Abstract
Since its initial misidentification as a trypanosome some 100 years ago, Pneumocystis has remained recalcitrant to study. Although we have learned much, we still do not have definitive answers to such basic questions as, where is the reservoir of infection, how does Pneumocystis reproduce, what is the mechanism of infection, and are there true species of Pneumocystis? The goal of this review is to provide the reader the most up to date information available about the biology of Pneumocystis and the disease it produces.
Pneumocystis is a fungus that causes pneumonia in people with weakened immune systems. As a nonculturable organism, it has been challenging to study, and key questions about its basic biology and pathogenic mechanisms remain.
Because it was first recognized in an animal also infected with Trypanosoma cruzi, Pneumocystis was initially mistaken for a morphologic form in the life cycle of T. cruzi. Antonio Carini, an Italian working in Brazil, saw the same organism-like cysts in the lungs of rats experimentally infected with Trypanosoma lewisi. The Delanoës and their colleagues at the Pasteur Institute recognized that these alveolar cysts were present in the lungs of local Parisian sewer rats, and established that the organisms were unique and distinct from trypanosomes. They proposed the name Pneumocystis carinii for the new species.
Over the next quarter century, there were occasional histological descriptions of Pneumocystis in the lungs of humans, but it was not until 1942 that Van der Meer and Brug reported the initial epidemics of interstitial plasma cell pneumonia among premature debilitated babies in nurseries and foundling homes in central Europe (Van der Meer and Brug 1942). In 1952 in Czechoslovakia, Vanek and Jirovec provided the most convincing demonstration of the etiologic relationship of Pneumocystis to this disease in an autopsy study of 16 cases (Vanek and Jirovek 1952).
Based on morphology, Pneumocystis was originally thought to be a protozoa. However, in 1988, DNA analyses revealed that the genomic structure of Pneumocystis was more closely related to fungi than to protozoa (Edman et al. 1988). Most recently a change in nomenclature for the organism infecting humans from P. carinii to Pneumocystis jirovecii has been proposed (Stringer et al. 2002). This name was chosen in honor of the parasitologist Otto Jirovec, who is credited by some, likely in error, with the original description of this organism in humans. The biologic rationale for this change is the unique antigenic and genetic properties, and restricted infectivity profile of the Pneumocystis organisms associated with each mammalian species.
SYSTEMATIC POSITION OF Pneumocystis SPECIES WITHIN THE KINGDOM FUNGI
The genus Pneumocystis represents related fungal species that are members of the phylum/division Ascomycota, the subphylum Taphrinomycotina, class Pneumocystidomycetes, order Pneumocystidales, and family Pneumocystidaceae, all within the kingdom Fungi. Genetic investigations indicate that individual Pneumocystis species coevolved and coexisted with their respective mammalian hosts over many thousands of years (reviewed in Aliouat-Denis et al. 2008). Indeed, the genus Pneumocystis encompasses a variety of ubiquitous fungi that colonize and infect a wide variety of mammalian host species. The species P. jirovecii specifically infects humans, whereas the well-studied species of P. carinii and Pneumocystis murina are the genus members associated with rats and mice, respectively. Relevant Pneumocystis species have been identified in rabbits, ferrets, sheep, monkeys, and even in waterborne mammalian hosts such as cetaceans. The various Pneumocystis species are also quite rigid with respect to their unique specificity for any given mammalian host. For example, the human-specific species P. jirovecii is only capable of infecting humans and is not capable of infecting other animals, and vice versa. Species specificity of Pneumocystis members across various host species was studied by Wakefield and colleagues using polymerase chain reaction (PCR) with primer pairs specific for the mitochondrial large subunit rRNA genes of all known species of Pneumocystis. When primers specific for nonhuman Pneumocystis species were used on human clinical isolates, no amplification was detected. Conversely, primers specific for human Pneumocystis failed to amplify any product from nonhuman isolates (reviewed in Stringer 2002). Interestingly, human P. jirovecii was found to have closest homology with Pneumocystis organisms derived from primates. Furthermore, cross-infection of a given Pneumocystis species into its non-native mammalian host has never been conclusively proven.
Additional investigations defining the molecular genetics of various Pneumocystis species have further indicated that rats can be infected with P. carinii, Pneumocystis wakefieldiae, or with both species concurrently (Icenhour et al. 2006). The general term Pneumocystis simply refers to the genus name, and unless otherwise specified, denotes qualities that are shared by all of the specific Pneumocystis species studied.
EPIDEMIOLOGY
There is no known environmental reservoir for Pneumocystis. DNA sequences identical to those of Pneumocystis have been detected by PCR in samples of ambient air, but intact organisms have not been visualized in environmental samples nor has disease been transmitted by such samples. Animal-to-animal transmission of Pneumocystis by the airborne route has been shown, and human-to-human transmission is presumed based on animal studies (Hendley and Weller 1971; Hughes 1982; Gigliotti et al. 2003). Consistent with this concept are the many clusters of cases of Pneumocystis pneumonia (PCP) that have been reported in immunocompromised patients (Sassi et al. 2012). As noted, animal experiments have shown that Pneumocystis from one mammalian host is not able to infect a different mammalian host, thereby making animal-to-human transmission unlikely (Gigliotti et al. 1993; Durand-Joly et al. 2002). Pneumocystis infection has global distribution among humans and most individuals show serologic evidence of infection by 2 years of age (Vargas et al. 2001). The incidence of PCP is related to the extent of immunosuppression, especially impairment in cell-mediated immunity, as evidenced by the frequent occurrence of PCP in patients with AIDS.
Pneumocystis SPECIES (LIFE CYCLE, MORPHOLOGICAL CHARACTERISTICS, SPECIES-SPECIFIC DIAGNOSTIC FEATURES DISTINGUISHING THE RELATED SPECIES)
The study of the Pneumocystis life cycle has remained a significant challenge over many years owing to the fact that an environmental reservoir or niche has still not been defined, and that a long-term in vitro culture system has also remained elusive. Transient proliferation of Pneumocystis has been achieved on lung epithelial and other feeder cells; however, such axenic cultures cannot be maintained beyond a few weeks (Cushion et al. 1985; Limper et al. 1997). Furthermore, despite scattered reports of short-term in vitro culture in systems that do not require feeder cells (Merali et al. 1999), continuous Pneumocystis cultures have not been consistently observed in major research laboratories. This may be explained by the observation that Pneumocystis appears to lack genes involved in de novo synthesis of amino acids (Hauser et al. 2010). Therefore, Pneumocystis investigators remain hamstrung without a valid and reproducible culture system, or with reliable methods to genetically manipulate this organism. Despite these obstacles, considerable advances have been made over the past decade using molecular approaches often in heterologous systems (Hauser et al. 2006; LoPressi et al. 2007). Immune-suppressed animal models of Pneumocystis pneumonia, particularly using rats and mice, continue to serve as a reliable source of organisms for study. In fact, all currently available agents for the treatment of P. jirovecii pneumonia in humans were initially derived from the study of P. carinii in rats and P. murina in mice (Thomas and Limper 2007).
