Abstract
Structure and function of the intestinal epithelial barrier (IEB) are dependent upon the integrity of junctional protein structures sealing the apical surface between epithelial cells. Tight junctions (TJ) and the surrounding apical F-actin cytoskeleton are involved in the regulation of paracellular permeability. The regulation of actin cytoskeleton organization by RhoA/Rho-kinase (ROCK) pathway plays an important role in TJ assembly and function. There is mounting evidence that the adipocyte-derived hormone leptin exerts pleiotropic effects on the intestinal epithelium including nutrient absorption, epithelial growth, inflammation and injury. Leptin activates multiple cell signaling pathways in intestinal epithelial cells (IEC) that can explain these pleiotropic effects. However, these pathways are also involved in the primary role of leptin that is the regulation of energy and glucose metabolism homeostasis. In this commentary, we examine how the interplay between leptin signaling pathways that regulate cell metabolism could impact upon IEB function.
Keywords: AMPK, barrier repair, intestinal epithelial barrier, JAK/STAT, leptin, metabolism, RhoA/ROCK, tight-junction
Abbreviations: IEB, intestinal epithelial barrier; TJ, tight junctions; ROCK, Rho-kinase; IEC, intestinal epithelial cells; LepR-b, leptin receptor; JAK, Janus kinase; STAT, signal transducer and activator of transcription; AMPK, AMP-activated protein kinase; MLC, myosin light chain; MEF, mouse embryonic fibroblast; VAT, visceral adipose tissue
Introduction
The intestinal epithelium is composed of a single monolayer of polarized epithelial cells that form a selective barrier allowing absorption of solutes and nutrients while excluding luminal microbes. Structure and function of the intestinal epithelial barrier (IEB) are dependent upon intercellular protein junctions, including adherens and tight junctions (TJ), also referred to as apical junctional complex. TJ are involved in the control of paracellular permeability between cells.1 TJ are associated with an apical meshwork of actin filaments enriched in myosin II.2 Organization and contraction of junction-associated actomyosin bundles play an important role in the formation, maintenance and permeability of TJ.2 The small GTPase RhoA and its effector Rho-kinase (ROCK) are important regulators of actin cytoskeleton organization under physiological and pathological stimuli.3,4 RhoA activity is finely tuned and depends on the nature and intensity of the stimuli. Both, up- or down-regulation of RhoA/ROCK pathway may result in similar effects (positive or negative) on TJ function.
The adipocyte-derived hormone leptin plays an essential role in energy homeostasis through its action on hypothalamic neurons but also by stimulating glucose and fatty acid oxidation in peripheral tissues such as skeletal muscle.5 Leptin binding to the long form leptin receptor (LepR-b) activates multiple cell signaling pathways, including phosphoinositide-3-kinase (PI3K), SH2-containing protein tyrosine phosphatase 2/extracellular signal regulated kinase (SHP2/ERK), Janus kinase 2/ signal transducer and activator of transcription (JAK2/STAT3), and AMP-activated protein kinase (AMPK).5,6 In addition, it was recently shown that hypothalamic activation of JAK2 by leptin is mediated by ROCK1.7 LepR-b is expressed throughout the intestinal epithelium, both in apical and basolateral membranes of intestinal epithelial cells (IEC)8,9 suggesting a physiological role of leptin in IEB. Accordingly, it has been shown that leptin reinforces IEB function by stimulating mucus secretion.10 The physiological role of leptin in IEB regulation appears rather complex because it may involve several cell signaling pathways.5 It is possible that leptin plays its specific role by regulating IEC metabolism using signal pathways that also regulate cellular processes involved in IEB function. This would be in agreement with the emerging concept of interplay between cellular metabolism, TJ formation and cell polarity.11,12 Several lines of evidence suggest that leptin activation of the RhoA/ROCK, JAK2/STAT3 and AMPK pathways are involved in the regulation of IEB structure and function. In this commentary, we examine how the interplay between leptin signaling pathways that regulate cell metabolism could impact upon IEB with a special focus on TJ permeability.
