Abstract
Postoperative effect of music listening has not been established in pediatric age. Response on postoperative distress and pain in pediatric day care surgery has been evaluated. Forty-two children were enrolled. Patients were randomly assigned to the music-group (music intervention during awakening period) or the non-music group (standard postoperative care). Slow and fast classical music and pauses were recorded and played via ambient speakers. Heart rate, blood pressure, oxygen saturation, glucose and cortisol levels, faces pain scale and Face, Legs, Activity, Cry, Consolability (FLACC) Pain Scale were considered as indicators of response to stress and pain experience. Music during awakening induced lower increase of systolic and diastolic blood pressure levels. The non-music group showed progressive increasing values of glycemia; in music-group the curve of glycemia presented a plateau pattern (P<0.001). Positive impact on reactions to pain was noted using the FLACC scale. Music improves cardiovascular parameters, stress-induced hyperglycemia. Amelioration on pain perception is more evident in older children. Positive effects seems to be achieved by the alternation of fast, slow rhythms and pauses even in pediatric age.
Key words: music therapy, children, surgery, pain, stress
Introduction
Listening to music is a complex phenomenon, involving psychological, emotional, neurological, endocrinological and cardiovascular changes.1,2
The physiological reaction to music is qualitatively similar in musicians and non-musicians. Although musicians respond better, the response seems not to be influenced by musical preferences. The response may be conditioned by musical style, melody, harmonic structure, rhythm, tempo, but also by verbal content. An arousal effect proportional to the speed of the music is described in the literature: with slower rhythms inducing relaxation; while a pause in the music induces a condition of relaxation greater than preceding the exposure to music.3
This evidence leads to the speculation that the music may give pleasure, and perhaps health benefit, as a result of controlled alternation between arousal and relaxation.4
According to the National Association for Music Therapy, music therapy (MT) consists of using music to achieve therapeutic objectives: restoration, maintenance, and increase in health, both physical and mental.
In recent years, music has been increasingly used as a therapeutic tool in the treatment of different diseases especially in intensive care medicine.5-12 More specifically; music has been shown to decrease pain, stress, anxiety and demand for analgesic and anesthetics drugs.9,11
Music seems to have measurable benefits on physiological and psychological outcomes in pediatric age. Although infant and children previously exposed to music treatment protocols could be distracted from unpleasant symptoms, calmed during stressful events such as invasive procedures, and less distressed while hospitalized,13 music listening effects after routinely day surgical procedures has not been established yet in pediatric patients.
The purpose of this study is to better understand the music benefits on postoperative stress and pain response in children.
Materials and Methods
Participants
Forty-two children, aged 3 to 14 years, having day surgical procedures (including orchidopexy, inguinal hernia repair, circumcision) were asked to participate in this study.
Surgery was performed between 8.30 am and 12 am and under general anesthesia, at Pediatric Surgery Unit. Exclusion criteria were: chronic illness, obesity, use of any medications and experience of prenatal music therapy.14-17 At admission, written and oral consent was obtained from all the children and their parents.
The ethics committee of the Department of Internal Medicine, University of Pavia, approved the study protocol and the researcher complied with the Helsinki Declaration.
Design and procedure
At admission, the axiological examination of children included evaluation of height, weight, body mass index. Height measurement was performed using a Harpenden stadiometer, performed with patients in an upright position, without shoes, with their heels together, arms extended down the sides of the body and head positioned parallel to the floor. Weight was made with the children barefooted and wearing light clothes, standing upright in the center of the scale platform with their arms extended down the sides of the body. Body mass index (BMI) was calculated as body weight in kilograms divided by body height squared in meters.
The children were randomly assigned to the music group when receiving music intervention during awakening period and to the non-music group when children had standard medical care. Randomization was carried out using opaque envelopes, half of which contained a paper that said music and half a paper that said no music.
Awakening was pursued at the recovery room, in comfortable conditions (e.g. temperature, light and noise).
Records were selected by expert musicians. Six tracks of slow (70-80 beats/min) and fast (140-150 beats/min) classical music were included; two minutes of silence, inserted after the slow and fast music sequence, were also recorded. Music tracks characteristics are reported in Table 1.
Table 1.
