesser motion artefacts and volume imaging. Volume imaging allows for the acquisition of either thinner or thicker sections from the same raw data, thus improving three-dimensional reconstructions and multiplanar reformation capability.(8)
In MDCT colonography, the volumetric data of the large bowel obtained via high-resolution helical CT is analysed using specialised computer software to generate endoluminal images. The advantages of MDCT colonography over conventional colonoscopy include the former’s noninvasive nature (which leads to better patient compliance), the ability to visualise the entire colon (conventional colonoscopy fails in 5% of cases), the absence of blind areas, and the ability to evaluate extracolonic pathology.(9) MDCT colonography also allows: (a) simultaneous assessment of colonic mucosal surface, depth of wall invasion, pericolic lymph nodes, surrounding structures and proximal colon in patients with occlusive carcinoma; and (b) the identification of synchronous carcinomas and/or coexisting adenomatous polyps, which could influence the treatment plan.(4,10)
While several studies have proven that CT colonography can be used to screen for colorectal cancer,(9-11) few studies have evaluated whether contrast-enhanced MDCT colonography (CEMDCTC) is valuable for preoperative staging of colorectal cancer.(9,11) The present study aimed to compare the accuracy of colorectal cancer staging done using CEMDCTC against that done using surgery and histopathology.
METHODS
This prospective study was approved by the Institute Review Board of the Post Graduate Institute of Medical Education and Research, Chandigarh, India. Over a period of three years, a total of 28 consecutive patients diagnosed with colorectal cancer on colonoscopy who were referred for CEMDCTC were included in the present study. Three patients were excluded due to the presence of renal failure (n = 2) and a history of an allergic reaction to iodinated contrast medium (n = 1). Thus, a total of 25 patients were enrolled in the present study. Informed consent was obtained from all the patients. All the patients underwent surgery within two weeks of CEMDCTC, and histopathological evaluation was done using the specimens collected during the surgeries. Curative resection was done in 23 patients, and palliative surgery (ileotransverse anastomoses) was performed for 2 patients with unresectable cancers.
Two packets of polyethylene glycol electrolyte solution (Peglec; Tablets (India) Limited, Chennai, India) in 2 L of water were given to the patients over 2 hours, 6 hours prior to the study. As 5 (20.0%) of our patients underwent conventional colonoscopy on the same day as CEMDCTC, we chose to use the wet preparation without faecal tagging for all our patients, in order to maintain uniformity in bowel preparation for all patients. All patients were given an intravenous (IV) injection of hyoscine butylbromide 20 mg (Buscopan; Biochem Pharmaceutical Industries Ltd, Mumbai, India) before rectal air insufflation to reduce bowel peristalsis and colonic spasm.
CT was performed using a 16-slice MDCT scanner (Siemens Sensation 16; Siemens Medical System, Forchheim, Germany), with the following settings: 120 kVp, 60 mAs in prone and 200 mAs in supine, 0.75 mm collimation, and 10 mm × 10 mm section width with 1 mm reconstruction thickness and 0.7 mm reconstruction interval. At the start of the procedure, each patient was placed in the left lateral decubitus position on the CT table, and the colon was insufflated with room air channelled through a rectal tube. Initially, 40 puffs (2 L) of air were used and the adequacy of large bowel distension achieved was evaluated using a CT scout film. If distension was inadequate, more air would be administered, according to patient tolerance. At the time of this study, dedicated carbon dioxide insufflation pump for CT colonography was not available in our country.
Unenhanced images were first acquired with the patient in prone position. Contrast-enhanced CT was subsequently performed with the patient in supine position, after the IV administration of 100 mL of non-ionic contrast at the rate of 3 mL/s, using an automated power injector in the portal venous phase, with a 70 s delay between the start of contrast administration and the start of helical scanning. CT acquisition was performed from the domes of diaphragm to the lower margin of the symphysis pubis during a single breath-hold. Two-dimensional multiplanar reformatted sagittal, coronal and oblique coronal images, and three-dimensional virtual colonography (endoluminal) images were generated.