Leptin, JAK2/STAT3 and RhoA/ROCK Pathways
Activation of RhoA/ROCK pathway by leptin and subsequent reorganization of actin cytoskeleton has been described in vitro in different cell types, including cardiomyocytes, fibroblasts and chondrocytes.13-15 We have recently shown that intraperitoneal injection of leptin increases intestinal paracellular permeability in rats.16 In vitro, leptin treatment of colonic epithelial HT29–19A cell monolayer increased TJ permeability by inducing TJ opening without delocalization of the TJ-associated protein ZO-1. This effect of leptin was attributed to a disorganization of F-actin cytoskeleton through a RhoA/ROCK-dependent pathway and was prevented by the ROCK inhibitor Y-27632. Another study reported that apical but not basal, leptin treatment of intestinal epithelial Caco2-BBe cells increased paracellular permeability and decreased mRNA expression of TJ proteins.17 The JAK2 inhibitor AG490 inhibited these effects suggesting the implication of this pathway. Interestingly, in human colonic epithelial cell lines, LS174T and HM7, leptin-induced RhoA activation was inhibited by AG490.18 On the contrary, JAK2 inhibition enhanced the stimulation of 2 other Rho family members, namely Rac1 and Cdc42. This data suggests that JAK2 could be a pivotal regulator in the coordinated activation and inhibition of small Rho GTPases. It should be underline that JAK signaling is also involved in TJ disassembly induced by other cytokines, such as IFN-γ and TNF-α.19 In fact, it appears that leptin shares similar barrier-disrupting signaling cascades with these pro-inflammatory cytokines. Intriguingly, it was recently demonstrated in hypothalamic neurons that leptin activation of JAK2/STAT3 pathway requires direct phosphorylation of JAK2 by ROCK1 and that ROCK1 is indispensable to the central satiety effect of leptin.7 Although the involvement of RhoA in the activation of ROCK1 was not investigated, this data could suggests that in other cell types, such as neurons, ROCK1 may phosphorylate JAK2, thereby providing a positive feedback loop during leptin signaling.20 This data also raises the question of the specific ROCK isoform, ROCK1 or ROCK2, involved in leptin-induced F-actin cytoskeleton rearrangement in IEC. It was suggested that ROCK2 could have a more important role in TJ disassembly induced by external stimuli including hormones.3
However, the physiological relevance of a crosstalk between these pathways in the regulation of IEB by leptin remains to be established. A beneficial effect of the crosstalk between RhoA/ROCK and JAK2/STAT3 pathways upon IEB could be to coordinate intestinal epithelial repair by activating cell motility and TJ formation.18,21,22 Inhibition of ROCK impaired TJ formation in intestinal T84 cells in the calcium switch assay,21 and actin rearrangement during wound healing.22 Convincing evidence for a role of leptin in IEB function came from mice with targeted deletion of LepR-b in IEC.23 Although, these mice do not present apparent alteration of intestinal functions under normal conditions, they were more susceptible to enteric infection by Entamoeba histolytica. Importantly, this study showed that infection of mice harboring a mutation of tyrosine 1138 in the intracellular domain of the leptin receptor, which mediates STAT3 signaling, have more severe destruction of mucosal architecture with large ulcerations. Similarly, mice with an IEC-specific deletion of STAT3 activity have a defective epithelial wound healing following experimental colitis.24 However, one must keep in mind that in this mice the Tyr1138 mutation affects whole-body leptin receptors and thus, leptin can indirectly impact the IEB by stimulating cytokine secretion by immune cells.25 Thus, animal models with targeted deletion of specific LepR-b signaling pathways in intestinal epithelium are useful to delineate the different pathophysiological roles of leptin in IEB function.
