Abstract
The epidermis of the skin is a highly polarized, metabolic tissue with important innate immune functions. The polarity of the epidermis is, for example, reflected in controlled changes in cell shape that accompany differentiation, oriented cell division, and the planar orientation of hair follicles and cilia. The establishment and maintenance of polarity is organized by a diverse set of polarity proteins that include transmembrane adhesion proteins, cytoskeletal scaffold proteins, and kinases. Although polarity proteins have been extensively studied in cell culture and in vivo in simple epithelia of lower organisms, their role in mammalian tissue biology is only slowly evolving. This article will address the importance of polarizing processes and their molecular regulators in epidermal morphogenesis and homeostasis and discuss how alterations in polarity may contribute to skin disease.
Cell polarity in the skin is organized by diverse proteins and pathways. Some (e.g., LKB1) are also involved in metabolism, growth control, or innate immunity, and thus may serve as central regulators of skin homeostasis.
Polarity is a fundamental property of cells and tissues that results from the differential distribution of cellular components (proteins, lipids, RNA, organelles) to promote asymmetry in form and/or function. This is important in a range of physiologically relevant processes such as oriented cell division, directed migration, barrier function, and recognition and adhesion of cells. In general, polarity can be achieved at the cellular level, known as cell polarity, or at the tissue level, known as tissue polarity or planar cell polarity. Perhaps the best-characterized example for cell polarity is epithelial polarity, in which simple epithelia such as the intestine establish two different membrane domains, the apical and basolateral domain (Roignot et al. 2013). This apicobasolateral polarity is important for barrier function, vectorial transport, and sensory and signal perception. In tissue polarity, cells or structures within cells orient in the plane of the tissue. This coordination of cell polarity in a tissue is crucial for proper tissue formation and function and regulates, for example, intercalation/convergence extension movements essential to shape the different body axes during development, the positioning of motile and sensory cilia as well as the polarization of the developing epidermis and hair follicles (Wang et al. 2006; Devenport et al. 2011; Wallingford 2012).
The most outer layer of the skin, the epidermis, is a multilayered stratifying epithelium and does not display the characteristic features of simple epithelial apicobasolateral polarity. The epidermis consists of the interfollicular epidermis (IFE) and epidermal appendages: hair follicles, sebaceous glands, and sweat glands. The continuous self-renewal of this tissue is driven by the existence of different stem and progenitor populations located in the basal layer of the IFE and in different locations in the hair follicle (Blanpain and Fuchs 2009; Watt and Jensen 2009). After exiting the cell cycle, basal keratinocytes undergo a terminal differentiation program to either form the stratum corneum, a dead, cornified, and water impermeable cell layer (Candi et al. 2005; Koster 2009), or another keratinized structure, the hair.
Many features within the epidermis are polarized (Fig. 1A) and, more importantly, this polarization is crucial for the formation and maintenance of the IFE and its appendages. For example, during stratification keratinocytes differentiate and undergo controlled cell shape changes until they reach the stratum corneum. This process requires intercellular rearrangements to allow cells to migrate through the layers. Another example is oriented cell division of basal cells in the IFE and in hair follicles. By orienting the mitotic spindle either parallel or perpendicular with respect to the underlying basement or hair follicle axis, stem and progenitor cells can control cell fate and differentiation while guaranteeing renewal. Wound closure is a highly polarized process that requires the coordinated secretion and deposition of the extracellular matrix to allow for directional migration of keratinocytes (Fig. 1B). Cilia are positioned in a polarized manner on keratinocytes and this is likely important for proper signal transduction. Not only individual cells or subcellular structures are highly polarized but the orientation of multicellular structures, such as sebaceous glands and hair follicles, are organized in the plane of the tissue. All of these processes depend on cell and tissue polarity and work in recent years has started to unravel how polarity genes contribute to these processes in the epidermis. In this article we will focus mostly on the role of cell polarity in the epidermis.
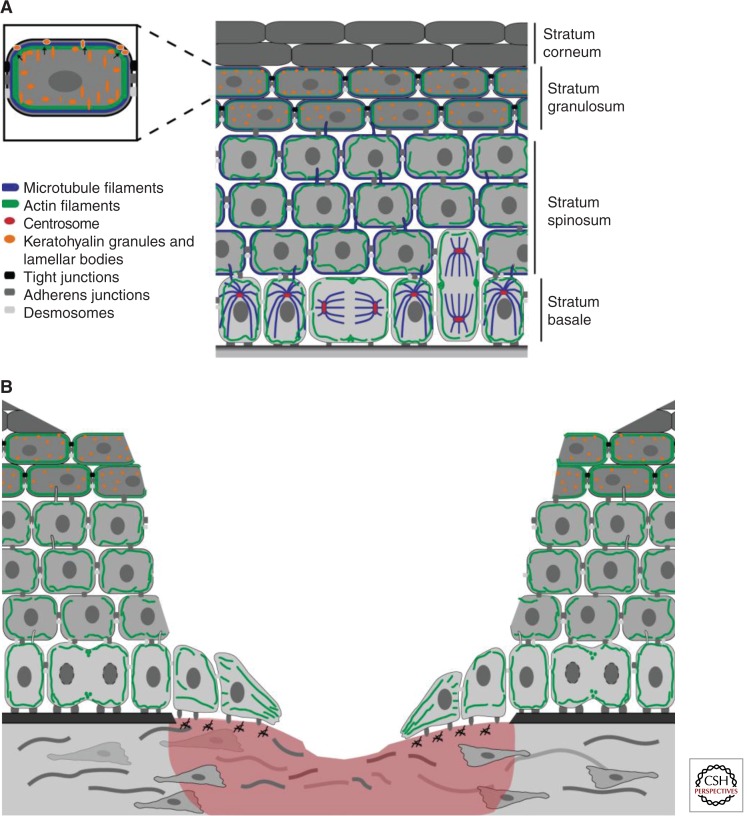
Figure 1.
The mammalian epidermis is a polarized stratified epithelium. (A) The interfollicular epidermis develops apicobasolateral polarity across the different layers. The last viable layer, the stratum granulosum, is forming the apical border. Polarization is reflected in the differential localization of integrin-based cell–matrix junctions and the cell–cell junctions desmosomes, adherens, and tight junctions (all in gray shades). Both the microtubule (blue) and actin (green) cytoskeleton show polarized distribution throughout the different layers. Microtubule-based cilia are positioned at the apical side of keratinocytes (blue protrusions) near the centrosome (red). (B) Wound healing requires the coordination of several polarized processes. After wounding, basal cells migrate in a directional manner into the wound bed on a provisional matrix that is secreted in a polarized manner by keratinocytes and fibroblasts. This migration required rearrangements of the actin cytoskeleton and formation of new contacts with the extracellular matrix (ECM).
