Abstract
Chamomile (Matricaria chamomilla L.) is a widely used medicinal plant possessing several pharmacological effects due to presence of active compounds. This study describes a method of using ultra performance liquid chromatography (UPLC) coupled with photodiode array (PDA) detector for the separation of phenolic compounds in M. chamomilla and its crude extracts. Separation was conducted on C18 column (150 mm × 2 mm, 1.8 μm) using a gradient elution with a mobile phase consisting of acetonitrile and 4% aqueous acetic acid at 25°C. The method proposed was validated for determination of free and total apigenin and apigenin 7-glucoside contents as bioactive compounds in the extracts by testing sensitivity, linearity, precision and recovery. In general, UPLC produced significant improvements in method sensitivity, speed and resolution. Extraction was performed with methanol, 70% aqueous ethanol and water solvents. Total phenolic and total flavonoid contents ranged from 1.77 to 50.75 gram (g) of gallic acid equivalent (GAE)/100 g and 0.82 to 36.75 g quercetin equivalent (QE)/100 g in dry material, respectively. There was a considerable difference from 40 to 740 mg/100 g for apigenin and 210 to 1110 mg/100 g for apigenin 7-glucoside in dry material.
Keywords: Matricaria chamomilla, Flavonoids, UPLC, Apigenin, Apigenin 7-glucoside
INTRODUCTION
Matricaria chamomilla L. (Asteraceae) is often used as a medicinal plant, due to its anti-inflammatory, analgesic, sedative, antimicrobial, anti-allergic, anti-hyperglycemia and anti-spasmodic effects. It is also used in a number of alimental, cosmetic, pharmaceutical industries. Recent research supports this use and shows these properties are partly due to its phenolic compounds (1,2,3,4). The spasmolytic and antiphlogistic activities are mainly attributed to the presence of apigenin, apigenin-7-O-glucoside and its acetylated derivatives (5). The flavones are also known to play an important role in the overall anti-inflammatory activity, chemopreventive activity against UV-radiation and/or anti-cancer properties against a number of tumor types and sedative effect of chamomile extracts (6,7,8). Due to its long history of use, there are phytochemical reports on the main constituents of chamomile including volatile oil, flavonoids, coumarins, mucilage and other constituents (9,10). According to the European Pharmacopeia (EP) chamomile flowers should contain at least 0.4% essential oil and at least 0.25% of apigenin 7-glucoside. Several techniques such as high performance thin layer chromatography (HPTLC), thin layer chromatography (TLC) (5), high performance liquid chromatography-ultraviolet (HPLC-UV) (5,11,12,13,14,15,16,17), HPLC-MS (mass spectrometry) (12,18,19,20,21), capillary electrophoresis (CE) (22), and capillary electrochromatography (CEC) (23) have been reported for the analysis of the chamomile extracts. The analysis time of HPLC method was long at a run time of about 50 min (11,12,13,14,19). Carnnat and coworkers analyzed several hydroxycinnamic acid derivatives and flavonoids of chamomile tea infusion by HPLC. The analysis time was within 45 min at a flow rate of 2 ml/min (12). In another report, Mulinacci and co-workers separated 24 phenolic compounds in flower extracts of Matricaria recutita using HPLC-DAD-MS at a long run time (14). Although phenolic compounds have been previously analyzed by HPLC, the technique of ultra performance liquid chromatography with UV detector has not been applied to M. chamomilla. In general, the UPLC technique decreases the solvent consumption, analysis time and increase resolution and sensitivity. Moreover, the equilibration time for bringing the column to the initial conditions after gradient analysis is short. The aim of this study was to develop a UPLC method coupled with photodiode array detector for the analysis of phenolic compounds in this herb and its extracts and to measure free apigenin and apigenin 7-glucoside and their total content in plant material and its extracts. Several flavonoid derivatives, many glycosides and some free aglycones have been previously reported in this plant, (14). Quantitative determination of individual apigenin glycosides is difficult because most reference compounds are not commercially available. However, in order to reduce the complexity of the analysis, total apigenin and apigenin 7-glucoside contents are quantified after both acid and alkaline hydrolysis by UPLC.