Leptin, AMPK and RhoA/ROCK Pathways
The AMP-activated protein kinase (AMPK) is a molecular sensor of cellular energy status that is activated by any stress that depletes cellular ATP content.5 AMPK is activated by conformational modification induced by AMP binding and through phosphorylation by upstream kinases, such as the tumor suppressor LKB1.5 AMPK phosphorylates downstream targets resulting in inhibition of ATP-utilizing pathways, such as fatty acid synthesis, and activation of ATP-generating pathways, including fatty acid and glucose oxidation. The demonstration that AMPK pathway mediates stimulation of fatty acid oxidation in skeletal muscle by leptin was the first indication of its important role in the regulation energy metabolism.26 However, the mechanism by which leptin activates AMPK (involvement of LKB1?) and the role of the leptin/AMPK pathway in regulating energy metabolism of IEC remain elusive (Fig. 1). A recent study suggested that AMPK could regulate fatty acid oxidation in intestinal epithelial Caco2/15 cells.27 Moreover, a proteomic analysis of intestinal tissues isolated from mice treated with leptin indicates that leptin modulates the expression of proteins involved in energy metabolism.28 Furthermore, it was reported that luminal leptin up-regulated the expression and activity of the sugar transporters GLUT2 and GLUT5 in rat jejunum through an AMPK-dependent pathway.29 In our hands, leptin treatment of colonic epithelial HT29–19A cells, in vitro, or rat colonic tissues, ex vivo, induces a rapid phosphorylation of AMPK (unpublished results).
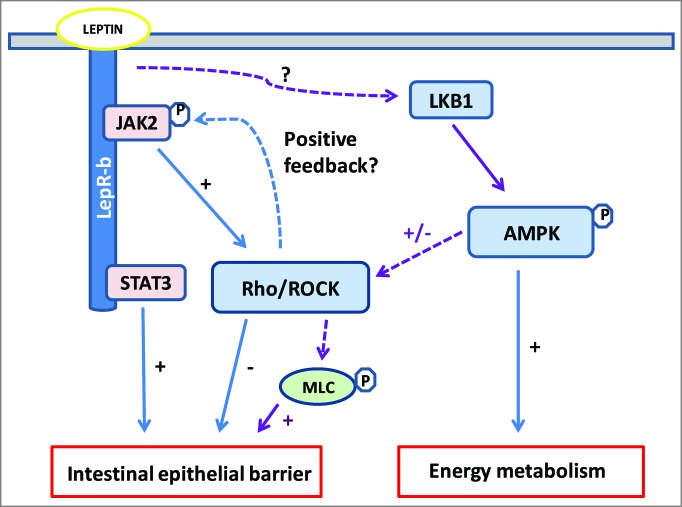
Figure 1.
Leptin signaling cascade at the crossroad of JAK/STAT3, Rho/ROCK and AMPK pathways in intestinal epithelial cell (IEC). Beneficial (+) and deleterious (-) effects of leptin upon intestinal epithelial barrier (IEB) involve the interplay of signaling pathways regulating cell metabolism and tight-junction (TJ) integrity. Leptin activates AMPK, probably via LKB, thereby stimulating IEC metabolism under physiological or cellular stress. Parallely or concomitantly, AMPK induces myosin light chain (MLC) phosphorylation, probably via Rho/ROCK, and thus regulates actomyosin contractility and TJ structure. Leptin activates RhoA/ROCK directly or via JAK, resulting in F-actin cytoskeleton reorganization and increased IEB permeability. As shown in neurons7, Rho/ROCK could activate of JAK in IEC. LepR-b signaling through the JAK2/STAT3 is important for IEB integrity and repair. See text for details. Dotted lines show hypothetical pathways.