The studies were evaluated by two radiologists (both with more than 7 years of experience in abdominal imaging) and their findings were recorded in consensus. Grading of the colonic distension seen on CEMDCTC was done according to the criteria used in previous studies.(12,13) Optimal colonic distension was deemed to have been achieved when the colonic wall was ‘pencil-thin’ throughout the segment, with thin, haustral folds that were less than 2 mm thick throughout their length. Axial and reformatted views were analysed and the findings of the 16-slice MDCT were recorded.
TNM staging (i.e. tumour, node, metastasis) in the present study was done based on the international TNM classification,(3) using both axial and reformatted multiplanar images. The TNM classification was compared against the surgical and histological findings. Synchronous lesions were also evaluated on CEMDCTC. As the T1 and T2 stages cannot be reliably differentiated from each other on CEMDCTC when bowel wall thickness exceeds 5 mm, they were grouped under one category (i.e. T1/T2 stage). A mass in the colon without pericolonic stranding (Fig. 1) would also be staged as T1/T2. A tumour is staged as T3 if pericolonic stranding and/or advancing nodular margin (Fig. 2) is present. Tumours which infiltrate the surrounding organs (Fig. 3) are staged as T4.
Fig. 1.

A 33-year-old man with a known case of ulcerative colitis presented with abdominal pain and per rectal bleeding. (a) Axial CEMDCTC image shows polypoidal obstructive growth (arrow) in the sigmoid colon, with increased vascularity in the adjacent mesentery. No pericolonic stranding or lymphadenopathy is seen. TNM staging for this patient was as follows: CT stage – T2N0M0, surgical stage – T2N0M0, and histological stage – T2N0M0. (b) CT colonographic image shows the presence of multiple pseudopolyps throughout the entire colon and (c) a polypoidal growth in the sigmoid colon.
Fig. 2.

A 46-year-old man presented with abdominal pain and weakness. (a) Axial and (b) coronal multiplanar reformatted CEMDCTC images show heterogeneously enhancing annular polypoidal growth in the proximal transverse colon with pericolonic stranding and advancing nodular margin (arrow). Enlarged pericolonic lymph nodes are also seen (arrowheads). TNM staging for this patient was as follows: CT stage – T3N2M0, surgical stage – T3N1M0, and histological stage – T3N0M0. (c) CT colonographic image shows an annular growth with a polypoidal component in the proximal transverse colon.
Fig. 3.

A 15-year-old boy presented with weight loss and a lump in the abdomen. (a) Coronal and (b) sagittal multiplanar reformatted CEMDCTC images show large heterogeneous transverse colon growth with multiple pericolonic lymphadenopathy (arrows) and loss of fat planes in segment V of the liver. TNM staging for this patient was as follows: CT stage – T4N2M0 and surgical stage – T4N2M0 (histological staging not done as the tumour was not resected). Axial CEMDCTC images show (c) loss of fat planes at the liver and gallbladder (arrow) and (d) thrombosis of the right portal vein (arrow).
These findings were compared with the findings on conventional colonoscopy, which was done within ten days of CEMDCTC. Surgical staging was done by gross palpation and intraoperative visual inspection. For histopathological analysis, the resected specimens were placed in 10% buffered formalin overnight for fixation. Representative tissue blocks, including the radial resection limit and the lymph nodes, were taken and processed routinely. Sections that were 3–4 µm in thickness were cut and stained with haematoxylin and eosin; these sections were used for the histopathological assessment of the tumours.
RESULTS
CEMDCTC was performed in the 25 cases of proven colorectal carcinomas. No procedural complications were encountered. As one patient had three synchronous cancers, a total of 28 cancers were evaluated in the 25 patients.
The mean age of the 25 patients was 50.3 ± 3.0 (15–80) years, and the male to female ratio was 3:2. Most of the cancers were seen in the sigmoid colon (29.0%), with sigmoid and rectosigmoid cancers accounting for 40.0% of the cases. Optimal colonic distension for the various segments was achieved 84.0%–96.0% and 76.0%–92.0% of the time in the supine and prone positions, respectively. The segment that had the least optimum distensibility was the sigmoid colon, reaching 84.0% and 76.0% in the supine and prone positions, respectively. The rectum and caecum had the highest percentage of optimal distensibility in both the supine and prone positions. Table I summarises the colonic distension grades of the various segments in the prone and supine positions. Table II summarises the TNM staging of the tumours on CEMDCTC and surgery.