MATERIALS AND METHODS
Chemicals and reagents
Acetonitrile and water (HPLC grade), acetic acid, hydrochloric acid, sodium hydroxide, and sodium carbonate were from Merck (Darmstadt, Germany). Chlorogenic acid, caffeic acid, quercetin, luteolin, apigenin, kaempferol, isorhamnetin, apigenin 7-glucoside p-coumaric acid, salicylic acid, rutin were obtained from Carl Roth (Karlsruhe, Germany).
Plant materials
M. chamomile aerial parts at flowering stage were gathered from Kashan vicinity, in July, 2012 and dried in a shaded and ventilated place for 48 h.
Standard solutions
Quantification of apigenin and apigenin 7-glucoside in the samples was carried out by the external standard method. The stock standard solutions of apigenin and apigenin 7-glucoside (1 mg/ml) were freshly prepared in methanol.
The working solutions apigenin and apigenin 7-glucoside were prepared by diluting the stock solution using methanol. These run solutions were further diluted with the same stock solutions for the UPLC system. One μl volume at each concentration level of standard materials was injected in triplicate into the UPLC system for the construction of the calibration curves. The calibration curves were plotted using various concentrations of standards (μg/ml) versus the peak area.
Extraction procedure
Dried and powdered chamomile aerial parts (5 g) were separately extracted by maceration with 150 ml of water, ethanol:water (7:3) and methanol using a shaker apparatus at room temperature for 24 h. The residual was rinsed with 50 ml of solvent and the recovered fractions were filtered and evaporated completely under vacuum.
Sample preparation
The dried and powdered aerial parts of M. chamomile (0.2 g) was transferred into a screw-capped extraction tube and extracted with 10 ml of 70% aqueous methanol at 75 °C for 60 min. After centrifuging at 4000 rpm for 5 min, 3 ml of supernatant was transferred to a 5 ml vial. The solution was filtrated through a syringe filter of 0.2 μm membrane for quantification of apigenin and apigenin 7-glucoside in plant material by UPLC.
Alkaline hydrolysis
Apigenin is normally present both free and as esters with glycoside in this herb (14,24). For measurement of total apigenin 7-glucoside, the herb and extracts were separately subjected to alkaline hydrolysis according to the procedure reported in British Pharmacopoeia (25). In this process, various acetylated derivatives of apigenin 7-glucoside are converted to apigenin 7-glucoside. The hydrolysates were filtrated through a syringe filter of 0.2 μm membrane to assay total apigenin 7-glucoside by UPLC.
Acid hydrolysis
A simple approach to obtain the aglycone is mild acid hydrolysis of the samples which releases the glycoside moiety without promoting decomposition of the remaining aglycone skeleton. Hydrolysis of apigenin glycosides in the extracts and herb were performed in acidic medium following the procedure described in the literature with minor modifications (26). A 100 mg of herb or 20 mg of dry extract were separately hydrolysed with 10 ml of a mixture of methanol:HCl (7:1) at 100 °C in a caped tube for 2 h. The solution was cooled, centrifuged for 5 min and filtered through a 0.2 μm filter for the measurement of total apigenin by UPLC method.
Total phenolic content (TPC)
Total phenolic content was estimated using the Folin–Ciocalteu colorimetric method as described elsewhere (27) with minor modification. 0.2 ml of each extract (1 mg/ml) was transferred into a 5 ml volumetric flask and swirled with 3 ml of deionised water. 0.25 ml of Folin-Ciocalteu's reagent was added and swirled. After 3 min, 0.75 ml of 20% (w/v) sodium carbonate solution was added and mixed. This was recorded as time zero. Deionised water was added to make up the volume to 5 ml exactly. The solution was mixed thoroughly and allowed to stand at ambient temperature for 2 h until the characteristic blue colour developed. Quantification was done on the basis of the standard curve of gallic acid at 760 nm by a spectrophotometer (Perkin-Elmer Lambda EZ-210 UV/VIS). All tests were conducted in triplicate and averaged. Results were expressed as gram of gallic acid equivalent (GAE) per 100 g of dry material.