Several lines of evidence suggest that AMPK pathway is involved in the regulation of TJ formation and cell polarity.11,12 Energy metabolism of epithelial cells is essential for maintaining the structure and function of the intestinal epithelial barrier. In kidney (MDCK) or intestinal epithelial cells (Caco2, LS174T), ATP depletion by inhibiting glycolysis or by cellular hypoxia, increases epithelial permeability associated with loss of TJ structure and cell polarity.30-32 Several studies using MDCK and LS174T cell monolayers have shown that AMPK is involved in TJ assembly and stability in recovery experiments after different cellular stresses.33-35 Inhibition of glycolysis in LS174T cells with 2-deoxy-D-glucose, induced an AMPK-dependent phosphorylation of myosin light chain (MLC) and reorganization of F-actin cytoskeleton and consequently cell polarization.34 However, a direct phosphorylation of MLC by AMPK is excluded,36 and several data suggest that RhoA/ROCK could regulate MLC downstream AMPK. Previous studies in MDCK cells demonstrated that RhoA protects TJ structure during ATP depletion,30 and that recovery of actomyosin cytoskeleton after ATP depletion is dependent on ROCK-induced phosphorylation of MLC.32 Another more recent study showed that pharmacological activation of AMPK in MDCK cells induced phosphorylation of MLC and other ROCK targets, including MLC phosphatase MYPT1 and cofilin.37 The ROCK inhibitor Y-27632 inhibited AMPK-induced phosphorylation of these proteins. Altogether, these data suggest that, under cellular stresses AMPK activates the RhoA/ROCK pathway to initiate organization of actomyosin cytoskeleton and TJ formation. As mentioned above, we have observed in HT29–19A that leptin activates AMPK, thus it is tempting to speculate that AMPK could be an upstream effector in leptin-induced RhoA/ROCK activation observed in IEC.16 Evidence for a role of a leptin/AMPK/RhoA/ROCK-dependent phosphorylation of MLC in actomyosin contraction and TJ opening16 needs further investigation. Similarly, it will be necessary to define the role of LKB in leptin-induced AMPK activation in IEC.
Recent data also suggest that AMPK may differentially regulate Rho and Rac/Cdc42. A role for AMPK in pulmonary endothelial barrier repair after LPS-induced injury was recently reported in vitro and in vivo.38,39 It was found that LPS-increased endothelial permeability was associated with a decrease in the phosphorylation of AMPK-α1 and that pharmacological activation of AMPK attenuated LPS-increased permeability.39 These effects were associated with Rac/Cdc42 activation and inhibition of MLC phosphorylation. Although not demonstrated, the authors proposed that Rho inhibition by AMPK was responsible for this hypo-phosphorylation of MLC. In agreement with this assumption, it was reported in vascular smooth muscle cells that estradiol inhibited RhoA/ROCK pathway through AMPK-α1-induced RhoA phosphorylation.40 Interestingly, a role for AMPK-induced activation of Rac1 in wound healing was clearly provided by Moser et al.41 in mouse embryonic fibroblasts (MEF) deficient for both AMPK-α1 and -α2 subunits and in human osteosarcoma U2OS cells treated with the AMPK inhibitor compound C. AMPK deficiency impaired Rac1-dependent actin remodeling and MEF motility. Although these data suggest that AMPK inhibits Rho while activating Rac/Cdc42, a subsequent activation of RhoA/ROCK contributing to TJ formation is also conceivable.
Thus, the role of AMPK in activation or inhibition of Rho family members and the consequences for actin cytoskeleton organization seems finely tuned and dependent on cell type and stimuli. Since leptin activates RhoA, Rac1 and Cdc42 in IEC,18 future studies have to establish the specific role of each AMPK subunits in leptin regulation of Rho family members and actin cytoskeleton organization.
Pathophysiological Regulation of Intestinal Permeability by Leptin
Whereas a role for leptin in regulating IEC metabolism needs further investigation, it is now demonstrated that leptin may increase IEB permeability.16,17 A direct consequence is that it allows the passage of luminal products such as endotoxin (LPS).16 However, leptin modulation of IEB permeability by leptin could be different between physiological and pathological situations, depending on several factors such as dose (low vs high), origin (gastric cells vs adipocytes) and exposure time (transient vs chronic) of leptin. Chronic secretion of high levels of leptin by visceral adipose tissue (VAT) in the context of aging or obesity would permanently impact IEB permeability by binding LepR on basolateral side of IEC and stimulating RhoA/ROCK.16 This in turn may contribute to metabolic endotoxemia as reported in high-fat diet-induced obesity42 and in the model of VAT hypertrophy in rat born with intrauterine growth retardation.16 However, in the context of intestinal inflammation, Sitaraman et al.43 have shown that colonocytes secreted high levels of luminal leptin that could stimulate the apical side of IEC. These authors demonstrated in vitro that apical leptin stimulated the NF-κB inflammatory signaling pathway in Caco2-BBE monolayer. Although, this study did not investigate the effect of leptin on epithelial paracellular permeability, it illustrates well that distinct pathways are probably activated by apical and basolateral leptin action.