Table I.
Colonic distension grades of the patients on contrast-enhanced multidetector computed tomography colonography (n = 25).

Table II.
TNM staging of the tumours (n = 28) on contrast-enhanced multidetector computed tomography colonography (CEMDCTC) and surgery.
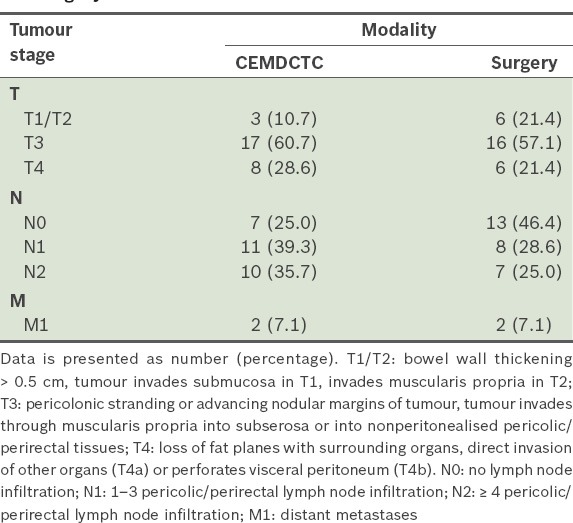
Two patients who had unresectable growths in their right colon were treated palliatively with ileotransverse anastomoses. As biopsies were taken from the resected tumours, histopathological staging could only be obtained for 26 of the 28 tumours. Only 1 (3.8%) tumour was staged as T1 (i.e. invasion into the submucosa on histopathology), and 2 (7.7%) tumours were staged as T2 (i.e. seen to invade the muscularis propria). The majority (n = 19, 73.1%) of the tumours were staged as T3 (i.e. invading through the muscularis propria and into the subserosa, nonperitonealised pericolic or perirectal tissues). The remaining 4 (15.4%) tumours were staged as T4, as they were seen to infiltrate the surrounding organs. Most (n = 20, 76.9%) of the tumours did not show any lymph node infiltration (i.e. staged as N0), while 3 (11.5%) tumours were staged as N1 and 3 (11.5%) tumours were staged as N2. Only one tumour had distant metastases and was staged as M1.
Table III shows the correlation between CEMDCTC and surgical T and N staging for the 28 tumours. Kendall’s tau-b coefficient and kappa value (measure of agreement) were calculated. There was excellent agreement between the two modalities for T staging (κ-value = 0.686, Kendall’s tau-b = 0.793, p < 0.0001), and good agreement between the two modalities for N staging (κ-value = 0.424, Kendall’s tau-b = 0.728, p < 0.0001).
Table III.
Correlation between contrast-enhanced multidetector computed tomography colonography (CEMDCTC) staging and surgical staging of the tumours (n = 28).

Table IV shows the correlation between CEMDCTC and histopathological T and N staging. There was excellent agreement between the two modalities for T staging (κ-value = 0.838, Kendall’s tau-b = 0. 870, p < 0.0001), suggesting a high level of significance. For T staging, the correlation between CEMDCTC and histopathology was stronger than that between CEMDCTC and surgery. Although there was poor agreement between CEMDCTC and histopathology with regard to N staging (κ-value = 0.186, Kendall’s tau-b = 0.320), the p-value obtained was < 0.05, suggesting a high level of significance. The negative predictive value was 100% for lymph node detection on CEMDCTC. With histopathological correlation as the standard reference, the overall diagnostic accuracies of TNM colorectalcancer staging by CEMDCTC for the 26 tumours were 92.3% for T staging, 42.3% for N staging and 96.1% for M staging.
Table IV.
Correlation between contrast-enhanced multidetector computed tomography colonography (CEMDCTC) staging and histopathological staging of the tumours (n = 26*).