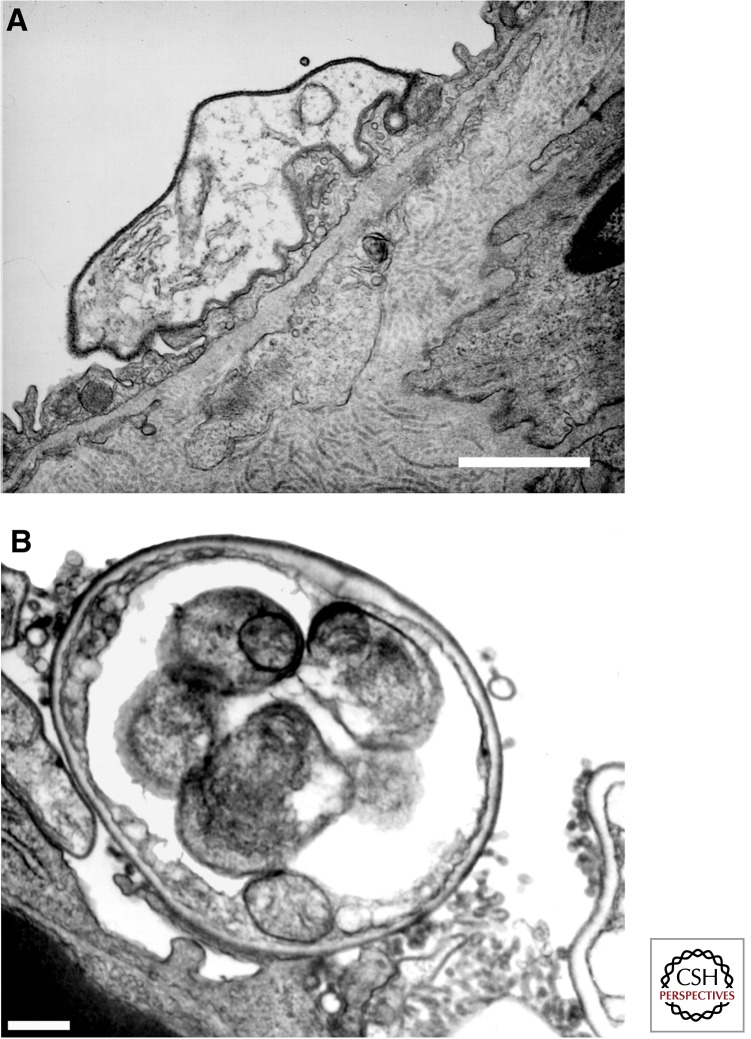
Light microscopic and ultrastructural analyses of animal- and human-derived materials have provided some initial insight into the life cycle of Pneumocystis. Such studies reveal that there are at least two different life cycle forms of Pneumocystis organisms, namely, the trophic form and the cyst (Fig. 1). The trophic form generally measured ∼2 µm in greatest diameter is relatively heterogonous in shape, and is surrounded only by a plasma membrane, lacking a rigid cell wall. The trophic forms contain a single haploid nucleus (1C), and are lacking in a rigid cell wall structure (Wyder et al. 1994). In contrast, the cyst is significantly larger than the trophic form, measuring ∼8–10 µm in greatest diameter, is more uniform in shape, and contains up to eight intracystic bodies. The nuclear content in cysts varies between diploid (2C), tetraploid (5C), or octaploid (8C) DNA content. Rupture of the mature cyst containing eight separate nuclear intracystic bodies, a process termed excystment, releases the intracystic bodies to become new trophic forms of the organism. The rigid Pneumocystis cyst wall is formed of β-glucan, a complex branching polysaccharide containing other components such as mannoproteins including glycoprotein A/major surface glycoprotein, chitins, and other proteins expressed on the surface of the organism. The main function of the cyst wall is to confer rigidity and support for the organism, and putatively, to protect the organism from harsh environmental conditions outside the host. Additional studies indicate that interactions of the cyst wall with lung cells can also activate innate immune responses in the host (Carmona et al. 2012; Evans et al. 2012).
Figure 1.
Electron micrographic visualization of typical Pneumocystis life cycle forms. (A) Reveals a trophic form of P. carinii adherent to a type I alveolar epithelial cell, mediated by interdigitation of the organism’s plasma membrane with that of the host cell. (B) Shows a thick-walled cyst form of P. carinii. Scale bars, ∼1 μm. (From Limper et al. 1997; reprinted, with permission, from the author.)
Unlike many other fungi, the route of Pneumocystis infection is not well defined. Current concepts suggest that infectious Pneumocystis forms are transmitted as aerosolized particles from host to host. Although some evidence supports the presence of Pneumocystis forms and DNA outside the host, an environmental reservoir or environmental phase of the life cycle has not been defined. Some investigators have proposed a model of initial infection early during infancy or childhood, with later reactivation during periods of immune suppression. Human serologic evidence of anti-Pneumocystis antibodies or positive PCR in healthy children supports early exposure to this organism (reviewed in Carmona and Limper 2011). Additional data further support the notion of colonization of patients with underlying lung disease such as chronic obstructive pulmonary disease (COPD) and also transiently in otherwise healthy individuals (Morris et al. 2004). Although some evidence suggests in utero transmission of Pneumocystis organisms, careful studies in rodents indicate that infants are likely exposed to the infectious agents very early in life (Icenhour et al. 2002).
Once inhaled, Pneumocystis has substantial tropism for alveolar epithelial cells of the lung and rarely disseminates to other organs, although occasional cases of extrapulmonary dissemination have been reported specifically in patients with profound immunosuppression such as during advanced HIV infection (Dieterich et al. 1992; Guttler et al. 1993; Bartlett and Hulette 1997; Ruggli et al. 1997; Hagmann et al. 2001; Panos et al. 2007). Pneumocystis trophic forms bind tightly to type I alveolar epithelial cells, in part utilizing extracellular matrix proteins (Walzer 1986). Interactions of Pneumocystis with extracellular matrix proteins, particularly fibronectin and vitronectin, facilitate binding of Pneumocystis to lung epithelial cells (Limper et al. 1993). A Pneumocystis surface integrin-like extracellular matrix adhesion receptor, termed PcInt1, has been described. This protein is expressed only on trophic forms and mediates attachment of the organism to fibronectin (Kottom et al. 2008). Adhesion of Pneumocystis to lung epithelial cells triggers several specific signaling pathways in the fungus, involving activation of conserved mitogen-activated protein (MAP) kinases, namely, PcSte20 and PcCbk1 that promote organism mating, proliferation, and cell wall remodeling (Kottom et al. 2003; Kottom and Limper 2013). It is proposed that a sexual conjugation mating of trophic forms occurs after binding of the organisms to lung cells, which is then followed by meiosis and mitosis. These processes result in generation of the mature cyst, containing the eight intracystic forms (Burgess et al. 2008). Pneumocystis life cycle forms that may represent conjugated trophic forms, zygotic forms, and other forms in transition to acquiring a mature cell wall have been generally referred to as “precyst” forms.
Pneumocystis cysts and trophic forms have identical morphology irrespective of whether they are derived from human (P. jirovecii), rat (P. carinii), mouse (P. murina), or other mammalian hosts. In human clinical samples, Pneumocystis trophic forms or cysts can be identified in induced sputum, bronchoalveolar lavage fluid, or lung tissue using a variety of tinctorial histochemical stains. Trophic forms can be detected with Papanicolaou, Gram-Weigert, or Wright Giemsa. Cysts can be stained with Gomori methenamine silver (GMS), cresyl echt violet, toluidine blue O, or calcofluor white (CW) fungal stains. The use of any of these tinctorial stains requires expertise on the part of the investigator to differentiate Pneumocystis from artifacts, from other endemic mycoses (such as Histoplasma capsulatum), and from nonspecific staining. Immunofluorescent antibody (IFA) stains, which use monoclonal antibodies directed against human Pneumocystis epitopes, can enhance direct detection of this organism in clinical specimens, because they have high specificity and because they detect both the cyst and trophic forms of Pneumocystis.
DISEASE
Defects in CD4+ T-cell-mediated immunity render hosts susceptible to Pneumocystis infection. However, innate immune mechanisms also contribute to host defense by limiting fungal growth in immunocompromised hosts. For example, accelerated Pneumocystis growth has been observed in CD4+ T-cell-deficient mice that also lack components of innate immunity (Inoue et al. 2011; Kelly et al. 2013; Bello-Irizarry et al. 2014). Although hypoxia is the hallmark of PCP, the clinical features vary with immune status in individual patients. The disease onset may be insidious with a clinical course of 3 or more wk, or fulminant and rapidly progressive over a few days. Fever generally is present and often precedes the onset of nonproductive cough, tachypnea, and severe dyspnea. Classic physical findings at the time of initial evaluation include tachypnea, nasal flaring, and intercostal, subcostal, or supracostal retractions. Cyanosis may be present or may develop rapidly. Auscultation of the chest frequently is characterized by a conspicuous absence of adventitious sounds. Scattered rales, rhonchi, or wheezes most often are detected later in the clinical course as resolution occurs. Radiographic abnormalities vary. Bilateral diffuse parenchymal infiltrates occur most commonly, but no pattern is sufficiently specific either to exclude or to confirm a diagnosis of PCP. Although initially a reticulogranular interstitial process, Pneumocystis pneumonitis progresses to a predominantly alveolar process with coalescence and air bronchogram formation. Late in the course of the disease, lung fields may opacify completely. Residual interstitial fibrosis occurs in a small percentage of patients. The timing of onset of clinical disease in non-AIDS, high-risk patients is unpredictable, but disease often occurs after discontinuance or reduction in the dose of corticosteroid therapy or after engraftment in bone marrow transplant recipients. These observations support the hypothesis that the clinical features early in the course of PCP are dependent to a large extent on the patient’s ability to mount an inflammatory response. A more robust pulmonary inflammatory response, rather than organism burden, portends a worse prognosis. For example, AIDS patients who develop PCP typically have higher organism burdens, but lower mortality rates than non-AIDS patients (Limper et al. 1989). This is further evidence that the patient’s inflammatory response plays an important role in the pathogenesis of PCP.