Characteristics of music tracks.
| Source | Time (min) | Style | Meter | Tempo (beats/min) | Pulsation perceived (beats/min) | Tonality rhythm | Comments about |
|---|---|---|---|---|---|---|---|
| Andante Molto A. Vivaldi: Concerto for strings and continuo RV 158, II Andante Molto | 2’20’’ | Baroque | 2/4 | 39 | 78 (eighth note) | A minor | Pulsations marked in the bass |
| Silence | 2’00’’ | - | - | - | - | - | - |
| Suite J. S. Bach: BWV 1010 for cello, Giga |
2’38’’ | Baroque | 12/8 | 144 | 144 | Eb major | Constant density rhythm |
|
Suite E. Grieg Holberg’s Rigaudon |
3’57’’ | Romantic | 2/2 | 72 | 144 (quarter note) | G major | High density rhythm in the first section and low density rhythm in the second section |
| Andante W. A. Mozart: Eine Kleine Nachtmusik K 525, II Andante | 4’07’’ | Classic | 2/2 | 34 | 68 (quarter note) | C major | Pulsations marked in the bass |
|
Suite J. S. Bach: BWV 1007 for cello, Preludio |
2’38’’ | Baroque | 4/4 | 70 | 70 rubato | G major | Constant density rhythm |
| Silence | 2’00’’ | - | - | - | |||
|
Allegro A. Corelli: Sonata op.5 n°4, V Allegro |
2’20’’ | Baroque | 2/2 | 79 | 158 (quarter note) | F major | Alternation between low density rhythm and high density rhythm |
Music was played for 20 minutes during awakening, via ambient speakers, in the music group.
Heart rate (HR), blood pressure (BP), oxygen saturation (SpO2), glucose and cortisol levels, faces pain scale (FPS) and Face, Legs, Activity, Cry, Consolability (FLACC) Pain Scale were considered as indicators of physiological response to stressful and pain experience.18,19
Few time points including evaluation were considered: i) admission (T0); ii) end of surgical procedure (T1) at recovery room; iii) awakening (T2) at recovery room; iv) re-admission to the Unit (T3). At T0, T1 and T2 and during music exposition the parents were not present.
Vitals signs
HR, BP and SpO2 were monitored (Primus, Dräger, Lübeck, Germany) and recorded as following: i) T0 (HR every 2 minutes and BP and SpO2 every 5 minutes, for 10 minutes. Mean values were used for statistical analysis); ii) T1 (HR every 2 minutes and BP and SpO2 every 5 minutes, for 10 minutes. Mean values were used for statistical analysis); iii) T2 (HR every 2 minutes and BP and SpO2 every 5 minutes, for 20 minutes. Mean values were used for statistical analysis) with the children listening or not listening to music according to their groups.
Metabolic and endocrinological parameters
Serum glucose at T0, T1 and T2 were measured using the hexokinase-G-6-PDH method (Abbott Diagnostics, Wiesbaden, Germany) with a chemistry analyzer (Architect c-16000); intra- and interassay CVs were 1.98% and 2.15%, respectively, at 4.4 mmol/L and 0.65% and 1.51% at 15.5 mmol/L.
Serum cortisol levels in both groups were measured at T0 between 8.00-9.00, at T1 between 12.00-14.00 pm and at the end of T2 with an immunochemistry analyzer (Immulite 2000 XP, Siemens Healthcare Diagnostics, Erlangen, Germany) using a solid-phase competitive method and chemiluminescent tracer; intra- and interassay CVs were 5 and 6.5%, at 8 g/dL. Serum cortisol was used for its ease collection in the post operative period, in respect to salivary cortisol.
Pain scales
FPS was used to measure the child’s self-reported pain during 2 phases: at T0 and at T3. This scale consisted of 6 cartoon faces with varying expressions ranging from very happy to very sad. The child rated the pain intensity on a scale, with point 0 being no pain and point 10 being the worst pain.
At T0, at the end of T2 and at T3 the FLACC was used to assess the behavioral reactions to pain. The FLACC pain scale assesses five behavioral areas (facial expression of the child, the position of the legs, activity, crying, and consolability) with scores ranging from 0 to 2 for each item; assessment of behavioral scores was 0=no pain; 1-3=mild pain, 4-6= moderate pain; 7-10=severe pain. All measurements were conducted by the same psychologist that was blind to randomization group. In Table 2 are summarized the measurements of the parameters at time points.
Table 2.