Conventional colonoscopy was done for all the 25 patients. However, due to obstructive growth, the scope was not negotiable beyond the growth in 20 (80.0%) patients. Complete colonoscopy could be done in only 5 (20.0%) patients – 3 patients had caecal growth, 1 had a rectosigmoid growth and 1 had a splenic flexure growth. Synchronous lesions were detected in 7 (28.0%) and 9 (36.0%) patients on conventional colonoscopy and CEMDCTC, respectively. For 1 (4.0%) patient who had three synchronous tumours, conventional colonoscopy was not able to detect one polyp (measuring > 1 cm) and one synchronous mass lesion due to incomplete colonoscopy; the polyp and synchronous mass lesion were detected on CEMDCTC, and subsequently confirmed on surgery and histopathology. None of the polyps that were seen on conventional colonoscopy were missed on CEMDCTC. All incidentally detected extraluminal findings (n = 71) were documented according to the CT Colonography Reporting and DataSystem (C-RADS)(14) (Table V). Potentially important findings accounted for 39.4% of the total extraluminal findings, including significant lymphadenopathy, metastases, vascular thrombosis, psoas abscess, colovesical fistula and ascites.
Table V.
Extraluminal findings (n = 71) on contrast-enhanced multidetector computed tomography colonography.

DISCUSSION
CEMDCTC is a noninvasive technique that can be used to evaluate the entire colon of patients with colorectal cancer, including for patients in whom a successful and complete colonoscopic examination is not possible. TNM staging can also be done using CEMDCTC. This is important as cancer stage is the strongest predictor of survival for patients with colorectal cancer.(2) In the present study, we evaluated the diagnostic accuracy of colorectal cancer staging using CEMDCTC by comparing it with the results of surgical and histopathological staging. Calculated with histopathology as the standard reference, the overall diagnostic accuracies of TNM colorectal cancer staging using CEMDCTC were 92.3% for T staging, 42.3% for N staging and 96.1% for M staging.
Tumours staged as T1/T2 using CEMDCTC (Fig. 1) were staged correctly, with reference to both surgical and histopathological staging. On CEMDCTC, the 17 tumours staged as T3 (Fig. 2) had 100% correlation with histopathological staging. However, surgical staging was found to have downstaged 3 of these 17 tumours to T2. On CEMDCTC, eight tumours were reported to be in the T4 stage (Fig. 3). Six of them were confirmed on surgery and four on histopathology. Two of the eight tumours could not be histopathologically assessed as they were unresectable, and in two cases, the loss of fat planes with the adjacent organs on CEMDCTC was secondary to adhesions and adjacent reactive changes (Fig. 4), with no direct tumour infiltration demonstrated by both surgery and histopathology. This limitation of CT had previously been described.(15) In one case of a T4 tumour, there was a loss of fat planes with the duodenum. For this T4 tumour, surgery and histopathology also demonstrated a duodenocolic fistula. However, this was not seen on CEMDCTC, as oral contrast was not routinely given to patients in the present study.
Fig. 4.

A 75-year-old woman presented with per rectal bleeding and abdominal pain. Coronal multiplanar reformatted CEMDCTC image shows annular growth involving the hepatic flexure, with a loss of fat planes with the duodenum (arrow). Cholelithiasis was also detected incidentally (arrowhead). The loss of fat planes seen on CT was due to inflammatory changes. T staging of the tumour is as follows: CT stage – T4, surgical stage – T3, histological stage – T3.
Using CEMDCTC, pericolonic lymphadenopathy was found to be absent in seven cases. Since this was confirmed on surgery and histopathology, the negative predictive value for lymph node detection using CEMDCTC was 100%. However, out of the 11 cases staged as N1 using CEMDCTC, only 4 cases correlated with the surgical findings, and only 2 cases were confirmed as true N1 stage on histopathology. In 8 of these 11 cases, the lymph nodes showed benign reactive enlargement. Of all the cases staged as N2 using CEMDCTC, only 25% correlated with histopathology, and the remaining 75% were found to be overstaged. This is because even if the dimensional criterion (nodes measuring > 1 cm in the long-axis diameter) is integrated with other information, such as number and clustering, CT is not able to distinguish malignant nodes from enlarged (> 1 cm) reactive benign nodes.(11) The accuracy of N staging using CEMDCTC was lower in the present study than in previous studies (accuracy of 80%–85%).(9,11) The previous studies were done on 4-slice(11) and 16-slice(9) CT scanners. However, in the present study, not a single case of lymph node metastases was missed on CEMDCTC. We hypothesise that with MDCT scanners, which have improved axial and spatial resolution compared to conventional CT, the detection rate of lymph nodes has actually improved. The inherent inability of CT to distinguish between benign and malignant nodes resulted in the lower accuracy for N staging in the present study, as the majority of the patients (76.9%) in the present study had N0 stage on histopathology vis-à-vis another study(9) that used a 16-slice CT scanner and had only 55% N0 stage patients on histopathology.