Diagnosis of PCP requires the demonstration of Pneumocystis in the lungs of a patient with compatible pulmonary signs and symptoms. An open lung biopsy is the most reliable method, although bronchoalveolar lavage is generally more practical and nearly as sensitive. Induced sputum samples are gaining popularity, but are helpful only if positive. The yield of various diagnostic specimens is approximately as follows: induced sputum 20%–40%, tracheal aspirate 50%–60%, bronchoalveolar lavage 75%–95%, transbronchial biopsy 75%–95%, and open lung biopsy 90%–100%. Four commonly used stains to identify Pneumocystis are GMS and toluidine blue that stain only cyst forms, polychrome stains such as Giemsa that stain both trophozoites and sporozoites, and fluorescein-labeled monoclonal antibody that stains for both trophozoites and cysts (Procop et al. 2004). PCR analysis of respiratory specimens offers promise as a rapid diagnostic method, but a standardized system for clinical use has not been established.
The drug of choice for prophylaxis against or treatment of PCP is trimethoprim-sulfamethoxazole (tmp-smx). The duration of treatment is generally 3 wk for patients with AIDS and 2 wk for other patients. Adverse reactions occur frequently, more so in adults than children. These include rash, fever, and neutropenia. These side effects are less common in non-AIDS patients. For patients who cannot tolerate or fail to respond to tmp-smx after 5–7 d, pentamidine isethionate may be used. Atovaquone has been used primarily in adults with mild to moderate disease. Administration of corticosteroids in addition to anti-Pneumocystis drugs increases the chances for survival in moderate and severe cases of PCP. Detailed guidelines for the management of PCP can be found at www.aidsinfo.nih.gov.
PATHOGENESIS
In the immunocompromised host, PCP is uniformly fatal if untreated. However, in contrast to conventional thinking, recent evidence indicates that immunocompetent hosts are also transiently infected with Pneumocystis. Infection of the normal host results in a self-limited mild or subclinical lower respiratory tract infection, and the organism is not overtly pathogenic in hosts with intact immune systems (Vargas et al. 2001; Gigliotti et al. 2003; Gigliotti and Wright 2012). Sequencing of the P. jirovecii genome determined that the organism lacks obvious virulence genes or toxins, and does not possess many metabolic enzymes typically found in free-living fungi (Cisse et al. 2012). In contrast to other human fungal pathogens, Pneumocystis grows slowly and noninvasively in its permissive host. These findings suggest that Pneumocystis have adapted to survive and propagate within mammalian lungs without causing obvious cellular damage, which is clearly beneficial for organisms that rely on the host lung environment for survival.
Early ultrastructural studies described the tight attachment of Pneumocystis to type I pneumocytes in vitro as well as in vivo (Murphy et al. 1977; Lanken et al. 1980; Yoneda and Walzer 1980; Long et al. 1986; Benfield et al. 1997). Despite this close host–pathogen interaction and the observation of projections emanating from the organism to the host cell, these early studies indicated that Pneumocystis causes little damage to the host and incites remarkably little inflammation. Only during advanced infection were pathogenic changes such as degeneration of type I pneumocytes, hyperplasia of type II pneumocytes, and disruption of the alveolar–capillary barrier noted. In the chronically immunosuppressed host, the pathophysiology of PCP appears related to physical disruption of gas exchange through direct attachment of Pneumocystis to alveolar epithelial cells (AECs), and the accumulation of organisms, cell debris, and a characteristic foamy exudate in the alveolar lumen. As the infection progresses, the interaction of Pneumocystis with lung epithelial cells may impede lung repair by inhibiting epithelial proliferation (Limper et al. 1998).
Pneumocystis lacks many characteristics that contribute to the virulence of other human fungal pathogens, but nonetheless PCP remains a life-threatening opportunistic respiratory disease. An intact immune system resists Pneumocystis infection, and fulminant PCP is only observed in immunocompromised individuals. However, the host’s immune system is also a major contributor to the pathogenesis of PCP. Clinical evidence has offered insight into the nature of PCP-related lung injury. For example, the severity of PCP correlates positively with the degree of pulmonary inflammation, but not lung fungal burden (Limper et al. 1989; Benfield et al. 1995; Bang et al. 2001). Comparison of AIDS and non-AIDS PCP patients revealed that profoundly immunosuppressed AIDS patients present with higher lung burdens, but display a subtler onset of disease with better pulmonary function and improved prognosis relative to non-AIDS patients (Limper et al. 1989). The onset of clinical PCP has also been noted when corticosteroids are tapered, or when bone marrow engraftment is evident (Sepkowitz 1992, 1993), suggesting that restoration of the immune system drives pathogenesis. The significance of immunopathogenesis has gained more attention with the recognition of PCP-related immune reconstitution inflammatory syndrome (IRIS) in HIV-positive patients (Barry et al. 2002; Koval et al. 2002; Jagannathan et al. 2009). The rapid recovery of CD4+ T cells following initiation of combined antiretroviral therapy causes an intense pulmonary immune response and rapid pulmonary decompensation. This pathological immune response is driven by a preexisting pulmonary infection, and Pneumocystis has been identified as one of the causative agents. These observations support the immunopathogenic nature of PCP in that infection with Pneumocystis is necessary to cause pneumonia, but the Pneumocystis-driven immune response is responsible for the associated pathophysiology. The failure of current therapies to adequately combat immunopathogenesis during PCP may partly explain treatment failures in patients. Even in the absence of viable organisms, the presence of Pneumocystis antigen may continue to drive immunopathogenesis.
Animal models of PCP have proven remarkably accurate mimics of human disease, and have provided a platform for the identification of immune effector mechanisms that contribute to immunopathogenesis. Severe combined immunodeficient (SCID) mice are extremely susceptible to Pneumocystis infection because they are unable to mount an adaptive immune response. Roths and Sidman found that transferring functional lymphocytes to Pneumocystis-infected SCID mice causes a lethal hyperinflammatory response in the lung (Roths and Sidman 1992). Subsequent studies show that immune reconstitution of infected SCID mice induces a rapid pulmonary inflammatory response with deleterious effects on pulmonary function and oxygenation (Wright et al. 1997, 1999). Although immune reconstitution restores an effective CD4+ T-cell-dependent immune response against Pneumocystis, it also has profound effects on physiology, including severe weight loss, tachypnea, and hypoxia. Nonreconstituted SCID mice with similar Pneumocystis burdens exhibited few signs of PCP-related respiratory disease, again supporting the concept that Pneumocystis itself is not potently toxic or damaging to the lung. The immune reconstituted SCID mouse model of PCP is similar to the IRIS presentation of PCP in human patients, and provides a model for elucidating mechanisms of PCP-related IRIS. Recent discoveries using this model have determined that CD4+ T cells are critical for mediating immunopathogenesis, whereas CD8+ T cells serve a regulatory function by limiting the CD4+ T-cell response (Bhagwat et al. 2006; Swain et al. 2006). Pharmacological manipulation of the organism in vivo found that the cyst form of Pneumocystis, which contains proinflammatory cell wall components, drives immunopathogenesis during IRIS (Linke et al. 2013). Immunomodulatory strategies that alter T-cell and macrophage effector phenotype during IRIS hold promise for enhancing host defense while reducing disease severity and promoting resolution (Wang et al. 2010, 2011).