Total flavonoid content (TFC)
Stock solution of quercetin (0.5 mg/ml) prepared in methanol was diluted with 80% (v/v) ethanol (ranging from 25 to 100 μg/ml) to construct the calibration curve. A 0.5 ml of standard solutions or the crude extract were separately transferred into a 5 ml volumetric flask containing 2 ml of 80% ethanol, 0.1 ml of 10% (w/v) aluminum chloride and 0.1 ml of 1 M potassium acetate, diluted with distilled water to the mark and mixed. After 30 min, the absorbance was measured at 415 nm with a Perkin-Elmer Lambda EZ-210 spectro-photometer. The TFC in the crude extracts (1 mg/ml) were determined as above method. TFC expressed as g of quercetin equivalent per 100 g of dry material (28).
Chromatographic conditions
The UPLC analysis was performed using a Knauer UHPLC/HPLC PLATIN blue system (Knauer, Berlin, Germany), equipped with a binary pump, column compartment, and PDA detector. Optimal separations were achieved using linear gradient elution with 4% (v/v) aqueous acetic acid (eluent A) and acetonitrile (eluent B) on a Blue Orchid column (150 × 2 mm, 1.8 μm) at a flow rate of 0.4 ml/min at 25 °C. The gradient elution was started with eluent A from 7 to 21% at 18 min, held for 3 min then, 21 to 48% at 4 min. All samples were injected three times (loop 1 μl). The spectral data of signals from the PDA detector were collected during the whole run in the range of 220-400 nm and detection set at 340 nm. The analytes were assigned by comparing retention times, UV spectra, spiking the samples with standard compounds.
Method validation
The validation parameters assessed were: linearity, sensitivity, precision and recovery. Detection limits (LODs) and quantification limits (LOQs) were determined by injecting a series of dilute solutions with known concentrations on the basis of a signal-to-noise ratio equal to about 3 and 10, respectively. Intra-and inter-day variation of the assay was determined on 1 and 3 consecutive days with 3 repetitions each. Three replicate recovery samples were prepared for analysis. A blank recovery sample was prepared for comparative analysis. Each sample was analyzed three times.
RESULTS
Determination of total phenolic, total flavonoid contents and analytes
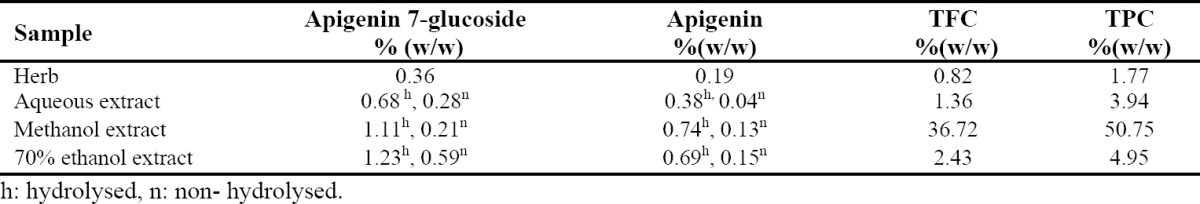
The dry extract yield of dry material was 24.4% for water, 21.7% for 70% aqueous ethanol and 15.2% for methanol. The total phenolic and total flavonoid contents were calculated on the basis of gallic acid and quercetin and expressed as equivalents of gram gallic acid and quercetin per 100 g of dry material, respectively. The amounts of total phenolics, total flavonoid and analytes are shown in Table 1. The analysis revealed that total phenolic and total flavonoid contents in methanol extract were higher than other extracts. The highest amount of phenolic and flavonoid was detected in the methanol extract with a mean value of 50.7 and 36.7% and the lowest in the aqueous extract with a mean value of 3.94 and 1.36%, respectively. The apigenin 7-glucoside content in the crude extracts was much higher than the free apigenin. As it is seen in Table 1, the apigenin 7-glucoside and apigenin ranged from 0.21 to 1.23 and 0.04 to 0.74 per 100 gram in dry samples, respectively.
Table 1.
Results obtained for analytes and total phenolic and total flavonoid contents.