In the present study, two cases were staged as M1 using CEMDCTC. Of these, one case was confirmed on both surgery and histopathology. In the other case, fine needle aspiration cytology of the hepatic lesion was negative and omental deposits were suspected surgically. The omental deposits were confirmed to be benign on histopathology. No false negatives were identified in all the cases that were staged as M0 using CEMDCTC.
Imaging was done in two positions (i.e. prone and supine) for all patients. Scanning in the prone position was done without contrast and at low mAs (i.e. 60 mAs) to reduce radiation exposure (routine scanning is usually done at 200 mAs). Imaging in two positions decreases the number of collapsed colonic segments and increases sensitivity for polyp detection. Fletcher et al, in a prospective study involving 180 patients, concluded that the acquisition and review of both supine and prone CT colonography images significantly improves the likelihood of identifying patients with polyps measuring ≥ 0.5 cm in diameter.(16) Dual positioning with better luminal distension was one of the factors that contributed to the improved performance of CEMDCTC in the present study.(16)
Nonionic IV contrast was used for all the patients in the present study to enhance bowel wall conspicuity and improve the detection of lesions. CEMDCTC also has the ability to provide images of the liver and extracolonic tissues for staging colorectal cancers. However, unlike conventional colonoscopy, where adherent stool or intraluminal pools of fluid can easily be irrigated or aspirated to reveal the underlying mucosal surface, CT colonography is dependent on proper bowel preparation. Dual positioning may not always overcome the problems associated with poor bowel preparation. It also may not always sufficiently displace fluid to permit visualisation of the entire circumference. In such cases, IV contrast helps improve the detection of submerged enhancing masses that might otherwise be obscured by residual colonic fluid.(17-19)
Conventional colonoscopy was done in all patients in the present study. However, due to obstructive growth in 20 of the 25 patients (80.0%), the scope was not negotiable beyond the growth. In a study by Chung et al, 55% of their patients had an incomplete colonoscopy due to occlusive growth.(9) In the present study, synchronous lesions were detected in 7 (28.0%) patients using conventional colonoscopy and in 9 (36.0%) patients using CT colonography. No polyp seen on conventional colonoscopy was missed by CT colonography. In the present study, 1 of the 25 patients (4.0%) had three synchronous tumours. In that one patient, conventional colonoscopy had missed one polyp that measured > 1 cm and one synchronous mass lesion due to incomplete colonoscopy; these were detected on CEMDCTC, and subsequently confirmed on surgery and histopathology. The data obtained on synchronous lesions in the present study correlates with the data found in the literature. That is, 1.5%–9.0% of patients with colorectal carcinoma have a second synchronous cancer and 27%–55% have multiple coexistent adenomatous polyps.(4)
The present study was not without limitations. A limitation of the present study was its small sample size. The number of patients who had distant metastasis was also very small. Other than that, the use of hand insufflations could have affected the uniformity of colonic distension. Another limitation was the low complete conventional colonoscopy rate. However, none of the lesions that were detected on conventional colonoscopy were missed on CEMDCTC.