POLARITY PROTEIN SIGNALING NETWORKS
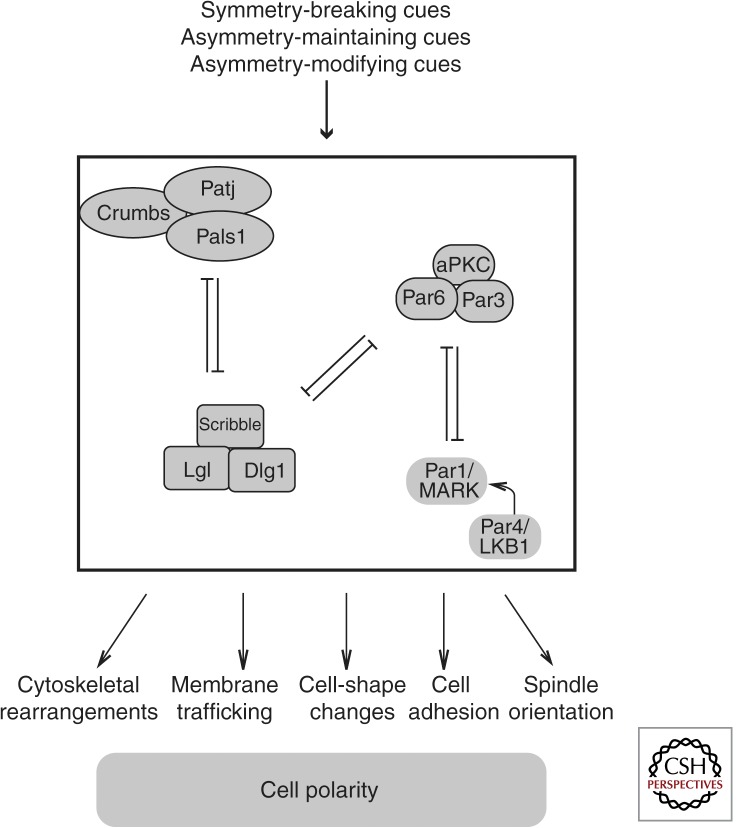
A highly conserved set of proteins, the so-called polarity proteins, orchestrates the setup, maintenance, and reorganization of polarity. These polarity proteins integrate upstream signals of various kinds to instruct regulators of the cytoskeleton to control, for example, polarized membrane trafficking, adhesive interactions, and signal complex localization (Fig. 2). In this article, we will focus mostly on the role of cell polarity proteins in the epidermis.
Figure 2.
Schematic overview of the interactions between the main cell polarity proteins and how they establish, maintain, and modify polarity.
Three main cell polarity complexes have been described that are implicated in different aspects of establishing and maintaining asymmetry: the Scribble/Disc large (Dlg)/Lethal Giant Larvae (Lgl) complex, the atypical PKC (aPKC)/Par3/Par6 complex, and the Crumbs/Patj/Pals complex (Fig. 2). In addition, Par4, known as Lkb1 in mammals, and Par5 (14-3-3 proteins in mammals) engage with these complexes to regulate polarity. Several excellent reviews describe the structure and cell biological role of these proteins, which include adhesion, scaffold, kinase, and regulatory functions (Goldstein and Macara 2007; Hurov and Piwnica-Worms 2007; Assemat et al. 2008). Initially predominantly identified in Caenorhabditis elegans and Drosophila (Bulgakova and Knust 2009; St Johnston and Ahringer 2010), it is now clear that the mammalian counterparts of these proteins play similar essential roles in morphogenesis and tissue homeostasis.
BARRIER FORMATION AND FUNCTION IN THE EPIDERMIS
A crucial function of the epidermis is the establishment and maintenance of a lifelong self-renewing barrier that does not only provide protection against water loss and mechanical insults, but also guards against UV-light, pathogens, and temperature changes. Keratinocytes must undergo a spatiotemporal highly controlled differentiation program to establish and maintain this barrier. Disturbance in this program resulting in an impaired barrier function has been implicated in a range of diseases (e.g., atopic dermatitis, psoriasis, and ichthyosis) (De Benedetto et al. 2012; Kubo et al. 2012) and can contribute to a cancer permissive microenvironment (Demehri et al. 2009).
Although the epidermis does not establish apicobasolateral polarity as observed in simple epithelia, many processes in the stratifying IFE barrier resemble features of polarized simple epithelial cells (Fig. 1A): (1) Basal cells have a highly polarized appearance with asymmetric distribution of integrin cell matrix receptors and polarity proteins as well as a polarized positioning of the nucleus, mitochondria, and the apically localized centrosome. (2) Both the actin and microtubule cytoskeleton show a polarized distribution in the epidermis. In basal cells, microtubules are organized in a radial array around the centrosome but translocate to the cortex in suprabasal layers (Lechler and Fuchs 2007). Actin is most strongly organized at the cortex in the most upper (“apical”) viable layer of the epidermis, the stratum granulosum. (3) Barrier-forming tight junctions (TJs) are only present in the stratum granulosum. These junctions are essential for the epidermal water barrier (Furuse et al. 2002; Tunggal et al. 2005) and regulate immune responses as Langerhans cells use the TJ pore to take up external antigens (Kubo et al. 2009). (4) Formation of the stratum corneum depends on the fusion of lamellar bodies (LBs) and keratohyalin granules with plasma membranes at the transition between the stratum granulosum and corneum layers (Lippens et al. 2009).
The stratum granulosum might thus be considered the viable apical boundary that contains TJs. As in simple epithelia these may serve as a landmark to separate the basolateral layers from the most apical layer and therefore control polarized targeting of LBs and keratohyalin granules toward the more apically localized stratum corneum.
The mechanisms that coordinate the polarization of the epidermis across the tissue are mostly unknown. As in simple epithelia, important cues are provided by adhesion and signaling from cell–matrix and cell–cell junctions. Loss of β1-integrin cell–matrix adhesion receptors or the integrin-linked kinase (ILK) interferes with proper polarization of basal cells and disturbs epidermal differentiation (Brakebusch et al. 2000; Raghavan et al. 2000; Lorenz et al. 2007). Epidermal loss of the AJ proteins E-cadherin or β-catenin results in a leaky TJ epidermal barrier (Tunggal et al. 2005; Ray et al. 2013). β-Catenin serves as a mechanosensor necessary to strengthen adhesion and TJs, likely by increasing their interaction with the actin cytoskeleton.