Evaluations of vital signs, metabolic and endocrinological parameters and pain scales at time points.
| Time | T0 Admission | T1 End of surgical procedure | T2 Awakening | T3 Re-admission to the Unit |
|---|---|---|---|---|
| Heart rate | X | X | X | |
| Blood pressure | X | X | X | |
| Oxygen saturation | X | X | X | |
| Glucose levels | X | X | X | |
| Cortisol levels | X | X | X | |
| Faces pain scale | X | X | ||
| Face, legs, activity, cry, consolability pain scale | X | X | X |
Anesthesia protocol
After obtaining the written consents from the parents the children were scheduled for surgical procedure. All subjects were in good physical state. Anesthesia was induced with propofol (2-2.5 mh/g) as sedative-hypnotic agent and fentanyl (1-1.5 mcg/kg) as analgesic. After laryngeal mask or tracheal tube positioning, patients underwent volume controlled mechanical ventilation with an inspired mixture of air and oxygen using a closed breathing system (fresh gas flow of 0.75l min–1 oxygen and 1.5l min–1 air during anesthesia) adjusted to achieve an end-tidal carbon dioxide of 32-35 mmHg. Anesthesia was maintained via volatile anesthetic based on the administration of Sevoflurane. The Sevoflurane was administered in a 0.75 to 1.25 MAC range. Twenty minutes before the end of intervention, all patients received Paracetamol 15 mg/kg, as analgesic. At the end of the operation the patient was transferred from the theater to the recovery room.
The awakening period started at the stop of Sevofluorane administration and finished when the child was totally conscious.
Statistical analysis
Continuous variables were described as mean and standard deviation (SD) and categorical variables as counts and percentages. Data were compared between groups with the Student t test or the Mann Whitney U test if continuous and the Fisher exact test if categorical. To test the effects of music on vital signs, on metabolic and endocrinological parameters in different groups, the mean differences were verified with repeated measures analysis of variance. Probability values less than 0.05 were considered statistically significant. All statistical analysis were performed using SPSS statistical package (SPSS, Chicago IL, USA) and Stata 8.0
Results
The 42 children (40 boys and 2 girls; mean age 6.7±4.1 years), were randomly assigned to 1 of the 2 groups: the music group (n=21) or the no-music group (n=21). No significant differences were found between the two groups with respect to age, sex, weight, BMI. Awakening time were slower in music group (P<0.001) (Table 3).
Table 3.
Demographic, auxological and procedural characteristics.
| Characteristics | No-music group (n=21) | Music group (n=21) | P |
|---|---|---|---|
| Age (yrs) | 6.8±3.9 | 6.6±4.4 | 0.87 |
| Sex (M/F) | 19/2 | 21/0 | 0.46 |
| Weight (kg) | 28.3±15.1 | 27.7±13.7 | 0.89 |
| Body mass index (kg/m2) | 16.9±2.4 | 17.8±5.2 | 0.46 |
| Duration of awakening (min) | 9.8±3.9 | 16.04±4.1 | <0.001 |
Vital signs data
Heart rate. HR at T0 and T1 were not significantly different (P=0.8). At T2 HR was higher compared to T0 and T2 (P=0.03 and P=0.01, respectively) without no difference between the two groups (P=0.15).
Systolic (SBP) and diastolic blood pressure (DBP). At T2 decreased SBP and DBP levels were found compared to T0 (P=0.008 and P<0.001, respectively). At T2 an increase of SBP and DBP levels were noted compared to T1 (P<0.001); these were lower in music group (P=0.09 and P=0.003, respectively; Figure 1).
Figure 1.
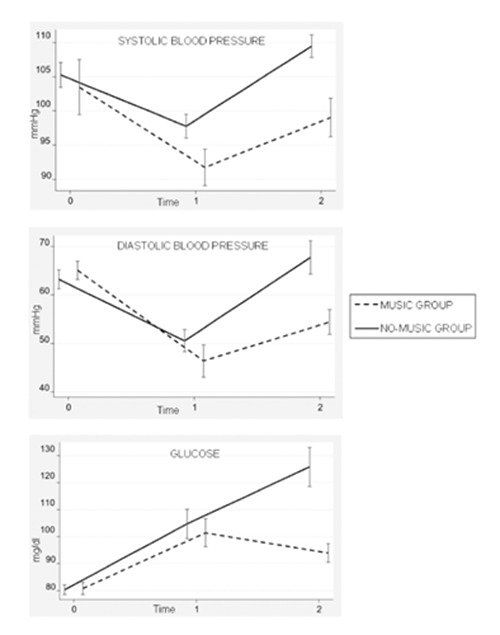
Comparison of the blood pressure and glucose levels at admission (Time 0), at the end of surgical procedure (Time 1) and during awakening (Time 2), in music and no-music groups.