The classic presentation of AIDS-related PCP has also been effectively modeled in animals. Although much work has been performed in rodents, SIV-infected nonhuman primates have also been used to model PCP (Board et al. 2003). Systemic administration of anti-CD4 monoclonal antibody maintains mice in a chronic CD4+ T-cell-depleted state. CD4-depleted mice develop a clinical syndrome very similar to AIDS-related PCP in humans (Harmsen and Stankiewicz 1990; Shellito et al. 1990; Wright et al. 1999). In the absence of CD4+ lymphocytes, CD8+ T cells are recruited to the lung in response to Pneumocystis infection (Wright et al. 1999, 2004; Meissner et al. 2005). These lymphocytes are unable to control Pneumocystis infection, but directly contribute to PCP-related lung injury. Infected mice depleted of both CD4+ and CD8+ lymphocytes are significantly healthier than mice depleted of only CD4+ lymphocytes (Wright et al. 1999). The mechanism of CD8+ T-cell-mediated lung injury during PCP is antigen specific and requires TNF receptor signaling (Wright et al. 2004), MHC class I expression on resident lung cells (Meissner et al. 2005), and the presence of Pneumocystis antigen in the lung (Meissner et al. 2005). It has also been suggested that Pneumocystis itself enhances the immunosuppressive environment in CD4-depleted mice by inducing alveolar macrophage apoptosis (Lasbury et al. 2006, 2007). Despite the fact that neutrophil numbers in the lungs of humans and animals with PCP strongly correlate with the severity of disease, these cells do not appear to directly contribute to either host defense or immunopathogenesis (Swain et al. 2004).
Surfactant dysfunction has been noted in humans and animals suffering from PCP (Sheehan et al. 1986; Escamilla et al. 1992; Hoffman et al. 1992; Aliouat et al. 1998; Atochina et al. 2000; Schmidt et al. 2006), and surfactant therapy has shown therapeutic benefit in both patients and experimental animals (Eijking et al. 1992; Creery et al. 1997). Dramatic alterations in surfactant phospholipid and protein composition, as well as impairment of physiological surfactant function are associated with PCP. Pneumocystis may disrupt the pulmonary surfactant system through several mechanisms. Pneumocystis may act directly on AECs to modulate surfactant phospholipid and protein synthesis; Pneumocystis organisms may directly interact with surfactant components in the alveolus to disrupt function; or Pneumocystis may affect surfactant through disruption of the alveolar–capillary barrier resulting in leakage of inhibitory plasma proteins into the alveolar lumen (Wang et al. 2005). Furthermore, a potential mechanism of PCP-related immunopathogenesis is impairment of normal surfactant function. Pulmonary inflammation elicited during Pneumocystis infection was found to directly disrupt surfactant function, contributing to PCP-related respiratory impairment (Wright et al. 2001). Thus, surfactant dysfunction is a physiological consequence of Pneumocystis infection, which likely contributes to impaired gas exchange and hypoxia that is a hallmark of PCP.
Current research suggests that in addition to causing active PCP, Pneumocystis infection may also be an underappreciated determinant of chronic lung disease. Recent clinical evidence suggests that Pneumocystis infection or colonization exacerbates other forms of chronic lung disease. COPD patients who are also colonized with Pneumocystis show an accelerated disease progression with worse pulmonary function compared with noncolonized controls (Morris and Norris 2012). Recent work in a nonhuman primate model of SIV infection determined that Pneumocystis colonization drives the development of COPD (Shipley et al. 2010). Pneumocystis colonization has also been reported in cystic fibrosis patients (Calderon et al. 2010), although the significance of this finding is unclear. Animal studies have shown that transient Pneumocystis infection can promote allergic sensitization of the lung (Swain et al. 2011), induce chronic pulmonary hypertension (Swain et al. 2007), and also exacerbate pulmonary fibrosis (Bruckner et al. 2006). Pneumocystis may also affect HIV-related disease by exerting unique effects on bone marrow precursor cells (Taylor et al. 2011). Many of these potential complicating effects persist long after the infection has been cleared.
Although the specific mechanisms of PCP-related immunopathogenesis are not fully defined, the contribution of inflammation and the immune response to this disease process is clear, and standard treatment of moderate to severe PCP typically includes corticosteroids as adjunctive therapy to dampen inflammation. Although infection is necessary to cause PCP, certain aspects of the immune response cause or exacerbate disease symptoms. Differences in the degree of immunosuppression among PCP patients likely affect their ability to produce an immunopathogenic response to infection, and may account for variability in the severity of PCP between different patient groups.
UNIQUE CHARACTERISTICS DISTINGUISHING Pneumocystis FROM OTHER RELATED MOLDS OR YEASTS
As ascomycetous fungi, Pneumocystis species bear certain morphological similarities to other related organisms. Although an extremely close fungal relative to Pneumocystis has not yet been defined, sequencing of rRNA from P. carinii, as well as more extensive sequencing studies undertaken during the P. carinii genome project, indicates the closest phylogenetic relative to be Schizosaccharomyces pombe. In addition, some genetic similarities have also been noted to Neurospora species. Functional genetic studies further show close parallels in certain Pneumocystis functional metabolic systems (e.g., cell wall generation and integrity) to those systems utilized by Saccharomyces cerevisiae. Similar to other ascomyctes, Pneumocystis have a life cycle form consisting of an ascus or spore case, which in the case of Pneumocystis has classically been referred to as the cyst. However, unlike other phylogenetically related organisms, Pneumocystis also has the separate life cycle stage, the trophic form, that lacks a rigid cell wall. The trophic form is known to extend cell membrane projections, which interdigitate with host lung cells and mediate firm attachment of the Pneumocystis tropic forms to the alveolar epithelium (Limper and Martin 1990). The Pneumocystis trophic forms, however, do not proceed to show either pseudohyphal or true hyphal growth morphologies. Hence, Pneumocystis cannot be viewed as a dimorphic fungus in the classical sense. Although the typical Pneumocystis cyst forms that are observed on silver staining are structurally reminiscent of yeast forms present in other fungal infections, during active infection, the overwhelming majority (>90%) of Pneumocystis organisms present in the lung are actually of the trophic form morphology.
In further contrast to other ascomycetous fungi, and despite extensive and ongoing efforts, Pneumocystis remains nonculturable in defined media. Although this may in part be nutritional, efforts to supplement defined media with lung extracts and with varying oxygen tensions and pH have been disappointingly unsuccessful (Thomas and Limper 2007). One clue to the elusive life cycle of Pneumocystis may be the organism’s ability to sense contact with lung epithelial cell surfaces. This contact sensing is termed thigmotropism, and is a phenotypic quality not widely observed in other ascomycetous fungi. Contact sensing by Pneumocystis initiates signaling pathways in the organism essential for cell wall remolding and fungal proliferation (Kottom et al. 2003, 2011a; Kottom and Limper 2013). Also distinguishing Pneumocystis from other ascomycetes is a lack of response to traditional antifungal agents, including polyenes such as amphotericin and extended spectrum azoles. The lack of responsiveness of Pneumocystis to amphotericin and other polyenes is likely related to the lack of ergosterol in the organism’s cell wall. In addition, sequencing data indicate that Pneumocystis natively shows resistance to extended spectrum azole such as itraconazole widely used against other fungi (Morales et al. 2003). Therapies against Pneumocystis largely rely on treatment with trimethoprim-sulfamethoxazole or with pentamidine (Thomas and Limper 2004).
ANALYTICAL MANIPULATION (MOLECULAR GENETICS INCLUDING PARADIGMS THAT DEFINE VIRULENCE FACTORS OR DRUG TARGETS)
Even though Pneumocystis is nonculturable, and is not genetically tractable, significant advances in understanding the molecular genetics of the organism have occurred over the past decade by directly sequencing Pneumocystis genes, and by studying Pneumocystis protein functions using heterologous gene expression, largely in S. pombe and S. cerevisiae (Burgess et al. 2008; Kottom and Limper 2013). The rat-derived P. carinii genome project (pgp.cchmc.org) has been widely utilized by the Pneumocystis research community. More recently, sequencing of both the P. murina and the P. jirovecii genomes has been reported (Cisse et al. 2012; Ma et al. 2013). The P. carinii genome contains ∼8 million base pairs of DNA divided into 15 linear chromosomes ranging from 300 to 700 kb (Stringer and Cushion 1998). The genes have a high A:T content of 65%, and the majority of genes are interrupted by introns ranging from 38 to 424 bp (Thomas et al. 1999). Using these sequence resources and heterologous expression strategies, key molecules in the organism’s mitotic cell cycle, cell wall assembly, signal transduction cascades, and metabolic pathways have been identified.