The desmosomal protein desmoplakin I (DPI) coordinates the organization of cortical microtubules in suprabasal layers by recruiting a subset of centrosomal proteins, such as Lis1, a protein implicated in the organization of microtubules. Epidermal loss of Lis1 did not only result in a lack of cortical microtubule recruitment to desmosomes in the suprabasal layers but, surprisingly, impaired desmosomal stability (Sumigray et al. 2011). The cortical localization of microtubules is also necessary to recruit myosin II that strengthens AJs, which in turn promotes TJs epidermal barrier function (Sumigray et al. 2012). Vice versa, AJs are necessary for desmosome assembly in the epidermis (Michels et al. 2009). Together these results indicate that the coordinated formation of junctions and their association with the different cytoskeletal networks are crucial for proper epidermal differentiation and barrier formation.
Polarity protein signaling may orchestrate the interplay between epidermal junctions and the cytoskeleton. Loss of or interference with Par3 or aPKCs alter the microtubule and actin cytoskeleton, impair AJs, and inhibit TJ function (Helfrich et al. 2007; Iden et al. 2012). Similarly the small GTPase Rac regulates keratinocyte TJ barrier function through the aPKC/Par3 complex (Mertens et al. 2005). Cell adhesion itself may positively enforce polarity signaling as loss of E-cadherin or CD44 alters aPKC activity and localization associated with reduced TJs function (Tunggal et al. 2005; Kirschner et al. 2011). Given their important role in several key aspects of epidermal barrier function, assessing the contribution of polarity proteins to human skin barrier diseases will be an important avenue for the future.
PRIMARY CILIA: COORDINATION OF POLARITY AND GROWTH FACTOR SIGNALING?
Primary cilia are small microtubule-based cylindrical membrane organelles that project into the extracellular space. Through enrichment of receptors, for example, Wnt, Hedgehog (HH), and Notch, cilia function as signal centers in sensation, signal reception, and mechanical cues (Goetz and Anderson 2010). Cilia dysfunction is associated with a range of (developmental) disease syndromes, generally referred to as ciliopathies (Hildebrandt et al. 2011). Recently, a direct role for cilia dysfunction in human skin disease was suggested as mutations in core cilia proteins were found in rare cranioectodermal dysplasia syndromes (Ruiz-Perez and Goodship 2009; Walczak-Sztulpa et al. 2010). Moreover, the Birt–Hogg–Dubé syndrome, which among others is associated with an increased risk of skin cancer, was recently linked to alterations in ciliogenesis (Luijten et al. 2013).
Within the skin, primary cilia are found on most dermal and epidermal cell populations. By sensing Hedgehog signals, primary cilia on dermal cells are required for hair follicle morphogenesis (Lehman et al. 2009). Epidermal inactivation of cilia components has revealed different roles for cilia in the developing epidermis. On interfollicular keratinocytes cilia control Notch signaling to balance IFE proliferation and differentiation during morphogenesis. At a later stage, epidermal cilia are necessary for the transduction of HH signals to promote hair follicle morphogenesis (Ezratty et al. 2011). Cilia also control adult epidermal homeostasis perhaps by balancing HH, which promotes hair follicle identity, versus p63 signaling, which stimulates IFE fate (Croyle et al. 2011).
Epidermal-derived polarity cues provided by the extracellular matrix are important for the formation of dermal cilia. Epidermal loss of the extracellular matrix protein laminin-511 resulted in shortened and structurally altered cilia on dermal papilla cells and a block in hair follicle formation, likely as a result of altered HH signaling (Gao et al. 2008). Disturbed cell-matrix (loss of β1-integrin) or cell–cell (loss of α-catenin) adhesion also impairs cilia formation (Ezratty et al. 2011), although the mechanism is unclear. As both of these adhesive junctions interact with actin, they may regulate ciliogenesis through the actin regulatory protein “missing in metastasis” (MIM). MIM controls HH signaling and dermal cilia formation through regulation of the actin cytoskeleton (Bershteyn et al. 2010).
Recent evidence indicates that cilia formation in simple epithelia requires the activity of cell polarity proteins. In these cells, the Par/aPKC complex can localize to cilia through interaction with Crb3 and down-regulation of Par3 or Crb3 interfered with cilia formation (Fan et al. 2004; Schermer et al. 2006; Sfakianos et al. 2007). The role of cell polarity proteins in the regulation of skin cilia is less clear. In different skin cells MIM forms a complex with aPKC-λ/Par3 at the basal body to regulate HH signaling (Atwood et al. 2013). However, loss of aPKC-λ only interfered with cilia formation in transformed (Atwood et al. 2013) but not in primary keratinocytes (own unpublished observations). These results suggest a cell context-dependent function for aPKC-λ polarity signaling in cilia formation.
POLARITY AND THE REGULATION OF EPIDERMAL CELL FATE
The epidermis is derived from a single layer of ectoderm that ultimately gives rise to the formation of different populations of keratinocytes that constitute the interfollicular epidermis, hair follicles, sebaceous-, sweat-, and mammary glands (Watt 2001; Fuchs 2007; Doucet et al. 2013). This implies that during morphogenesis these differential fates have to be specified. Lineage tracing analysis revealed that in the adult epidermis different populations of stem cell/progenitor cells exist that replenish the different lineages on turnover (Van Keymeulen and Blanpain 2012). In adult skin, mechanisms must thus exist by which epidermal progenitors self-renew while also generating the appropriate differentiated cell types. From bacteria to mammals oriented cell divisions are used to produce daughter cells with similar or differential cell fate and/or to partition more damaged or older versus newer components in one of the daughter cells (Inaba and Yamashita 2012; Li 2013). Symmetric cell divisions (SCD) generate two daughter cells of the same fate, whereas asymmetric cell divisions (ACD) generate two daughters with differential fate. During development or regeneration oriented division can also be coupled to the expansion and/or elongation of the embryo or tissue along a specific axis.
Polarity proteins are key regulators of oriented cell divisions. Although the detailed mechanisms vary within different systems (Knoblich 2008; Lu and Johnston 2013), polarity proteins establish a polarity axis at the cortex that is essential to couple the distribution of cell fate determinants to the orientation of the spindle (intrinsic mechanism). ACD can also result in differential positioning of the two daughters in the tissue, thereby exposing these cells to different niche signals that promote or inhibit differentiation and/or cell specification (extrinsic mechanism). Although these two mechanisms are not necessarily exclusive, thus far an ACD-mediated differential separation of cell fate determinants has not been shown in the epidermis. Evidence does exist for extrinsic regulation of differential epidermal fate (e.g., through Insulin/IGF-1, Notch, or adhesive signals) (Lechler and Fuchs 2005; Williams et al. 2011; Günschmann et al. 2013).