Oxygen saturation. No significant difference between SpO2 at T0 and T1 was found (P=0.48). At T2 SpO2 decreased significantly respect to T0 and T1 (P<0.001), without differences between the two groups (P=0.9).
Metabolic and endocrinological data
Glucose levels. Increased levels of glucose at T1 compared to T0 levels were found in both groups (P<0.001). The non-music group showed a progressive increasing values of glycemia at T2, while in the music group the curve of glycemia presented a plateau pattern (P<0.001); Figure 1.
Serum cortisol. T0 cortisol levels were lower than T1 (P=0.01) and T2 values (P=0.06). A significant decrease at T2 respect to T1 was found (P=0.02), without difference between the two groups (P=0.6).
Findings from pain scale
FPS. No significant difference was found between no-music and music groups in average faces pain score at T0 (P=0.41) and T3 (P=0.78).
FLACC scale. No-music and music groups showed no significant difference in mean value of FLACC scores at the end of T2 (P=0.19) and T3 (P=0.79). The improvement in average pain score is higher in music than in no-music group (P=0.05).
No difference in pain intensity score was noted between the two groups at the end of T2 (P=0.2) and T3 (P=0.29). In the music group a higher number of children reported no or mild pain at T3 (P=0.008); these patients however resulted to be older than those with moderate or severe pain in the behavioral score (P=0.05).
Discussion
Surgery is one of the most stressful events. Stressors activate the hypothalamic-pituitaryadrenal (HPA) axis and the sympathetic nervous system. During stress, the HPA axis stimulates the adrenal cortex to secrete cortisol, to support and stimulate cardiovascular and inflammatory response.20-22 Listening to music could offer a wide range of therapeutic effects, such as helping patients to relax, enhancing sedation and relieving anxiety and pain.13 Music evokes desired emotional and stress responses that serve as distraction from unpleasant stimuli.23
Music induces physiological cardiovascular changes even in absence of conscious reactions. The subconscious autonomic response is independent of music preferences or previous training and involves respiration and cardiovascular parameters. Sympathetic nervous system response leads to a decrease in adrenergic activity. It induces altered status of consciousness delayed neuromuscular arousal. This relaxation response is manifested by physiologic indicators like decrease in heart rate, blood pressure, metabolic rate, oxygen consumption, respiratory rate, skeleton muscle tension, gastric acidity and motility and sweat gland activity.
Our study showed the positive impact of the music on blood pressure, postoperative glucose levels and pain experience in children undergoing day surgery procedures.
During awakening, the music group reported a lower increase of the DBP and SBP levels. The current findings confirm the benefits of music in reducing the sympathetic nervous system stress response.24
Music may stimulate physiological responses.3,4,23 This response seems to be strictly related to the music characteristics (tempo, style, harmonic and rhythmic structure), sequence and presence of other stimuli (such as colors, lights, sounds). Cardiovascular, respiratory and autonomic systems also respond to different genres of music, order of presentation and short interpolated pauses. Music induces an arousal effect, predominantly related to the tempo.3,4
Autonomic response is synchronized with music. Slow or meditative music can induce a relaxing effect particularly evident during pauses. In adults, the same effect has been achieved by alternating faster and slower rhythms or pauses.3 The use of the same method has led to a positive response in our report. Due to the different blood pressure and heart rate in childhood ages, it would be more convenient to use different music characteristics. Further studies are needed to define the most useful style of music with more benefits according to the child’s age.
Our results also document how glucose response after surgery is linked to music influence. This finding seems to have a possible impact on the stress-induced hyperglycemia (SIH). SIH is a transient condition, characterized by insulin resistance and an up regulated hepatic gluconeogenesis (in part through of cortisol) that occur during stressful situations such as trauma, surgery, stroke and sepsis.24-27 The improvement of SIH may have a positive impact on patients’ risks of infection and complications.