In conclusion, T staging of colorectal cancer is highly accurate with CEMDCTC. The detection rate of perilesional lymph nodes using CEMDCTC is also high. However, CEMDCTC is unable to distinguish malignant nodes from enlarged benign lymph nodes, and this may result in the overstaging of the N stage of colorectal cancer.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS. Adjuvant therapy of colon cancer. CA Cancer J Clin. 1999;49:202–19. doi: 10.3322/canjclin.49.4.202. [DOI] [PubMed] [Google Scholar]
- 3.Compton CC, Greene FL. The staging ofcolorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 4.Fenlon HM, McAneny DB, Nunes DP, Clarke PD, Ferrucci JT. Occlusive colon carcinoma: virtual colonoscopy in the preoperative evaluation of the proximal colon. Radiology. 1999;210:423–8. doi: 10.1148/radiology.210.2.r99fe21423. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad NA, Kochman ML, Ginsberg GG. Endoscopic ultrasound and endoscopic mucosal resection for rectal cancers and villous adenomas. Hematol Oncol Clin North Am. 2002;16:897–906. doi: 10.1016/s0889-8588(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 6.Minagawa M, Yamamoto J, Miwa S, et al. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006;141:1006–12. doi: 10.1001/archsurg.141.10.1006. [DOI] [PubMed] [Google Scholar]
- 7.Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology. 2004;232:335–46. doi: 10.1148/radiol.2322021326. [DOI] [PubMed] [Google Scholar]
- 8.Rydberg J, Buckwalter KA, Caldemeyer KS, et al. Multisection CT: scanning techniques and clinical applications. Radiographics. 2000;20:1787–806. doi: 10.1148/radiographics.20.6.g00nv071787. [DOI] [PubMed] [Google Scholar]
- 9.Chung DJ, Huh KC, Choi WJ, Kim JK. CT colonography using 16-MDCT in the evaluation of colorectal cancer. AJR Am J Roentgenol. 2005;184:98–103. doi: 10.2214/ajr.184.1.01840098. [DOI] [PubMed] [Google Scholar]
- 10.Iannaccone R, Laghi A, Passariello R. Colorectal carcinoma: detection and staging with multislice CT (MSCT) colonography. Abdom Imaging. 2005;30:13–9. doi: 10.1007/s00261-004-0245-9. [DOI] [PubMed] [Google Scholar]
- 11.Fillippone A, Ambrosini R, Fuschi M, et al. Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography--initial experience. Radiology. 2004;231:83–90. doi: 10.1148/radiol.2311021152. [DOI] [PubMed] [Google Scholar]
- 12.Taylor SA, Halligan S, Goh V, et al. Optimizing colonic distention for multi-detector row CT colonography: effect of hyoscine butylbromide and rectal balloon catheter. Radiology. 2003;229:99–108. doi: 10.1148/radiol.2291021151. [DOI] [PubMed] [Google Scholar]
- 13.Chen SC, Lu DS, Hecht JR, Kadell BM. CT colonography: value of scanning in both the supine and prone positions. AJR Am J Roentgenol. 1999;172:595–9. doi: 10.2214/ajr.172.3.10063842. [DOI] [PubMed] [Google Scholar]
- 14.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 15.Kalra N, Suri S, Gupta R, et al. MDCT in the staging of gallbladder carcinoma. AJR Am J Roentgenol. 2006;186:758–62. doi: 10.2214/AJR.04.1342. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher JG, Johnson CD, Welch TJ, et al. Optimization of CT colonography technique: prospective trial in 180 patients. Radiology. 2000;216:704–11. doi: 10.1148/radiology.216.3.r00au41704. [DOI] [PubMed] [Google Scholar]
- 17.Sosna J, Morrin MM, Kruskal JB, et al. Colorectal neoplasms: role of intravenous contrast-enhanced CT colonography. Radiology. 2003;228:152–6. doi: 10.1148/radiol.2281020950. [DOI] [PubMed] [Google Scholar]
- 18.Morrin MM, Farrell RJ, Kruskal JB, et al. Utility of intravenously administered contrast material at CT colonography. Radiology. 2000;217:765–71. doi: 10.1148/radiology.217.3.r00nv42765. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia A, Saxena AK, Kalra N, et al. Intravenous contrast enhanced computed tomography colonoscopy in children with suspected colonic polyps. Eur J Radiol. 2013;82:905–12. doi: 10.1016/j.ejrad.2012.12.017. [DOI] [PubMed] [Google Scholar]