Within the epidermis symmetric divisions are defined as parallel to the basement membrane or the long axis of the hair follicle, whereas ACDs are defined as perpendicular to these axes (Fig. 3A). A shift in the balance of SCD toward ACD drives the formation of a multilayered stratified interfollicular epidermis (Poulson and Lechler 2012). Asymmetric divisions also occur during the specification of sebaceous gland cell identity (Frances and Niemann 2012) and in the junctional zone (Niessen et al. 2013). In the hair follicle bulb, asymmetric and symmetric divisions may regulate the appropriate differentiation of the different hair follicle layers (Fig. 3A). Although ACDs do occur in the bulge (Niessen et al. 2013), lineage tracing analysis and life cell imaging indicates that bulge stem cells mostly rely on SCD for self-renewal (Zhang et al. 2009; Petersson et al. 2011), whereas on entry into anagen, the growth phase of the hair cycle, asymmetric divisions have been observed in the secondary hair germ (Rompolas et al. 2012), where activated progenitors reside (Fig. 3A), and in the proliferative zone of the outer root sheath (Rompolas et al. 2013).
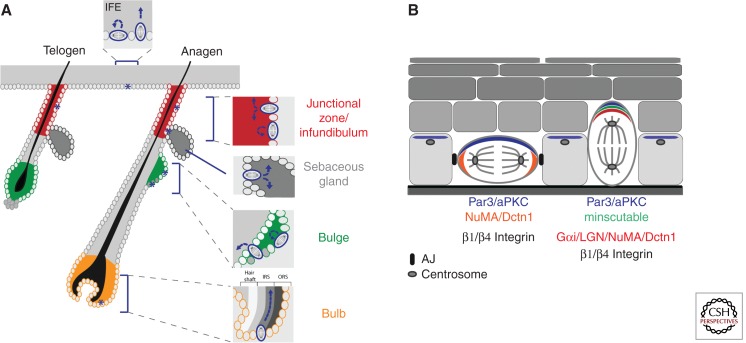
Figure 3.
Oriented cell divisions in the epidermis. (A) Schematic overview of asymmetric (ACD) and symmetric (SCD) cell divisions in interfollicular epidermis (IFE) and the hair follicle (HF). ACDs in the basal IFE keratinocytes give rise to one cell that remains in the basal layer and one differentiated cell. In contrast, symmetric cell divisions give rise to two basal cells. Both ACD and SCD were observed in the junctional zone (JZ, red) of telogen and anagen hair follicles. These divisions likely contribute to the renewal of these progenitors and also fuel the sebaceous gland and the IFE. SCD ensure the self-renewal of the HF stem cells in the bulge (green). In early anagen HFs, ACDs were observed at the border of the bulge and secondary hair germ (dark gray cells), where they may contribute to the expanding lower hair follicle population. ACDs have also been observed in the hair bulb, where they may drive the differentiation of outer root sheath cells (ORS) into the differentiated hair follicle layers: inner root sheath layers (IRS) and hair shaft layers. ACDs likely contribute to the formation of the sebaceous gland (SG) during epidermal morphogenesis. (B) Schematic overview of the polarized distribution of polarity proteins and spindle orientation machinery in symmetric and asymmetric dividing basal keratinocytes.
First evidence that implicated polarity proteins in the regulation of epidermal-oriented cell division came from a seminal study by Lechler and Fuchs (2005), in which they showed that the onset of stratification coincided with a shift from symmetric toward asymmetric division in basal cells of the developing epidermis. Both Par3 and aPKC were distributed asymmetrically at the apical pole of basal cells. In mitosis, these proteins colocalized with Inscutable (Insc) and the spindle orientation complex consisting of Gai, LGN, NuMA, and dynactin (Dctn) (Lechler and Fuchs 2005; Williams et al. 2011). In Drosophila neuroblasts Insc couples polarity to spindle orientation by binding both Par3 and Partner of Inscutable, Pins, the Drosophila homolog of LGN (Knoblich 2008). A similar Par3-Insc-LGN complex was found in the developing epidermis (Lechler and Fuchs 2005), indicating that the aPKC/Par3 complex may also interact with the spindle orientation complex in the epidermis to drive asymmetric division (Fig. 3B).
Adhesive cues within the epidermis are likely crucial as loss of either β1-integrin-mediated adhesion or the adherens junction protein α-catenin resulted in a loss of apical localization of aPKC, LGN, and NuMA (Lechler and Fuchs 2005), coinciding with disturbed differentiation and hyperproliferation. On in vivo knockdown of LGN, Numa1, or Dctn1 the spindle is biased toward SCD, resulting in impaired stratification and epidermal barrier formation (Williams et al. 2011). Similarly, in vivo overexpression of Inscutable is initially sufficient to promote ACDs, but the SCD/ACD ratio in these mice is restored later in development, suggesting the existence of a compensatory mechanism (Poulson and Lechler 2010).
A recent study implicated a direct role for polarity protein signaling in the regulation of epidermal cell fate and oriented division. Epidermal inactivation of aPKC-λ, the predominant aPKC isoform expressed in the epidermis, resulted in a gradual loss of hair follicle bulge stem cells accompanied by a temporary increase in more committed progenitors located in the isthmus/junctional zone, the IFE, and lower HF. Loss of aPKC-λ induced a shift toward more ACDs in the IFE, bulge, and the junctional zone/isthmus region. Most importantly, lineage tracing of lower hair bulge and hair germ stem cells showed that, on loss of aPKC-λ, these cells no longer exclusively contributed to the lower hair follicle but also repopulated the upper junctional zone, the IFE, and on occasion even the sebaceous glands (Niessen et al. 2013). Thus, aPKC-λ regulates epidermal homeostasis and cell fate likely by balancing SCD and ACD (Fig. 4A). How aPKC-λ regulates this balance is not clear. Although aPKC is apically localized in both asymmetrically dividing Drosophila neuroblasts and basal keratinocytes, the fate of the future daughter cells that inherit this domain is opposite: inheritance of stem cell properties in case of the neuroblast, whereas the future apical epidermal daughter will differentiate. This is similar to the situation in Drosophila intestinal stem cells, where the aPKC/Par3 domain marks the future differentiated daughter. Interestingly, in these cells, integrin-mediated adhesion was essential for asymmetric distribution and differentiation similar to the developing IFE. Moreover, increased aPKC activity enhanced Notch/Delta signaling to promote differentiation (Goulas et al. 2012). Although the latter is in contrast to the finding that loss of aPKC-λ promotes ACD in the epidermis, overall these findings show a strong parallel to the epidermis where loss of integrins results in loss of oriented cell division and Notch/Delta signaling is downstream from ACD (Williams et al. 2011). Thus, unlike neuroblasts, oriented division in the Drosophila intestine and in the epidermis appears to be regulated by both intrinsic and extrinsic signals.