As reported, cortisol levels increase quickly following surgical incision and remain elevated during and after surgery.28 Studies of the effect of music on cortisol levels before, during and after surgery have had mixed results.15,29-31 We showed that serum cortisol levels were higher in the immediate postoperative care compared to values found before surgical procedure. Music played for almost 20 minutes after surgery, does not influence the decrease of cortisol levels during awakening. The effect of music was probably less evident due to the high intraindividual biological variability of cortisol, although its gluconeogenic effect was found to be reduced in music group. To understand the real benefit of music on the cortisol response necessitates more than one measurement of its levels 24 hours post operatively.
Even though total pain scores were similar between music and no-music groups, a distinct reaction to pain in music group was noted using the behavioral FLACC scale. These results, which seem to be influenced by a wide age range, may confirm the pain gate control theory. Music may act as a distracter by blocking pain pathways thus diminishing the amount of perceived pain.32,33
Caprilli et al. have previously reported that age, but not gender, was found to have a significant effect on behavioral distress during venipuncture.9 In our study, an high number of boys is present and the gender was not included as a covariate in statistical analysis. A further evaluation including a more homogenous sample, could be useful to define any difference on music benefits on postoperative distress and control pain between males and females.
Conclusions
Music seems to improve postoperative cardiovascular parameters. While benefits on pain control are not so evident, positive effects on hyperglycemia stress-induced are relevant. According to previous reports in adults, relaxing effect are also achieved in children by the alternation of fast, slow rhythms and pauses.
Acknowledgments
The authors would thank A.B.C. Burlo Onlus for supporting psychological research in our Pediatric Surgery Unit; Dr. Ghassan Nakib for surgical support; Davide Bontempo (Associazione Culturale Musikademia, Vanzaghello, Milan); Fabio Gallazzi (Circolo Accademico Culturale di Vanzaghello, Milan) and Christian Silva (Associazione L’Ilopera, Lodi) for musical support; Dr Fiorenza Fava for support in the clinical research.
References
- 1.Orem J, Trotter RH.Behavioral control of breathing. News Physiol Sci 1994;9:228-32. [Google Scholar]
- 2.Shea SA.Behavioural and arousal-related influences on breathing in humans. Exp Physiol 1996;81:1-26. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi L, Porta C, Casucci G, et al. Dynamic interactions between musical, cardiovascular, and cerebral rhythms in humans. Circulation 2009;119:3171-80. [DOI] [PubMed] [Google Scholar]
- 4.Bernardi L, Porta C, Sleight P.Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: the importance of silence. Heart 2006;92:445-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trappe HJ.Role of music in intensive care medicine. Int J Crit Illn Inj Sci 2012;2:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dastgheib SS, Layegh P, Sadeghi R, et al. The effects of Mozart's music on interictal activity in epileptic patients: systematic review and meta-analysis of the literature. Curr Neurol Neurosci Rep 2014;14:420. [DOI] [PubMed] [Google Scholar]
- 7.Scalford D, Flynn-Roth R, Howard D, et al. Pain management of children aged 5 to 10 years after adenotonsillectomy. J Perianesth Nurs 2013;28:353-60. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald RA.Music, health, and well-being: a review. Int J Qual Stud Health Well-being 2013;8:20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caprilli S, Anastasi F, Grotto L, et al. Interactive music as a treatment for pain and stress in children during venipuncture: a randomized prospective study. J Dev Behav Pediatr 2007;28:399-403. [DOI] [PubMed] [Google Scholar]
- 10.Métayer S.Music therapy, a partner in patient care. Rev Infirm 2012;184:39-40. [PubMed] [Google Scholar]
- 11.Moris DN, Linos D.Music meets surgery: two sides to the art of healing. Surg Endosc 2013;27:719-23. [DOI] [PubMed] [Google Scholar]