Optimization of the UPLC method
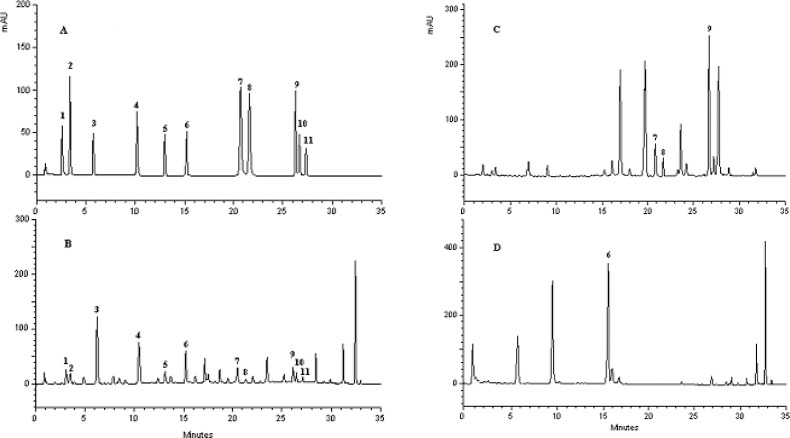
Optimal chromatographic conditions were obtained after multiple running different mobile phases with BlueOrchid C18 columns. In this study, acetonitrile was preferred over methanol as the mobile phase as its use slightly improved separation as well as a significantly reduced column back pressure. As a result, acetonitrile and water containing of 4% (v/v) acetic acid was chosen as mobile phase in linear gradient mode for a satisfactory separation of 30 compounds in the samples. The extracts were separately analysed on two columns of BlueOrchid 100 and 150 mm. UPLC chromatograms of the extracts on two columns were compared with each other. Although analysis time on column 100 mm was less than 20 min, but separation between peaks of apigenin and kaempferol was not desirable due to very similar retention properties. A sufficient separation was obtained using longer column, and its actual run time was less than 35 min. Chromatogram of reference standards and crude extract generated by PDA detection are presented in Figs. 1A and 1B. The hydrolysis of plant material showed noteworthy changes in the UPLC chromatogram, in particular with respect to the apigenin and apigenin 7-glucoside (Figs. 1C and 1D).
Fig. 1.
UPLC-PDA chromatograms of standard materials (A), 70% methanol extract (B), acid hydrolysis of plant material (C), alkaline hydrolysis of plant material (D). 1: chlorogenic acid, 2: caffeic acid, 3: p-coumaric acid, 4: salicylic acid, 5: rutin, 6: apigenin-7-glucoside, 7: quercetin, 8: luteolin, 9: apigenin, 10: kaempferol, 11: isorhamnetin.
Validation of proposed procedure
Quantitative analyses of both apigenin and apigenin 7-glucoside by UPLC-PDA were validated with respect to linearity, sensitivity, precision and recovery. Using the linear regression analysis, calibration curves for apigenin and apigenin 7-glucoside were established in the range of 5-50 and 5-100 μg/ml. The correlation coefficients (r2) of the calibration curves were higher than 0.999. The LODs and LOQs calculated for apigenin were 33 and 100 ng/ml, while for apigenin 7-glucoside were 46 and 140 ng/ml, respectively (Table 2). These numbers however were lower when compared with those from other techniques such as HPLC or CE.
Table 2.
Data for linearity and sensitivity.
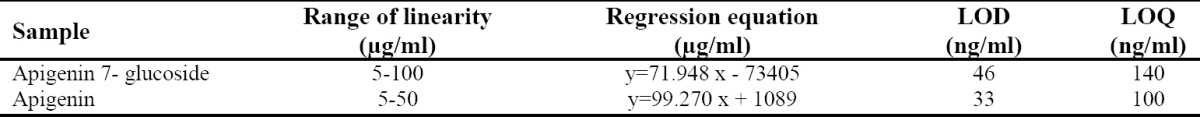
The intra-mediate precision (repeatability) was assessed by triplicate analysis of three same samples on one day. Each sample was analyzed three times. Intermediate precision was measured by analysis of three same samples on three consecutive days. Each sample was analyzed three times. The precision was calculated as relative standard deviation (RSD%). The RSD of the standards were between 2.8 and 3.4%. To assess the recovery, three replicate recovery samples including one an unspiked sample (blank) and three samples spiked with standards at three concentration levels were prepared and analyzed.
The recovery of the method was evaluated by spiking with known amounts of apigenin and apigenin 7-glucoside at three concentration levels under optimized conditions. The average percentage in the samples ranged from 97.7 to 99.4%.