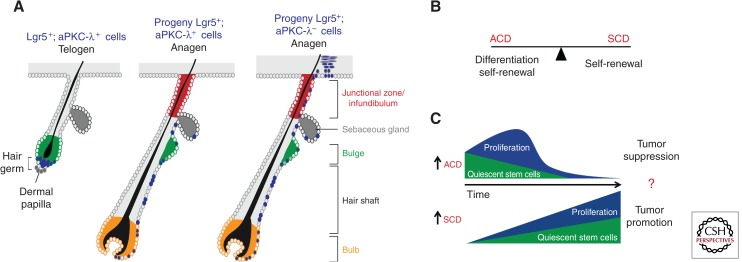
Figure 4.
The balance between ACD and SCD regulate epidermal homeostasis. (A) A schematic overview of the regulation of cell fate by aPKC-λ. Lineage tracing analysis using inducible Lgr5-CreERT2:Rosa26LacZ mice. In telogen follicles Lgr5-positive cells (blue) reside in the lower bulge (green) and hair germ. During anagen, the Lgr5+ progeny exclusively contributes to the down-growing hair follicles. Lgr5+; aPKC− progeny contribute not only to the lower newly forming hair follicle but also to the upper junctional zone (red) and the interfollicular epidermis, showing that aPKC-λ and thus polarity signaling determines cell fate in the epidermal lineage. (B) The ACD/SCD ratio balances differentiation and self-renewal to regulate epidermal homeostasis. (C) Model showing how an increase in ACD drives a gradual loss of quiescent stem cells and a transient increase in more committed proliferating progenitors that further differentiate over time. This is indeed observed upon loss of aPKC-λ, providing evidence for this model. Based on work in Drosophila, this model also predicts that an increase in ACD would suppress tumor formation and vice versa.
Understanding how the balance between ACD and SCD is regulated is most likely crucial for skin diseases, as an imbalance toward SCD may promote overgrowth and expansion of stem cells, perhaps leading to inappropriate healing and ultimately cancer (see below). On the other hand, a shift toward ACD might promote premature differentiation, resulting in a hypomorphic epidermis and altered sebaceous gland, HF and sweat gland function (Fig. 4B).
POLARIZATION IN EPIDERMAL REGENERATION AND MIGRATION
Cutaneous wound healing is a complex process necessary to efficiently restore skin barrier function. This process requires a tightly orchestrated spatiotemporal response of different skin cell types (Gurtner et al. 2008). Several of these responses involve polarization of cells in the plane of the tissue and this is likely essential for restoration of tissue architecture and homeostasis. Within the epidermis keratinocytes need to coordinate proliferation with cell migration. On wounding, leading edge keratinocytes migrate in a directional fashion to close the wound. This is accompanied by a polarized secretion of provisional matrix and the reorganization of intercellular and cell-matrix contacts (Fig. 1B). Polarity cues are derived from a combination of the provisional matrix deposited and remodeled by keratinocytes and fibroblasts, the absence of intercellular contacts at the leading edge as well as soluble signals. Polarized proliferation of keratinocytes occurs in the area directly after the leading edge (Fig. 4C) to provide sufficient new cells to restore surface coverage. This might involve a shift from ACD to SCD to promote divisions that expand the basal cell layer.
In vitro studies have shown that cell polarity proteins regulate front rear polarization and the reorientation of the nucleus and centrosome during directed migration in diverse cell types (Etienne-Manneville 2008). Epidermal deletion of the small GTPase Rac delayed in vivo wound healing likely as a combined result of a reduction in proliferation and migration. Rac activity has been implicated both upstream of and downstream from aPKC (Mertens et al. 2005; Scotti et al. 2010) and might thus regulate ACD/SCD decisions. In line, Rac inactivation is associated with epidermal stem cell loss (Benitah et al. 2005; Castilho et al. 2007), Rac mutant keratinocytes also manifested reduced persistence in lamella protrusion providing an explanation for the reduction in migration (Pegtel et al. 2007; Tscharntke et al. 2007). This may involve the Rac exchange factor TIAM1, which associates with Par3 and aPKC at the leading edge of keratinocytes to regulate persistent migration in vitro (Pegtel et al. 2007). Scribble may function as another coordinator of Rac activity in collective cell migration (Dow et al. 2007). Interestingly, Scribble may integrate cell and planar polarity signaling to regulate epidermal wound healing. In mice, mutations for Scribble genetically interact with mutations in different PCP genes resulting in strongly impaired embryonic wound healing (Caddy et al. 2010).
An important future research topic will be to examine whether altered polarity signaling contributes to impaired wound healing, a major and increasing socioeconomic problem caused by the increase in obesity-related skin problems (e.g., “diabetic ulcers”) and the aging population.
ALTERED POLARITY SIGNALING: A DRIVER OF NONMELANOMA SKIN CANCER?
Cancer initiation and progression is characterized by changes in cell adhesion and in cell and tissue architecture. This may not only drive migration and invasion of cancer cells but may also contribute to a loss of proliferation control owing to, for example, changes in the microenvironment of stem/progenitor cells resulting in altered division orientation. As polarity proteins are key determinants of cell and tissue architecture, it is perhaps not surprising that altered polarity signaling can contribute to and has been implicated in a range of human cancers (Ellenbroek et al. 2012; Martin-Belmonte and Perez-Moreno 2012; Muthuswamy and Xue 2012).
Mutations in LKB1 result in Peutz–Jegher syndrome (PJS), a rare autosomal dominant syndrome characterized by the development of gastrointestinal polyps and mucocutaneous pigmentation abnormalities (Jansen et al. 2009). These patients are also more susceptible to a range of malignant epithelial tumors. In mice, either haploinsufficiency or epidermal inactivation of LKB1 strongly promotes DMBA-induced SCC not only in the skin but also in the lung (Gurumurthy et al. 2008). Interestingly, the SCCs did not originate from papillomas, as is usually the case in DMBA protocols. Moreover, haploinsufficient LKB1-derived SCCs showed loss of heterozygosity. LKB1-negative tumors were associated with increased Ras pathway activity, suggesting that loss of LKB1 promotes SCC formation at least in part through the Ras pathway.
In general, the Lgl/Scribble/Dlg polarity complex proteins show a reduced expression in human tumors and are considered potential tumor suppressors. In line, re-expression of Hugl2, a human homolog for Lgl in melanoma cell lines inhibits migration, restored E-cadherin expression, and decreases MMP expression (Kuphal et al. 2006). Loss of Lgl1 in mice induced brain hyperplasia (Klezovitch et al. 2004), similar to what has been observed in Drosophila Lgl mutants (Bilder 2004). A recent study in zebrafish epidermis provides a potential mechanism by which Lgls may serve as a tumor suppressor in the skin. Interestingly, loss of Lgl2 also induced epidermal overgrowth and epithelial to mesenchymal transition (EMT), which were both driven by enhanced ErbB2 signaling (Reischauer et al. 2009).