Physiologically, post-prandial release of luminal leptin by gastric cells44 could also transiently increase IEB permeability by stimulating apical LepR of IEC in a JAK2/STAT3-dependent pathway.17 It is tempting to speculate that the post-prandial-increased leptinemia could be involved in the fluctuation of endotoxemia observed between fasted and fed mice.42 In addition, it has been proposed that physiological levels of luminal leptin may contribute to IEB function in rat colon by stimulating mucus secretion in a JAK/STAT-independent pathway.10 Furthermore, activation of AMPK by luminal leptin in IEC could contribute to glucose and fatty acid metabolism29 and thus in the production of ATP indispensable to the rapid turnover of intestinal epithelium. In parallel, AMPK could participate to TJ formation by regulating actomyosin contractility in a RhoA/ROCK-dependent pathway.37 However, the respective role of AMPK-α1 and AMPK-α2 subunits in the regulation of metabolism and actomyosin contractility needs to be established. Further studies are needed to clarify whether leptin action on apical and basolateral side of IEC stimulates different intracellular signaling pathways and differently affects IEB.
Conclusion
In conclusion, the physiological role of leptin in IEB function appears complex and involves crosstalk between numerous signaling pathways (Fig. 1). Both JAK2 and AMPK that are implicated in metabolic action of leptin in many cells could differentially regulate Rho family members to coordinate actin cytoskeleton organization that are involved in TJ formation and in establishment and maintenance of IEC polarity. There is compelling evidence that cell signaling pathways that regulate cellular metabolism are also involved in remodeling actin cytoskeleton, concomitantly or in parallel. Future studies need to more precisely determine how these pathways and the different partners are coordinately regulated in time and space.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol 2005; 17:453-8; PMID:; http://dx.doi.org/ 10.1016/j.ceb.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci 2008; 13:6662-81; PMID: http://dx.doi.org/ 10.2741/3180 [DOI] [PubMed] [Google Scholar]
- 3.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 2010; 177:512-24; PMID:; http://dx.doi.org/ 10.2353/ajpath.2010.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citalán-Madrid AF, García-Ponce A, Vargas-Robles H, Betanzos A, Schnoor M. Small GTPases of the Ras superfamily regulate intestinal epithelial homeostasis and barrier function via common and unique mechanisms. Tissue Barriers 2013; 1:e26938; PMID:; http://dx.doi.org/ 10.4161/tisb.26938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 2009; 297:E1247-59; PMID:; http://dx.doi.org/ 10.1152/ajpendo.00274.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005; 1:15-25; PMID:; http://dx.doi.org/ 10.1016/j.cmet.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Kong D, Byun KH, Ye C, Koda S, Lee DH, Oh BC, Lee SW, Lee B, Zabolotny JM, et al. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat Neurosci 2012; 10:1391-8; PMID:; http://dx.doi.org/ 10.1038/nn.3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drew JE, Farquharson AJ, Padidar S, Duthie GG, Mercer JG, Arthur JR, Morrice PC, Barrera LN. Insulin, leptin, and adiponectin receptors in colon: regulation relative to differing body adiposity independent of diet and in response to dimethylhydrazine. Am J Physiol Gastrointest Liver Physiol 2007; 293: G682-91; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00231.2007 [DOI] [PubMed] [Google Scholar]
- 9.Barrenetxe J, Villaro AC, Guembe L, Pascual I, Muñoz-Navas M, Barber A, Lostao MP. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut 2002; 50:797-802; PMID:; http://dx.doi.org/ 10.1136/gut.50.6.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Homsi M, Ducroc R, Claustre J, Jourdan G, Gertler A, Estienne M, Bado A, Scoazec JY, Plaisancié P. Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am J Physiol Gastrointest Liver Physiol. 2007; 293:G365-73; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00091.2007 [DOI] [PubMed] [Google Scholar]