A significant fraction of the Pneumocystis genome encodes for surface membrane proteins and glycoconjugates. The most plentiful surface antigens, termed glycoprotein A (gpA) or major surface glycoprotein (MSG), have molecular masses of 95–120 kDa and are heavily mannosylated. These glycoproteins are proposed to participate in attachment of Pneumocystis to host lung cells, as well as in evading host defenses (Gigliotti et al. 1986, 1988; Linke et al. 1989; Lundgren et al. 1993; O’Riordan et al. 1995; Vuk-Pavlovic et al. 2001). The gpA/MSG isoform expressed is immunogenic and antigenically distinct in the different species of Pneumocystis that infect different mammalian hosts (Kovacs et al. 1989; Gigliotti 1992; Kovacs et al. 1993; Stringer and Keely 2001). Of the >80 gpA/MSG found in the P. carinii genome, only a single isoform of gpA/MSG is expressed at any given time. This may enable the organism to evade host defense and establish infection. Also expressed by the organism is the subtilisin-like serine proteases Prt1 (also termed Kex1) in both human and mouse-derived Pneumocystis (Lugli et al. 1997, 1999; Lee et al. 2000; Kutty and Kovacs 2003). These proteases are thought to function in processing of the Pneumocystis surface antigens.
The major structural component of the Pneumocystis cyst wall is β-glucan. This form of glucan consists of polymers of d-glucose arranged with a β-1,3-linked carbohydrate core and side chains of β-1,6-linked glucosyl residues (Douglas 2001). The cyst wall also contains other complex carbohydrate polymers, chitins, and accessory proteins. P. carinii β-glucan provides stability and structural support in the infected lung, but also elicits significant inflammatory responses in the infected host, which enhance immunological damage to the alveolar epithelium (Vassallo et al. 2000). Isolated P. carinii β-glucans elicit inflammatory responses by alveolar macrophages and respiratory epithelial cells, promoting respiratory damage in patients with severe PCP (Limper et al. 1989; Vassallo et al. 1999a,b; Lebron et al. 2003; Carmona et al. 2006). In addition, Pneumocystis β-glucans also activate dendritic cells and T-cell responses (Carmona et al. 2006) that participate in the clearance of Pneumocystis organisms. Pneumocystis β-glucans further prime dendritic cells in a manner that polarizes T cells toward Th17 differentiation, further augmenting host antifungal resistance (Zelante et al. 2007). The synthesis of Pneumocystis β-glucan is mediated by Pcgsc1, a β-1-3-glucan synthetase gene and Pckre6, which confers β-1-6-glucan synthetic activity. Inhibition of the P. jirovecii cell wall with agents that suppress these pathways is a potential novel treatment target for Pneumocystis pneumonia, although this has yet to be studied in humans (Schmatz et al. 1990; Powles et al. 1998).
Additional studies have focused on the mechanisms that regulate Pneumocystis cell-cycle control and proliferation, as these might provide new understanding for treating these infections. Pneumocystis shows both mitotic and meiotic cell replication machinery (Kottom et al. 2000; Burgess et al. 2008). Analogous to other ascomyctes, particularly S. pombe, Pneumocystis cell-cycle control is precisely regulated by cell division cycle (cdc) cyclin-dependent kinases and their related cyclins and regulatory inhibitors. These molecules include P. carinii cdc2 cyclin-dependent kinase, the cdc13 B-type cyclin, and the regulatory cdc25 mitotic phosphatase (Thomas et al. 1998; Kottom et al. 2000; Gustafson et al. 2001). These molecules are essential in mitotic proliferation. In addition, regulated gene expression and chromosomal replication further requires regulation of Pneumocystis histone acetylation, which is mediated both in P. carinii and P. jirovecii by Rtt109 histone acetyltransferases and related proteins (Kottom et al. 2011b). These molecules appear to be a novel new treatment target with activity in many major fungi (Pupaibool et al. 2013). Additional evidence supports the existence of meiotic life cycle machinery in Pneumocystis species required for sexual reproduction. This system in P. carinii utilizes PcMei2 and PcRan1, molecules highly analogous to the meiotic regulatory systems present in S. pombe (Burgess et al. 2008, 2009). Taken together, these studies indicate that Pneumocystis species have a life cycle with both mitotic and meiotic phases analogous to other ascomycetous fungi, but with unique adaptations to interact with host cells and proliferate within the unique niche of the mammalian lung.
Footnotes
Editors: Arturo Casadevall, Aaron P. Mitchell, Judith Berman, Kyung J. Kwon-Chung, John R. Perfect, and Joseph Heitman
Additional Perspectives on Human Fungal Pathogens available at www.perspectivesinmedicine.org
REFERENCES
- Aliouat EM, Escamilla R, Cariven C, Vieu C, Mullet C, Dei-Cas E, Prevost MC 1998. Surfactant changes during experimental pneumocystosis are related to Pneumocystis development. Eur Respir J 11: 542–547. [PubMed] [Google Scholar]
- Aliouat-Denis CM, Chabe M, Demanche C, Aliouat eM, Viscogliosi E, Guillot J, Delhaes L, Dei-Cas E 2008. Pneumocystis species, co-evolution and pathogenic power. Infect Genet Evol 8: 708–726. [DOI] [PubMed] [Google Scholar]
- Atochina EN, Beers MF, Scanlon ST, Preston AM, Beck JM 2000. P. carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am J Physiol Lung Cell Mol Physiol 278: L599–L609. [DOI] [PubMed] [Google Scholar]
- Bang D, Emborg J, Elkjaer J, Lundgren JD, Benfield TL 2001. Independent risk of mechanical ventilation for AIDS-related Pneumocystis carinii pneumonia associated with bronchoalveolar lavage neutrophilia. Respir Med 95: 661–665. [DOI] [PubMed] [Google Scholar]
- Barry SM, Lipman MC, Deery AR, Johnson MA, Janossy G 2002. Immune reconstitution pneumonitis following Pneumocystis carinii pneumonia in HIV-infected subjects. HIV Med 3: 207–211. [DOI] [PubMed] [Google Scholar]
- Bartlett JA, Hulette C 1997. Central nervous system pneumocystosis in a patient with AIDS. Clin Infect Dis 25: 82–85. [DOI] [PubMed] [Google Scholar]
- Bello-Irizarry SN, Wang J, Johnston CJ, Gigliotti F, Wright TW 2014. MyD88 signaling regulates both host defense and immunopathogenesis during Pneumocystis infection. J Immunol 192: 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfield TL, Vestbo J, Junge J, Nielsen TL, Jensen AB, Lundgren JD 1995. Prognostic value of interleukin-8 in AIDS-associated Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 151: 1058–1062. [DOI] [PubMed] [Google Scholar]
- Benfield TL, Prento P, Junge J, Vestbo J, Lundgren JD 1997. Alveolar damage in AIDS-related Pneumocystis carinii pneumonia. Chest 111: 1193–1199. [DOI] [PubMed] [Google Scholar]
- Bhagwat SP, Gigliotti F, Xu H, Wright TW 2006. Contribution of T cell subsets to the pathophysiology of Pneumocystis-related immunorestitution disease. Am J Physiol Lung Cell Mol Physiol 291: L1256–L1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board KF, Patil S, Lebedeva I, Capuano S III, Trichel AM, Murphey-Corb M, Rajakumar PA, Flynn JL, Haidaris CG, Norris KA 2003. Experimental Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. J Infect Dis 187: 576–588. [DOI] [PubMed] [Google Scholar]
- Bruckner L, Gigliotti F, Wright T, Harmsen A, Notter RH, Chess P, Wang Z, Finkelstein J 2006. Pneumocystis carinii infection sensitizes the lung to radiation-induced injury after syngeneic marrow transplantation: Role of CD4+ T-cells. Am J Physiol Lung Cell Mol Physiol 290: L1087–L1096. [DOI] [PubMed] [Google Scholar]