- 12.Thrane S.Effectiveness of integrative modalities for pain and anxiety in children and adolescents with cancer: a systematic review. J Pediatr Oncol Nurs 2013;30:320-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stouffer JW, Shirk BJ, Polomano RC.Practice guidelines for music interventions with hospitalized pediatric patients. J Pediatr Nurs 2007;22:448-56. [DOI] [PubMed] [Google Scholar]
- 14.Granier-Deferre C, Bassereau S, Ribeiro A, et al. A melodic contour repeatedly experienced by human near-term fetuses elicits a profound cardiac reaction one month after birth. PLoS One 2011;6:e17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhury S, Jain S, Wadhwa S.Expression of synaptic proteins in the hippocampus and spatial learning in chicks following prenatal auditory stimulation. Dev Neurosci 2010;32:114-24. [DOI] [PubMed] [Google Scholar]
- 16.Sanyal T, Kumar V, Nag TC, et al. Prenatal loud music and noise: differential impact on physiological arousal, hippocampal synaptogenesis and spatial behavior in one day-old chicks. PLoS One 2013;8:e67347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S, Nag TC, Upadhyay AD, et al. Prenatal music stimulation facilitates the postnatal functional development of the auditory as well as visual system in chicks (Gallus domesticus). J Biosci 2014;39:107-17. [DOI] [PubMed] [Google Scholar]
- 18.Garra G, Singer A, Taira B, et al. Validation of the Wong-Baker FACES pain rating scale in pediatric emergency department patients. Acad Emerg Med 2010;17:50-4. [DOI] [PubMed] [Google Scholar]
- 19.Suranivongse S, Santawat U, Kraiprasit K, et al. Cross-validation of a composite pain scale for preschool children within 24 hours of surgery. Br J Anaesth 2001;87:400-05. [DOI] [PubMed] [Google Scholar]
- 20.Arafah BM.Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab 2006;91:3725-45. [DOI] [PubMed] [Google Scholar]
- 21.Desborough JP.The stress response to trauma and surgery. Br J Anaesth 2000;85:109-17. [DOI] [PubMed] [Google Scholar]
- 22.Good M, Albert JM, Arafah B, et al. Effects on postoperative salivary cortisol of relaxation/music and patient teaching about pain management. Biol Res Nurs 2013;15:318-29. [DOI] [PubMed] [Google Scholar]
- 23.Klassen JA, Liang Y, Tjosvold L, et al. Music for pain and anxiety in children undergoing medical procedures: a systematic review of randomized controlled trials. Ambul Pediatr 2008;8:117-28. [DOI] [PubMed] [Google Scholar]
- 24.Bernardi L, Porta C, Spicuzza L, et al. Cardiorespiratory interactions to external stimuli. Arch Ital Biol 2005;143:215-21. [PubMed] [Google Scholar]
- 25.Pei D, Chen TW, Kuo YL, et al. The effect of surgical stress on insulin sensitivity, glucose effectiveness and acute insulin response to glucose load. J Endocrinol Invest 2003;26:397-402. [DOI] [PubMed] [Google Scholar]
- 26.Sebranek JJ, Lugli AK, Coursin DB.Glycaemic control in the perioperative period. Br J Anaesth 2003;111:i18-i34. [DOI] [PubMed] [Google Scholar]
- 27.Bosarge PL, Kerby JD.Stress-induced hyperglycemia: is it harmful following trauma? Adv Surg 2013;47:287-97. [DOI] [PubMed] [Google Scholar]
- 28.Bai J, Hsu L, Tang Y, Van Dijk M.Validation of the comfort behaviour scale and FLACC scale for pain assessment in Chinese children after cardiac surgery. Pain Manag Nurs 2012;13:18-26. [DOI] [PubMed] [Google Scholar]
- 29.Wang SM, Kulkarni L, Dolev J, Kain ZN.Music and preoperative anxiety: a randomized, controlled study. Anesth Analg 2002;94:1489-94. [DOI] [PubMed] [Google Scholar]
- 30.Megneault B, Girard F, Albert C, et al. The effect of music on the neurohormonal stress response to surgery under general anesthesia. Anesth Analg 2004;98:527-32. [DOI] [PubMed] [Google Scholar]
- 31.Miluk-Kolasa B, Obminski Z, Stupnicki R, Golec L.Effects of music treatment on salivary cortisol in patients exposed to presurgical stress. Exp Clin Endocrinol 1994;102:118-20. [DOI] [PubMed] [Google Scholar]
- 32.Locsin RG.The effect of music on the pain of selected post-operative patients. J Adv Nurs 1981;6:19-25. [DOI] [PubMed] [Google Scholar]
- 33.Melzack R, Wall PD.Pain mechanisms: a new theory. Science 1965;150:971-9. [DOI] [PubMed] [Google Scholar]