- 11.Caplan MJ, Seo-Mayer P, Zhang L. Epithelial junctions and polarity: complexes and kinases. Curr Opin Nephrol Hypertens 2008; 17:506-12; PMID:; http://dx.doi.org/ 10.1097/MNH.0b013e32830baaae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirouse V, Billaud M. The LKB1/AMPK polarity pathway. FEBS Lett 2011; 585:981-5; PMID:; http://dx.doi.org/ 10.1016/j.febslet.2010.12.025 [DOI] [PubMed] [Google Scholar]
- 13.Zeidan A, Javadov S, Karmazyn M. Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc Res 2006; 72:101-11; PMID:; http://dx.doi.org/ 10.1016/j.cardiores.2006.06.024 [DOI] [PubMed] [Google Scholar]
- 14.Schram K, Ganguly R, No EK, Fang X, Thong FS, Sweeney G. Regulation of MT1-MMP and MMP-2 by leptin in cardiac fibroblasts involves Rho/ROCK-dependent actin cytoskeletal reorganization and leads to enhanced cell migration. Endocrinology 2011; 152:2037-47; PMID:; http://dx.doi.org/ 10.1210/en.2010-1166 [DOI] [PubMed] [Google Scholar]
- 15.Liang J, Feng J, Wu WK, Xiao J, Wu Z, Han D, Zhu Y, Qiu G. Leptin-mediated cytoskeletal remodeling in chondrocytes occurs via the RhoA/ROCK pathway. J Orthop Res 2011; 29:369-74. PMID:; http://dx.doi.org/ 10.1002/jor.21257 [DOI] [PubMed] [Google Scholar]
- 16.Le Dréan G, Haure-Mirande V, Ferrier L, Bonnet C, Hulin P, de Coppet P, Segain JP. Visceral adipose tissue and leptin increase colonic epithelial tight junction permeability via a RhoA-ROCK-dependent pathway. FASEB J 2014; 28:1059-70; PMID:; http://dx.doi.org/ 10.1096/fj.13-234203 [DOI] [PubMed] [Google Scholar]
- 17.Kim CY, Kim KH. Curcumin prevents leptin-induced tight junction dysfunction in intestinal Caco-2 BBe cells. J Nutr Biochem 2014; 25:26-35; PMID:; http://dx.doi.org/ 10.1016/j.jnutbio.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 18.Jaffe T, Schwartz B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int J Cancer 2008; 123, 2543-56; PMID:; http://dx.doi.org/ 10.1002/ijc.23821 [DOI] [PubMed] [Google Scholar]
- 19.Naydenov NG, Baranwal S, Khan S, Feygin A, Gupta P, Ivanov AI. Novel mechanism of cytokine-induced disruption of epithelial barriers: Janus kinase and protein kinase D-dependent downregulation of junction protein expression. Tissue Barriers 2013; 1:e25231; PMID:; http://dx.doi.org/ 10.4161/tisb.25231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peelman F, Tavernier J. ROCKing the JAKs. JAKSTAT 2013; 2:e24074; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh SV, Hopkins AM, Chen J, Narumiya S, Parkos CA, Nusrat A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology 2001; 121:566-79; PMID:; http://dx.doi.org/ 10.1053/gast.2001.27060 [DOI] [PubMed] [Google Scholar]
- 22.Russo JM, Florian P, Shen L, Graham WV, Tretiakova MS, Gitter AH, Mrsny RJ, Turner JR. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology 2005; 128:987-1001; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2005.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC, Jr, Myers MG, Jr, Duggal P, Houpt ER, Petri WA, Jr. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol 2011; 4:294-303; PMID:; http://dx.doi.org/ 10.1038/mi.2010.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 2009; 206:1465-72; PMID:; http://dx.doi.org/ 10.1084/jem.20082683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol 2000; 68:437-46; PMID: [PubMed] [Google Scholar]
- 26.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002; 415:339-43; PMID:; http://dx.doi.org/ 10.1038/415339a [DOI] [PubMed] [Google Scholar]
- 27.Harmel E, Grenier E, Bendjoudi Ouadda A, El Chebly M, Ziv E, Beaulieu JF, Sané A, Spahis S, Laville M, Levy E. AMPK in the small intestine in normal and pathophysiological conditions. Endocrinology 2014; 155:873-88; PMID:; http://dx.doi.org/ 10.1210/en.2013-1750 [DOI] [PubMed] [Google Scholar]