- Burgess JW, Kottom TJ, Limper AH 2008. Pneumocystis carinii exhibits a conserved meiotic control pathway. Infect Immun 76: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess JW, Kottom TJ, Villegas LR, Lamont JD, Baden EM, Ramirez-Alvarado M, Limper AH 2009. The Pneumocystis meiotic PCRan1p kinase exhibits unique temperature-regulated activity. Am J Respir Cell Mol Biol 41: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon EJ, Friaza V, Dapena FJ, de la HC 2010. Pneumocystis jirovecii and cystic fibrosis. Med Mycol 48: S17–S21. [DOI] [PubMed] [Google Scholar]
- Carmona EM, Limper AH 2011. Update on the diagnosis and treatment of Pneumocystis pneumonia. Ther Adv Respir Dis 5: 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona EM, Vassallo R, Vuk-Pavlovic Z, Standing JE, Kottom TJ, Limper AH 2006. Pneumocystis cell wall β-glucans induce dendritic cell costimulatory molecule expression and inflammatory activation through a Fas-Fas ligand mechanism. J Immunol 177: 459–467. [DOI] [PubMed] [Google Scholar]
- Carmona EM, Kottom TJ, Hebrink DM, Moua T, Singh RD, Pagano RE, Limper AH 2012. Glycosphingolipids mediate Pneumocystis cell wall β-glucan activation of the IL-23/IL-17 axis in human dendritic cells. Am J Respir Cell Mol Biol 47: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse OH, Pagni M, Hauser PM 2012. De novo assembly of the Pneumocystis jirovecii genome from a single bronchoalveolar lavage fluid specimen from a patient. mBio 4: e00428–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creery WD, Hashmi A, Hutchison JS, Singh RN 1997. Surfactant therapy improves pulmonary function in infants with Pneumocystis carinii pneumonia and acquired immunodeficiency syndrome. Pediatr Pulmonol 24: 370–373. [DOI] [PubMed] [Google Scholar]
- Cushion MT, Ruffolo JJ, Linke MJ, Walzer PD 1985. Pneumocystis carinii: Growth variables and estimates in the A549 and WI-38 VA13 human cell lines. Exp Parasitol 60: 43–54. [DOI] [PubMed] [Google Scholar]
- Dieterich DT, Lew EA, Bacon DJ, Pearlman KI, Scholes JV 1992. Gastrointestinal pneumocystosis in HIV-infected patients on aerosolized pentamidine: Report of five cases and literature review. Am J Gastroenterol 87: 1763–1770. [PubMed] [Google Scholar]
- Douglas CM 2001. Fungal β(1,3)-d-glucan synthesis. Med Mycol 39: 55–66. [DOI] [PubMed] [Google Scholar]
- Durand-Joly I, Aliouat EM, Recourt C, Guyot K, Francois N, Wauquier M, Camus D, Dei-Cas E 2002. Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J Clin Microbiol 40: 1862–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JC, Kovacs JA, Masur H, Santi DV, Elwood HJ, Sogin ML 1988. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature 334: 519–522. [DOI] [PubMed] [Google Scholar]
- Eijking EP, van Daal GJ, Tenbrinck R, Sluiters JF, Hannappel E, Erdmann W, Lachmann B 1992. Improvement of pulmonary gas exchange after surfactant replacement in rats with Pneumocystis carinii pneumonia. Adv Exp Med Biol 316: 293–298. [DOI] [PubMed] [Google Scholar]
- Escamilla R, Prevost MC, Hermant C, Caratero A, Cariven C, Krempf M 1992. Surfactant analysis during Pneumocystis carinii pneumonia in HIV-infected patients. Chest 101: 1558–1562. [DOI] [PubMed] [Google Scholar]
- Evans SE, Kottom TJ, Pagano RE, Limper AH 2012. Primary alveolar epithelial cell surface membrane microdomain function is required for Pneumocystis β-glucan-induced inflammatory responses. Innate Immun 18: 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F 1992. Host species-specific antigenic variation of a mannosylated surface glycoprotein of Pneumocystis carinii. J Infect Dis 165: 329–336. [DOI] [PubMed] [Google Scholar]
- Gigliotti F, Wright TW 2012. Pneumocystis: Where does it live? PLoS Pathog 8: e1003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F, Stokes DC, Cheatham AB, Davis DS, Hughes WT 1986. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis 154: 315–322. [DOI] [PubMed] [Google Scholar]
- Gigliotti F, Ballou LR, Hughes WT, Mosley BD 1988. Purification and initial characterization of a ferret Pneumocystis carinii surface antigen. J Infect Dis 158: 848–854. [DOI] [PubMed] [Google Scholar]
- Gigliotti F, Harmsen AG, Haidaris CG, Haidaris PJ 1993. Pneumocystis carinii is not universally transmissible between mammalian species. Infect Immun 61: 2886–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F, Harmsen AG, Wright TW 2003. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect Immun 71: 3852–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson MP, Thomas CF Jr, Rusnak F, Limper AH, Leof EB 2001. Differential regulation of growth and checkpoint control mediated by a Cdc25 mitotic phosphatase from Pneumocystis carinii. J Biol Chem 276: 835–843. [DOI] [PubMed] [Google Scholar]
- Guttler R, Singer PA, Axline SG, Greaves TS, McGill JJ 1993. Pneumocystis carinii thyroiditis. Report of three cases and review of the literature. Arch Intern Med 153: 393–396. [DOI] [PubMed] [Google Scholar]
- Hagmann S, Merali S, Sitnitskaya Y, Fefferman N, Pollack H 2001. Pneumocystis carinii infection presenting as an intra-abdominal cystic mass in a child with acquired immunodeficiency syndrome. Clin Infect Dis 33: 1424–1426. [DOI] [PubMed] [Google Scholar]
- Harmsen AG, Stankiewicz M 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med 172: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser PM, Burdet FX, Cisse OH, Keller L, Taffe P, Sanglard D, Pagni M 2010. Comparative genomics suggests that the fungal pathogen pneumocystis is an obligate parasite scavenging amino acids from its host's lungs. Plos ONE 5: e15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendley JO, Weller TH 1971. Activation and transmission in rats of infection with Pneumocystis. Proc Soc Exp Biol Med 137: 1401–1404. [DOI] [PubMed] [Google Scholar]
- Hoffman AG, Lawrence MG, Ognibene FP, Suffredini AF, Lipschik GY, Kovacs JA, Masur H, Shelhamer JH 1992. Reduction of pulmonary surfactant in patients with human immunodeficiency virus infection and Pneumocystis carinii pneumonia. Chest 102: 1730–1736. [DOI] [PubMed] [Google Scholar]
- Hughes WT 1982. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J Infect Dis 145: 842–848. [DOI] [PubMed] [Google Scholar]
- Icenhour CR, Rebholz SL, Collins MS, Cushion MT 2002. Early acquisition of Pneumocystis carinii in neonatal rats as evidenced by PCR and oral swabs. Eukaryot Cell 1: 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenhour CR, Arnold J, Medvedovic M, Cushion MT 2006. Competitive coexistence of two Pneumocystis species. Infect Genet Evol 6: 177–186. [DOI] [PubMed] [Google Scholar]
- Inoue M1, Moriwaki Y, Arikawa T, Chen YH, Oh YJ, Oliver T, Shinohara ML 2011. Cutting edge: Critical role of intracellular osteopontin in antifungal innate immune responses. J Immunol 186: 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan P, Davis E, Jacobson M, Huang L 2009. Life-threatening immune reconstitution inflammatory syndrome after Pneumocystis pneumonia: A cautionary case series. AIDS 23: 1794–1796. [DOI] [PubMed] [Google Scholar]
- Kelly MN, Zheng M, Ruan S, Kolls J, D’Souza A, Shellito JE 2013. Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J Immunol 190: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottom TJ, Limper AH 2013. The Pneumocystis Ace2 transcription factor regulates cell wall-remodeling genes and organism virulence. J Biol Chem 288: 23893–23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottom TJ, Thomas CF Jr, Mubarak KK, Leof EB, Limper AH 2000. Pneumocystis carinii uses a functional cdc13 B-type cyclin complex during its life cycle. Am J Respir Cell Mol Biol 22: 722–731. [DOI] [PubMed] [Google Scholar]
- Kottom TJ, Kohler JR, Thomas CF Jr, Fink GR, Limper AH 2003. Lung epithelial cells and extracellular matrix components induce expression of Pneumocystis carinii STE20, a gene complementing the mating and pseudohyphal growth defects of STE20 mutant yeast. Infect Immun 71: 6463–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottom TJ, Kennedy CC, Limper AH 2008. Pneumocystis PCINT1, a molecule with integrin-like features that mediates organism adhesion to fibronectin. Mol Microbiol 67: 747–761. [DOI] [PubMed] [Google Scholar]