Two-stage DMBA/TPA nonmelanoma skin carcinogenesis mouse experiments revealed a dual role for Par3 in skin tumorigenesis (Iden et al. 2012). Epidermal loss of Par3 inhibited papilloma formation accompanied by increased apoptosis and a reduction in Ras-driven proliferation. The latter was dependent on intact cell–cell contacts. In contrast, loss of Par3 promoted the formation of keratoacanthomas (KA), a tumor type that is frequent in humans but rarely observed in mice. In agreement, Par3 is at sites of cell–cell contacts in human papillomas but is lost in human KA. Interestingly, Par3 is essential to localize its binding partner aPKC at the membrane (Iden et al. 2012). aPKC-λ is overexpressed in human cancer and shown to be a strong tumor promoter in a range of epithelial cancer models (Murray et al. 2011). It is thus tempting to speculate that in papilloma formation, aPKC exerts its tumor promoting activity at the membrane, whereas in KAs aPKC may drive tumor formation in the cytoplasm. Evidence for the latter was provided in a breast cancer model, in which loss of Par3 promoted tumor initiation and invasion likely as a result of cytoplasmic aPKC activation (McCaffrey et al. 2012). Par3 may thus function either as a tumor promoter or tumor suppressor in the skin, perhaps depending on the cell of origin within the epidermis.
In line with these observations is the recent finding that overactivation of the Par3 binding partner aPKC-λ promoted basal cell carcinoma (BCC). This study identified aPKC-λ as a direct target of HH signaling, a major driver of BCC (Atwood et al. 2013). In turn, aPKC-λ provides a positive-feedback loop by phosphorylating Gli1, thereby promoting DNA binding of Gli and thus its transcriptional output. More importantly, chemical inhibition of aPKC blocked BCC tumor growth also in lines that were resistant to the inhibition of the HH receptor Smoothened (Atwood et al. 2013). This study thus identifies polarity signaling as a potential novel target in the treatment of BCC.
As Par3 can also have functions independent of aPKC/Par6 it will be important to dissect the role of Par3 in aPKC driven BCC skin cancer and vice versa to ask whether the Par3 tumor promoting and suppressive functions depend on aPKC. Loss of aPKC-λ promotes ACD, differentiation, and loss of stem cells (Niessen et al. 2013), whereas in Drosophila, constitutive aPKC membrane expression drives SCD and overgrowth of stem cells, resulting in tumor formation (Fig. 4B). It will thus be important to determine whether Par3 and aPKC-λ promote epidermal tumorigenesis through control of cell fate, differentiation status, and division orientation within the different epidermal compartments.
Overall, these data indicate that altered polarity protein signaling directly contributes to nonmelanoma skin carcinogenesis and that identification of the underlying mechanisms may provide potential novel targets for tumor therapy.
LINKING CELL POLARITY TO GROWTH, IMMUNITY, AND ENERGY METABOLISM
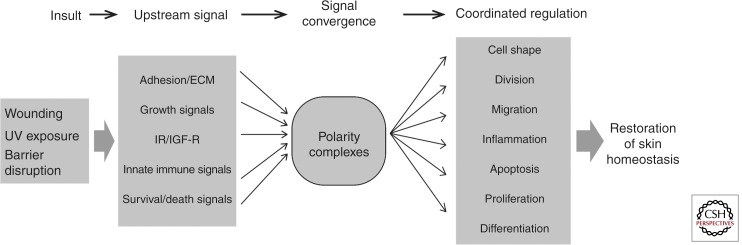
Cell polarity proteins not only regulate cell and tissue architecture but also are intermediates in pathways that control growth, metabolism, and inflammation, suggesting a direct link between these processes (Fig. 5).
Figure 5.
Polarity proteins as central integrators of cell architecture, innate immunity, metabolism, and growth. Schematic overview that illustrates how polarity protein signaling may integrate upstream signals and serve as central coordinators of the epidermal response to restore tissue homeostasis on different epidermal insults.
The Hippo pathway is perhaps the best example how cytoarchitectural status controls growth. The Hippo tumor suppressor negatively regulates the activity of the transcription factor Yap to control cell proliferation and thus organ size. Several recent papers identified a role for polarity proteins and intercellular junctions in the regulation of Hippo signaling (Boggiano and Fehon 2012). In the epidermis, the AJs protein α-catenin binds Yap and regulates its subcellular localization. Epidermal loss of α-catenin results in nuclear localization and transcriptional activation of Yap to promote overgrowth resulting in skin tumors (Schlegelmilch et al. 2011; Silvis et al. 2011). The exact mechanism by which α-catenin controls Yap is not clear. One potential mechanism might be through its interaction with merlin, a Ferm domain containing protein, which in Drosophila is an upstream regulator of Yap. Similar to α-catenin merlin mediates contact-mediated suppression of proliferation in cultured epithelial cells (Lallemand et al. 2003). In the epidermis, merlin connects α-catenin to Par3 and thus aPKC to regulate adherens junction maturation and spindle orientation (Gladden et al. 2010). Nevertheless, a direct link to regulation of Yap in the epidermis has not yet been reported.
Polarity may also directly link to the metabolic status of cells. The Par4/LKB1 serine/threonine kinase is a positive upstream regulator of at least 14 AMPK-related kinases, including the polarity protein Par1/MARK. These kinases thus couple LKB1 to a range of pathways that regulate diverse processes, such as cellular responses to metabolic stress, cell size, cell-cycle regulation, and cell polarity (Jansen et al. 2009). On low ATP conditions, LKB1 cooperated with the metabolic stress kinase AMPK to regulate epithelial polarization (Lee et al. 2007; Mirouse et al. 2007). LKB1 may thus directly couple energy status to the regulation of cell shape and cellular interactions.
The Par3/Par6/aPKC complex may also control metabolic signaling downstream from the insulin receptor as insulin treatment stimulated aPKC kinase activity (Kanzaki et al. 2004). In line, specific loss of aPKC-λ in classical insulin-sensitive tissues, such as pancreas or muscle, resulted in impaired insulin sensitivity (Hashimoto et al. 2005) and mimicked human metabolic syndrome (Farese et al. 2007). Interestingly, increased insulin sensitivity was observed on liver-specific loss of aPKC-λ, suggesting a cell-type-specific regulation of this pathway by aPKC-λ (Matsumoto et al. 2003). Finally, aPKCs have been implicated in the regulation of innate and adaptive immune signaling (Moscat et al. 2009). For example, inactivation of aPKC-ζ in all tissues of the mice results in impaired B-cell survival and altered NF-κB signaling (Leitges et al. 2001). At present, it is unclear whether in the epidermis polarity signaling regulates metabolic activity and innate immunity.