- 28.Padidar S, Farquharson AJ, Williams LM, Hoggard N, Reid MD, Duncan GJ, Drew JE. Impact of obesity and leptin on protein expression profiles in mouse colon. Dig Dis Sci 2011; 56:1028-36; PMID:; http://dx.doi.org/ 10.1007/s10620-010-1394-z [DOI] [PubMed] [Google Scholar]
- 29.Sakar Y, Nazaret C, Lettéron P, Ait Omar A, Avenati M, Viollet B, Ducroc R, Bado A. Positive regulatory control loop between gut leptin and intestinal GLUT2/GLUT5 transporters links to hepatic metabolic functions in rodents. PlosOne 2009; 4:e7935; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0007935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gopalakrishnan S, Raman N, Atkinson SJ, Marrs JA. Rho GTPase signaling regulates tight junction assembly and protects tight junctions during ATP depletion. Am J Physiol 1998; 275:C798-809; PMID: [DOI] [PubMed] [Google Scholar]
- 31.Unno N, Menconi MJ, Salzman AL, Smith M, Hagen S, Ge Y, Ezzell RM, Fink MP. Hyperpermeability and ATP depletion induced by chronic hypoxia or glycolytic inhibition in Caco-2BBe monolayers. Am J Physiol 1996; 270:G1010-21; PMID: [DOI] [PubMed] [Google Scholar]
- 32.Sutton TA, Mang HE, Atkinson SJ. Rho-kinase regulates myosin II activation in MDCK cells during recovery after ATP depletion. Am J Physiol Renal Physiol 2001; 281:F810-18; PMID: [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci USA 2006; 103:17272-7; PMID:; http://dx.doi.org/ 10.1073/pnas.0608531103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 2007; 447:1017-20; PMID:; http://dx.doi.org/ 10.1038/nature05828 [DOI] [PubMed] [Google Scholar]
- 35.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci U S A 2007; 104:819-22; PMID:; http://dx.doi.org/ 10.1073/pnas.0610157104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bultot L, Horman S, Neumann D, Walsh MP, Hue L, Rider MH. Myosin light chains are not a physiological substrate of AMPK in the control of cell structure changes. FEBS Lett 2009; 583:25-28; PMID:; http://dx.doi.org/ 10.1016/j.febslet.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 37.Miranda L, Carpentier S, Platek A, Hussain N, Gueuning MA, Vertommen D, Ozkan Y, Sid B, Hue L, Courtoy PJ, et al. AMP-activated protein kinase induces actin cytoskeleton reorganization in epithelial cells. Biochem Biophys Res Commun 2010; 396:656-61; PMID:; http://dx.doi.org/ 10.1016/j.bbrc.2010.04.151 [DOI] [PubMed] [Google Scholar]
- 38.Creighton J, Jian M, Sayner S, Alexeyev M, Insel PA. Adenosine monophosphate-activated kinase alpha1 promotes endothelial barrier repair. FASEB J 2011; 25:3356-65; PMID:; http://dx.doi.org/ 10.1096/fj.10-179218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol 2013; 182:1021-30; PMID:; http://dx.doi.org/ 10.1016/j.ajpath.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gayard M, Guilluy C, Rousselle A, Viollet B, Henrion D, Pacaud P, Loirand G, Rolli-Derkinderen M. AMPK alpha 1-induced RhoA phosphorylation mediates vasoprotective effect of estradiol. Arterioscler Thromb Vasc Biol 2011; 31:2634-42; PMID:; http://dx.doi.org/ 10.1161/ATVBAHA.111.228304 [DOI] [PubMed] [Google Scholar]
- 41.Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog 2010; 6:e1000954; PMID:; http://dx.doi.org/ 10.1371/journal.ppat.1000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56:1761-72; PMID:; http://dx.doi.org/ 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 43.Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, Merlin D. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J 2004; 18:696-8; PMID: [DOI] [PubMed] [Google Scholar]
- 44.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, et al. The stomach is a source of leptin. Nature 1998; 394:790-3; PMID:; http://dx.doi.org/ 10.1038/29547 [DOI] [PubMed] [Google Scholar]