- Kottom TJ, Burgess JW, Limper AH 2011a. Pneumocystis carinii interactions with lung epithelial cells and matrix proteins induce expression and activity of the PcSte20 kinase with subsequent phosphorylation of the downstream cell wall biosynthesis kinase PcCbk1. Infect Immun 79: 4157–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottom TJ, Han J, Zhang Z, Limper AH 2011b. Pneumocystis carinii expresses an active Rtt109 histone acetyltransferase. Am J Respir Cell Mol Biol 44: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JA, Halpern JL, Lundgren B, Swan JC, Parrillo JE, Masur H 1989. Monoclonal antibodies to Pneumocystis carinii: Identification of specific antigens and characterization of antigenic differences between rat and human isolates. J Infect Dis 159: 60–70. [DOI] [PubMed] [Google Scholar]
- Kovacs JA, Powell F, Edman JC, Lundgren B, Martinez A, Drew B, Angus CW 1993. Multiple genes encode the major surface glycoprotein of Pneumocystis carinii. J Biol Chem 268: 6034–6040. [PubMed] [Google Scholar]
- Koval CE, Gigliotti F, Nevins D, Demeter LM 2002. Immune reconstitution syndrome after successful treatment of Pneumocystis carinii pneumonia in a man with human immunodeficiency virus type 1 infection. Clin Infect Dis 35: 491–493. [DOI] [PubMed] [Google Scholar]
- Kutty G, Kovacs JA 2003. A single-copy gene encodes Kex1, a serine endoprotease of Pneumocystis jiroveci. Infect Immun 71: 571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanken PN, Minda M, Pietra GG, Fishman AP 1980. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am J Pathol 99: 561–588. [PMC free article] [PubMed] [Google Scholar]
- Lasbury ME, Durant PJ, Ray CA, Tschang D, Schwendener R, Lee CH 2006. Suppression of alveolar macrophage apoptosis prolongs survival of rats and mice with Pneumocystis pneumonia. J Immunol 176: 6443–6453. [DOI] [PubMed] [Google Scholar]
- Lasbury ME, Merali S, Durant PJ, Tschang D, Ray CA, Lee CH 2007. Polyamine-mediated apoptosis of alveolar macrophages during Pneumocystis pneumonia. J Biol Chem 282: 11009–11020. [DOI] [PubMed] [Google Scholar]
- Lebron F, Vassallo R, Puri V, Limper AH 2003. Pneumocystis carinii cell wall β-glucans initiate macrophage inflammatory responses through NF-κB activation. J Biol Chem 278: 25001–25008. [DOI] [PubMed] [Google Scholar]
- Lee LH, Gigliotti F, Wright TW, Simpson-Haidaris PJ, Weinberg GA, Haidaris CG 2000. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene 242: 141–150. [DOI] [PubMed] [Google Scholar]
- Limper AH, Martin WJ 1990. Pneumocystis carinii: Inhibition of lung cell growth mediated by parasite attachment. J Clin Invest 85: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limper AH, Offord KP, Smith TF, Martin WJ 1989. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis 140: 1204–1209. [DOI] [PubMed] [Google Scholar]
- Limper AH, Standing JE, Hoffman OA, Castro M, Neese LW 1993. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect Immun 61: 4302–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limper AH, Thomas CF Jr, Anders RA, Leof EB 1997. Interactions of parasite and host epithelial cell cycle regulation during Pneumocystis carinii pneumonia. J Lab Clin Med 130: 132–138. [DOI] [PubMed] [Google Scholar]
- Limper AH, Edens M, Anders RA, Leof EB 1998. Pneumocystis carinii inhibits cyclin-dependent kinase activity in lung epithelial cells. J Clin Invest 101: 1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke MJ, Cushion MT, Walzer PD 1989. Properties of the major antigens of rat and human Pneumocystis carinii. Infect Immun 57: 1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke MJ, Ashbaugh A, Collins MS, Lynch K, Cushion MT 2013. Characterization of a distinct host response profile to Pneumocystis murina asci during clearance of Pneumocystis pneumonia. Infect Immun 81: 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EG, Smith JS, Meier JL 1986. Attachment of Pneumocystis carinii to rat pneumocytes. Lab Invest 54: 609–615. [PubMed] [Google Scholar]
- Lo Presti L, Cockell M, Cerutti L, Simanis V, Hauser PM 2007. Functional characterization of Pneumocystis carinii brl1 by transspecies complementation analysis. Eukaryot Cell 6: 2448–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli EB, Allen AG, Wakefield AE 1997. A Pneumocystis carinii multi-gene family with homology to subtilisin-like serine proteases. Microbiology 143: 2223–2236. [DOI] [PubMed] [Google Scholar]
- Lugli EB, Bampton ET, Ferguson DJ, Wakefield AE 1999. Cell surface protease PRT1 identified in the fungal pathogen Pneumocystis carinii. Mol Microbiol 31: 1723–1733. [DOI] [PubMed] [Google Scholar]
- Lundgren B, Koch C, Mathiesen L, Nielsen JO, Hansen JE 1993. Glycosylation of the major human Pneumocystis carinii surface antigen. APMIS 101: 194–200. [PubMed] [Google Scholar]
- Ma L, Huang DW, Cuomo CA, Sykes S, Fantoni G, Das B, Sherman BT, Yang J, Huber C, Xia Y, et al. 2013. Sequencing and characterization of the complete mitochondrial genomes of three Pneumocystis species provide new insights into divergence between human and rodent Pneumocystis. FASEB J 27: 1962–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner NN, Lund FE, Han S, Harmsen A 2005. CD8 T cell-mediated lung damage in response to the extracellular pathogen Pneumocystis is dependent on MHC class I expression by radiation-resistant lung cells. J Immunol 175: 8271–8279. [DOI] [PubMed] [Google Scholar]
- Merali S, Frevert U, Williams JH, Chin K, Bryan R, Clarkson AB Jr 1999. Continuous axenic cultivation of Pneumocystis carinii. Proc Natl Acad Sci 96: 2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales IJ, Vohra PK, Puri V, Kottom TJ, Limper AH, Thomas CF Jr 2003. Characterization of a lanosterol 14 α-demethylase from Pneumocystis carinii. Am J Respir Cell Mol Biol 29: 232–238. [DOI] [PubMed] [Google Scholar]
- Morris A, Norris KA 2012. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev 25: 297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA 2004. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med 170: 408–413. [DOI] [PubMed] [Google Scholar]
- Murphy MJ, Pifer LL, Hughes WT 1977. Pneumocystis carinii in vitro: A study by scanning electron microscopy. Am J Pathol 86: 387–401. [PMC free article] [PubMed] [Google Scholar]
- O’Riordan DM, Standing JE, Limper AH 1995. Pneumocystis carinii glycoprotein A binds macrophage mannose receptors. Infect Immun 63: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panos GZ, Karydis I, Velakoulis SE, Falagas ME 2007. Multi-skeletal Pneumocystis jiroveci (carinii) in an HIV-seropositive patient. Int J STD AIDS 18: 134–137. [DOI] [PubMed] [Google Scholar]
- Powles MA, Liberator P, Anderson J, Karkhanis Y, Dropinski JF, Bouffard FA, Balkovec JM, Fujioka H, Aikawa M, McFadden D, et al. 1998. Efficacy of MK-991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob Agents Chemother 42: 1985–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procop GW, Haddad S, Quinn J, Wilson ML, Henshaw NG, Reller LB, Artymyshyn RL, Katanik MT, Weinstein MP 2004. Detection of Pneumocystis jiroveci in respiratory specimens by four staining methods. J Clin Microbiol 42: 3333–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupaibool J, Kottom TJ, Bouchonville K, Limper AH 2013. Characterization of the Pneumocystis carinii histone acetyltransferase chaperone proteins PcAsf1 and PcVps75. Infect Immun 81: 2268–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roths JB, Sidman CL 1992. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J Clin Invest 90: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggli GM, Weber R, Messmer EP, Font RL, Moll C, Bernauer W 1997. Pneumocystis carinii infection of the conjunctiva in a patient with acquired immune deficiency syndrome. Ophthalmology 104: 1853–1856. [DOI] [PubMed] [Google Scholar]