CONCLUDING REMARKS
Polarity protein signaling is slowly emerging as a central pathway important for the regulation of diverse processes. In many skin diseases, impaired epidermal barrier function is associated with an inflammatory and hyperproliferative response (Kubo et al. 2012). Wounding also elicits a spatiotemporal coordinated inflammatory and proliferative response that is integrated with changes in cell shape, adhesion, and migration. As polarity proteins have been implicated not only in the regulation of the cytoarchitecture but also in growth control, innate immunity, and metabolic signaling, these proteins may thus serve as central integrators of different upstream signals to coordinate the cell and tissue response to maintain and restore tissue homeostasis (Fig. 5). Key future questions will be whether these functions of polarity are indeed coupled and how altered polarity signaling disturbs skin homeostasis leading to disease.
ACKNOWLEDGMENTS
We thank Terry Lechler, Sara Wickström, Sandra Iden, and the members of the Niessen laboratory for fruitful discussions on the role of polarity proteins in epidermal biology. Work in the Niessen laboratory is supported by the German Research Community (DFG), SFB829, SFB832, and German Cancer Aid.
Footnotes
Editors: Anthony E. Oro and Fiona M. Watt
Additional Perspectives on The Skin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D 2008. Polarity complex proteins. Biochim Biophys Acta 1778: 614–630. [DOI] [PubMed] [Google Scholar]
- Atwood SX, Li M, Lee A, Tang JY, Oro AE 2013. GLI activation by atypical protein kinase C ι/λ regulates the growth of basal cell carcinomas. Nature 494: 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah SA, Frye M, Glogauer M, Watt FM 2005. Stem cell depletion through epidermal deletion of Rac1. Science 309: 933–935. [DOI] [PubMed] [Google Scholar]
- Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE 2010. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D 2004. Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes Dev 18: 1909–1925. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E 2009. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano JC, Fehon RG 2012. Growth control by committee: Intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev Cell 22: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, et al. 2000. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J 19: 3990–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova NA, Knust E 2009. The Crumbs complex: From epithelial-cell polarity to retinal degeneration. J Cell Sci 122: 2587–2596. [DOI] [PubMed] [Google Scholar]
- Caddy J, Wilanowski T, Darido C, Dworkin S, Ting SB, Zhao Q, Rank G, Auden A, Srivastava S, Papenfuss TA, et al. 2010. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev Cell 19: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G 2005. The cornified envelope: A model of cell death in the skin. Nat Rev Mol Cell Biol 6: 328–340. [DOI] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Patel V, Millar SE, Zheng Y, Molinolo A, Gutkind JS 2007. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene 26: 5078–5085. [DOI] [PubMed] [Google Scholar]
- Croyle MJ, Lehman JM, O’Connor AK, Wong SY, Malarkey EB, Iribarne D, Dowdle WE, Schoeb TR, Verney ZM, Athar M, et al. 2011. Role of epidermal primary cilia in the homeostasis of skin and hair follicles. Development 138: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetto A, Kubo A, Beck LA 2012. Skin barrier disruption: A requirement for allergen sensitization? J Invest Dermatol 132: 949–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S, Turkoz A, Kopan R 2009. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell 16: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Oristian D, Heller E, Fuchs E 2011. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol 13: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet YS, Woo SH, Ruiz ME, Owens DM 2013. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Rep 3: 1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, Russell SM, Humbert PO 2007. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: Regulation of Rho GTPase recruitment to the leading edge. Oncogene 26: 2272–2282. [DOI] [PubMed] [Google Scholar]
- Ellenbroek SI, Iden S, Collard JG 2012. Cell polarity proteins and cancer. Semin Cancer Biol 22: 208–215. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S 2008. Polarity proteins in migration and invasion. Oncogene 27: 6970–6980. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E 2011. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145: 1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B 2004. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol 14: 1451–1461. [DOI] [PubMed] [Google Scholar]
- Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR Jr, Nimal S, Choi CS, Kim S, Shulman GI, et al. 2007. Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117: 2289–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances D, Niemann C 2012. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev Biol 363: 138–146. [DOI] [PubMed] [Google Scholar]
- Fuchs E 2007. Scratching the surface of skin development. Nature 445: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S 2002. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J Cell Biol 156: 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, Harada K, Sekiguchi K, Morgan BA, Miner JH, et al. 2008. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev 22: 2111–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI 2010. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell 19: 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV 2010. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet 11: 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Macara IG 2007. The PAR proteins: Fundamental players in animal cell polarization. Dev Cell 13: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas S, Conder R, Knoblich JA 2012. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell 11: 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunschmann C, Stachelscheid H, Akyuz MD, Schmitz A, Missero C, Bruning JC, Niessen CM 2013. Insulin/IGF-1 controls epidermal morphogenesis via regulation of FoxO-mediated p63 inhibition. Dev Cell 26: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT 2008. Wound repair and regeneration. Nature 453: 314–321. [DOI] [PubMed] [Google Scholar]
- Gurumurthy S, Hezel AF, Sahin E, Berger JH, Bosenberg MW, Bardeesy N 2008. LKB1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res 68: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Kido Y, Uchida T, Matsuda T, Suzuki K, Inoue H, Matsumoto M, Ogawa W, Maeda S, Fujihara H, et al. 2005. PKC-λ regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic β cells. J Clin Invest 115: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich I, Schmitz A, Zigrino P, Michels C, Haase I, le Bivic A, Leitges M, Niessen CM 2007. Role of aPKC isoforms and their binding partners Par3 and Par6 in epidermal barrier formation. J Invest Dermatol 127: 782–791. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N 2011. Ciliopathies. N Engl J Med 364: 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurov J, Piwnica-Worms H 2007. The Par-1/MARK family of protein kinases: From polarity to metabolism. Cell Cycle 6: 1966–1969. [DOI] [PubMed] [Google Scholar]
- Iden S, van Riel WE, Schafer R, Song JY, Hirose T, Ohno S, Collard JG 2012. Tumor type-dependent function of the par3 polarity protein in skin tumorigenesis. Cancer Cell 22: 389–403. [DOI] [PubMed] [Google Scholar]
- Inaba M, Yamashita YM 2012. Asymmetric stem cell division: Precision for robustness. Cell Stem Cell 11: 461–469. [DOI] [PubMed] [Google Scholar]
- Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H 2009. LKB1 and AMPK family signaling: The intimate link between cell polarity and energy metabolism. Physiol Rev 89: 777–798. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Mora S, Hwang JB, Saltiel AR, Pessin JE 2004. Atypical protein kinase C (PKC-ζ/λ) is a convergent downstream target of the insulin-stimulated phosphatidylinositol 3-kinase and TC10 signaling pathways. J Cell Biol 164: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner N, Haftek M, Niessen CM, Behne MJ, Furuse M, Moll I, Brandner JM 2011. CD44 regulates tight-junction assembly and barrier function. J Invest Dermatol 131: 932–943. [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V 2004. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev 18: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA 2008. Mechanisms of asymmetric stem cell division. Cell 132: 583–597. [DOI] [PubMed] [Google Scholar]
- Koster MI 2009. Making an epidermis. Ann NY Acad Sci 1170: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M 2009. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med 206: 2937–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Nagao K, Amagai M 2012. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest 122: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal S, Wallner S, Schimanski CC, Bataille F, Hofer P, Strand S, Strand D, Bosserhoff AK 2006. Expression of Hugl-1 is strongly reduced in malignant melanoma. Oncogene 25: 103–110. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI 2003. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev 17: 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E 2007. Desmoplakin: An unexpected regulator of microtubule organization in the epidermis. J Cell Biol 176: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, et al. 2007. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447: 1017–1020. [DOI] [PubMed] [Google Scholar]
- Lehman JM, Laag E, Michaud EJ, Yoder BK 2009. An essential role for dermal primary cilia in hair follicle morphogenesis. J Invest Dermatol 129: 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J 2001. Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol Cell 8: 771–780. [DOI] [PubMed] [Google Scholar]
- Li R 2013. The art of choreographing asymmetric cell division. Dev Cell 25: 439–450. [DOI] [PubMed] [Google Scholar]
- Lippens S, Hoste E, Vandenabeele P, Agostinis P, Declercq W 2009. Cell death in the skin. Apoptosis 14: 549–569. [DOI] [PubMed] [Google Scholar]
- Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumailley M, Fassler R 2007. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol 177: 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MS, Johnston CA 2013. Molecular pathways regulating mitotic spindle orientation in animal cells. Development 140: 1843–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten MN, Basten SG, Claessens T, Vernooij M, Scott CL, Janssen R, Easton JA, Kamps MA, Vreeburg M, Broers JL, et al. 2013. Birt–Hogg–Dube syndrome is a novel ciliopathy. Hum Mol Genet 22: 4383–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno M 2012. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer 12: 23–38. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyake K, Furukawa K, Hayashi Y, Iguchi H, Matsuki Y, Hiramatsu R, et al. 2003. PKC-λ in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest 112: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Montalbano J, Mihai C, Macara IG 2012. Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 22: 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG 2005. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol 170: 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels C, Buchta T, Bloch W, Krieg T, Niessen CM 2009. Classical cadherins regulate desmosome formation. J Invest Dermatol 129: 2072–2075. [DOI] [PubMed] [Google Scholar]
- Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE 2007. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol 177: 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moscat J, Diaz-Meco MT, Wooten MW 2009. Of the atypical PKCs, Par-4 and p62: Recent understandings of the biology and pathology of a PB1-dominated complex. Cell Death Differ 16: 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NR, Kalari KR, Fields AP 2011. Protein kinase Ciota expression and oncogenic signaling mechanisms in cancer. J Cell Physiol 226: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy SK, Xue B 2012. Cell polarity as a regulator of cancer cell behavior plasticity. Annu Rev Cell Dev Biol 28: 599–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen MT, Scott J, Zielinski JG, Vorhagen S, Sotiropoulou PA, Blanpain C, Leitges M, Niessen CM 2013. aPKC-λ controls epidermal homeostasis and stem cell fate through regulation of division orientation. J Cell Biol 202: 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG 2007. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol 17: 1623–1634. [DOI] [PubMed] [Google Scholar]
- Petersson M, Brylka H, Kraus A, John S, Rappl G, Schettina P, Niemann C 2011. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J 30: 3004–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson ND, Lechler T 2010. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol 191: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson ND, Lechler T 2012. Asymmetric cell divisions in the epidermis. Int Rev Cell Mol Biol 295: 199–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E 2000. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150: 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Foote HP, Lechler T 2013. β-Catenin protects the epidermis from mechanical stresses. J Cell Biol 202: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischauer S, Levesque MP, Nusslein-Volhard C, Sonawane M 2009. Lgl2 executes its function as a tumor suppressor by regulating ErbB signaling in the zebrafish epidermis. PLoS Genet 5: e1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roignot J, Peng X, Mostov K 2013. Polarity in mammalian epithelial morphogenesis. Cold Spring Harb Perspect Biol 5: a13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V 2012. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487: 496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V 2013. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Goodship JA 2009. Ellis–van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am J Med Genet C Semin Med Genet 151C: 341–351. [DOI] [PubMed] [Google Scholar]
- Schermer B, Ghenoiu C, Bartram M, Muller RU, Kotsis F, Hohne M, Kuhn W, Rapka M, Nitschke R, Zentgraf H, et al. 2006. The von Hippel–Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol 175: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. 2011. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144: 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR 2010. Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res 70: 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakianos J, Togawa A, Maday S, Hull M, Pypaert M, Cantley L, Toomre D, Mellman I 2007. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J Cell Biol 179: 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V 2011. α-Catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal 4: ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J 2010. Cell polarity in eggs and epithelia: Parallels and diversity. Cell 141: 757–774. [DOI] [PubMed] [Google Scholar]
- Sumigray KD, Chen H, Lechler T 2011. Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J Cell Biol 194: 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumigray KD, Foote HP, Lechler T 2012. Noncentrosomal microtubules and type II myosins potentiate epidermal cell adhesion and barrier formation. J Cell Biol 199: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharntke M, Pofahl R, Chrostek-Grashoff A, Smyth N, Niessen C, Niemann C, Hartwig B, Herzog V, Klein HW, Krieg T, et al. 2007. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J Cell Sci 120: 1480–1490. [DOI] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM 2005. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J 24: 1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Blanpain C 2012. Tracing epithelial stem cells during development, homeostasis, and repair. J Cell Biol 197: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, et al. 2010. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genetics 86: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB 2012. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol 28: 627–653. [DOI] [PubMed] [Google Scholar]
- Wang Y, Badea T, Nathans J 2006. Order from disorder: Self-organization in mammalian hair patterning. Proc Natl Acad Sci 103: 19800–19805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM 2001. Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev 11: 410–417. [DOI] [PubMed] [Google Scholar]
- Watt FM, Jensen KB 2009. Epidermal stem cell diversity and quiescence. EMBO Mol Med 1: 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, Fuchs E 2011. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 470: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T 2009. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 5: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]