- Sassi M, Ripamonti C, Mueller NJ, Yazaki H, Kutty G, Ma L, Huber C, Gogineni E, Oka S, Goto N, et al. 2012. Outbreaks of Pneumocystis pneumonia in 2 renal transplant centers linked to a single strain of Pneumocystis: Implications for transmission and virulence. Clin Infect Dis 54: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz DM, Romancheck MA, Pittarelli LA, Schwartz RE, Fromtling RA, Nollstadt KH, Vanmiddlesworth FL, Wilson KE, Turner MJ 1990. Treatment of Pneumocystis carinii pneumonia with 1,3-β-glucan synthesis inhibitors. Proc Natl Acad Sci 87: 5950–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Markart P, Ruppert C, Temmesfeld B, Nass R, Lohmeyer J, Seeger W, Gunther A 2006. Pulmonary surfactant in patients with Pneumocystis pneumonia and acquired immunodeficiency syndrome. Crit Care Med 34: 2370–2376. [DOI] [PubMed] [Google Scholar]
- Sepkowitz KA 1992. Pneumocystis carinii pneumonia among patients with neoplastic disease. Semin Respir Infect 7: 114–121. [PubMed] [Google Scholar]
- Sepkowitz KA 1993. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis 17: S416–S422. [DOI] [PubMed] [Google Scholar]
- Sheehan PM, Stokes DC, Yeh YY, Hughes WT 1986. Surfactant phospholipids and lavage phospholipase A2 in experimental Pneumocystis carinii pneumonia. Am Rev Respir Dis 134: 526–531. [DOI] [PubMed] [Google Scholar]
- Shellito J, Suzara VV, Blumenfeld W, Beck JM, Steger HJ, Ermak TH 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest 85: 1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley TW, Kling HM, Morris A, Patil S, Kristoff J, Guyach SE, Murphy JE, Shao X, Sciurba FC, Rogers RM, et al. 2010. Persistent Pneumocystis colonization leads to the development of chronic obstructive pulmonary disease in a nonhuman primate model of AIDS. J Infect Dis 202: 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JR 2002. Pneumocystis. Int J Med Microbiol 292: 391–404. [DOI] [PubMed] [Google Scholar]
- Stringer JR, Cushion MT 1998. The genome of Pneumocystis carinii. FEMS Immunol Med Microbiol 22: 15–26. [DOI] [PubMed] [Google Scholar]
- Stringer JR, Keely SP 2001. Genetics of surface antigen expression in Pneumocystis carinii. Infect Immun 69: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JR, Beard CB, Miller RF, Wakefield AE 2002. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg Infect Dis 8: 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG 2004. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect Immun 72: 5722–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SD, Meissner NN, Harmsen AG 2006. CD8 T cells modulate CD4 T-cell and eosinophil-mediated pulmonary pathology in Pneumocystis pneumonia in B-cell-deficient mice. Am J Pathol 168: 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SD, Han S, Harmsen A, Shampeny K, Harmsen AG 2007. Pulmonary hypertension can be a sequela of prior Pneumocystis pneumonia. Am J Pathol 171: 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SD, Meissner N, Han S, Harmsen A 2011. Pneumocystis infection in an immunocompetent host can promote collateral sensitization to respiratory antigens. Infect Immun 79: 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D, Wilkison M, Voyich J, Meissner N 2011. Prevention of bone marrow cell apoptosis and regulation of hematopoiesis by type I IFNs during systemic responses to Pneumocystis lung infection. J Immunol 186: 5956–5967. [DOI] [PubMed] [Google Scholar]
- Thomas CF Jr, Limper AH 2004. Pneumocystis pneumonia. N Engl J Med 350: 2487–2498. [DOI] [PubMed] [Google Scholar]
- Thomas CF Jr, Limper AH 2007. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol 5: 298–308. [DOI] [PubMed] [Google Scholar]
- Thomas CF, Anders RA, Gustafson MP, Leof EB, Limper AH 1998. Pneumocystis carinii contains a functional cell-division-cycle Cdc2 homologue. Am J Respir Cell Mol Biol 18: 297–306. [DOI] [PubMed] [Google Scholar]
- Thomas CF Jr, Leof EB, Limper AH 1999. Analysis of Pneumocystis carinii introns. Infect Immun 67: 6157–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer G, Brug SL 1942. Infection par Pneumocystis chez l’homme et chez les animaux. Ann Soc Belge Med Trop 22: 301–309. [Google Scholar]
- Vanek J, Jirovek O 1952. Parasitic pneumonia. Interstitial plasma cell pneumonia of premature, caused by Pneumocystis carinii. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg 158: 120–127. [PubMed] [Google Scholar]
- Vargas SL, Hughes WT, Santolaya ME, Ulloa AV, Ponce CA, Cabrera CE, Cumsille F, Gigliotti F 2001. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis 32: 855–861. [DOI] [PubMed] [Google Scholar]
- Vassallo R, Standing JE, Limper AH 1999a. β-glucan from Pneumocystis carinii stimulates TNF α release from alveolar macrophages. J Eukaryot Microbiol 46: 145S. [PubMed] [Google Scholar]
- Vassallo R, Thomas CF Jr, Vuk-Pavlovic Z, Limper AH 1999b. Alveolar macrophage interactions with Pneumocystis carinii. J Lab Clin Med 133: 535–540. [DOI] [PubMed] [Google Scholar]
- Vassallo R, Standing JE, Limper AH 2000. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J Immunol 164: 3755–3763. [DOI] [PubMed] [Google Scholar]
- Vuk-Pavlovic Z, Standing JE, Crouch EC, Limper AH 2001. Carbohydrate recognition domain of surfactant protein D mediates interactions with Pneumocystis carinii glycoprotein A. Am J Respir Cell Mol Biol 24: 475–484. [DOI] [PubMed] [Google Scholar]
- Walzer PD 1986. Attachment of microbes to host cells: Relevance of Pneumocystis carinii. Lab Invest 54: 589–592. [PubMed] [Google Scholar]
- Wang Z, Foye A, Chang Y, Chess PR, Wright TW, Bhagwat S, Gigliotti F, Notter RH 2005. Inhibition of surfactant activity by Pneumocystis carinii organisms and components in vitro. Am J Physiol Lung Cell Mol Physiol 288: L1124–L1131. [DOI] [PubMed] [Google Scholar]
- Wang J, Gigliotti F, Bhagwat SP, George TC, Wright TW 2010. Immune modulation with sulfasalazine attenuates immunopathogenesis but enhances macrophage-mediated fungal clearance during Pneumocystis pneumonia. PLoS Pathog 6: e1001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wright TW, Gigliotti F 2011. Immune modulation as adjunctive therapy for Pneumocystis pneumonia. Interdiscip Perspect Infect Dis 2011: 918038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TW, Johnston CJ, Harmsen AG, Finkelstein JN 1997. Analysis of cytokine mRNA profiles in the lungs of Pneumocystis carinii-infected mice. Am J Respir Cell Mol Biol 17: 491–500. [DOI] [PubMed] [Google Scholar]
- Wright TW, Gigliotti F, Finkelstein JN, McBride JT, An CL, Harmsen AG 1999. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest 104: 1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TW, Notter RH, Wang Z, Harmsen AG, Gigliotti F 2001. Pulmonary inflammation disrupts surfactant function during Pneumocystis carinii pneumonia. Infect Immun 69: 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TW, Pryhuber GS, Chess PR, Wang Z, Notter RH, Gigliotti F 2004. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J Immunol 172: 2511–2521. [DOI] [PubMed] [Google Scholar]
- Wyder MA, Rasch EM, Kaneshiro ES 1994. Assessment of Pneumocystis carinii DNA content. J Eukaryot Microbiol 41: 120S. [PubMed] [Google Scholar]
- Yoneda K, Walzer PD 1980. Interaction of Pneumocystis carinii with host lungs: An ultrastructural study. Infect Immun 29: 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, De LA, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 37: 2695–2706. [DOI] [PubMed] [Google Scholar]