DISCUSSION
UPLC method has been developed in recent years for pharmaceutical analysis but not yet utilized in quality control analysis of pharma-ceutical formulations. Although applications of UPLC have been reported in the past years, it is still new trend in separation sciences and has the potential for more pioneering research (29). Although this method separated about 30 peaks, but were unable to characterize all peaks due to lack of standard substances. UPLC separation identified some phenolic compounds especially flavonoids such as apigenin 7-glucoside, apigenin, quercetin, luteolin, kaempferol, rutin and isorhamnetin in the extract by matching UV spectra and retention times and co-elution with their standards (Figs. 1 A and 1 B). The peaks were eluted based on their polarity and molecule size, first chlorogenic and caffeic acids, second flavonoid glycosides as they contain polar groups and finally flavonoid aglycones as non-polar compounds. The analytes were characterized by comparing retention times and UV spectra of authentic compounds and literature data (13,15).
The extracts hydrolysis showed the larger peak area for quercetin, luteolin, apigenin and apigenin 7-glucoside than the non-hydrolysed extracts. Figs. 1C and 1D show the peaks of apigenin and apigenin 7-glucoside in the hydrolysed extracts. The analysis time of phenolic compounds was shorter than HPLC methods reported in the literature. For example, Mulinacci and coworkers have described a HPLC-DAD-MS method on separation of the flavonoids and phenypropanoid of Italian chamomile flowers (14). The analysis time in their study was 50 min.
Fonseca and Tavares reported the amounts of free and total apigenin in the methanolic, ethanolic and glycolic extracts. Their findings indicated that free and total apigenin content were 106 and 903 μg/g for methanolic extract and 11 and 247μg/g for ethanolic extract (22) which is in accordance with our results. In other investigation, the total polyphenols and total flavonols contents in commercial chamomile (Chamomilla recutita L. Rauschert) teas from different countries were found to be between 13.2 to 17.6 % and 0.39 to 1.21% (w/w) chlorogenic acid equivalent, respectively (11). The reported amount for total flavonols was consistent to average value of our results but a significant difference for total polyphenols content in plant material exist. This difference can be related to the genetic background of the chamomile, geographical origin, extraction procedure, solvent type, used standard material, drying condition, environment, cultivation, pick time, plant organ and other factors.
CONCLUSION
The proposed UPLC-UV method enables to separate about 30 compounds in M. chamomilla. The actual run time is about two times shorter than the conventional method of HPLC. Furthermore, the UPLC method requires lower amounts of solvents than the HPLC approaches. The developed method is a reliable procedure that shows satisfactory linearity, sensitivity, precision, and accuracy. This study revealed a major difference in total phenolic and flavonoid contents, and the amounts of apigenin 7-glucoside and apigenin in the extracts with respect to extraction solvent.
ACKNOWLEDGMENT
The authors gratefully acknowledge the financial support of the Barij Essence Pharmaceutical Company and Agriculture Group of Medicinal Plant Research Center of Jundi Shapour for providing of samples.
REFERENCES
- 1.Maschi O, Dal Cero E, Galli GV, Caruso D, Bosisio E, Dell’ Agli M. Inhibition of human cAMP-phosphodiesterase as a mechanism of the spasmolytic effect of Matricaria recutita L. J Agric Food Chem. 2008;56:5015–5020. doi: 10.1021/jf800051n. [DOI] [PubMed] [Google Scholar]
- 2.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother Res. 2006;20:519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 3.Bozorgmehr B, Mojab F, Faizi M. Evaluation of sedative-hypnotic effect of ethanolic extract of five medicinal plants; Nepeta menthoides, Matricaria chamomilla, Asperugo procumbens, Lippia citriodora and Withania somnifera. Res Pharm Sci. 2012;7:S831. [Google Scholar]
- 4.Darvishpadok A, Azemi M, Namjoyan F, Khodayar M, Ahmadpour F, Panahi M. Effect of Matricaria chamomilla L. on blood glucose and glycosylated hemoglobin in female fertile diabetic rats. Res Pharm Sci. 2012;7:S19. [Google Scholar]
- 5.Zekovic Z, Peki B, Lepojevi Z, Petrovi L. Chromatography in our investigations of chamomile (Matricaria chamomilla L) Chromatographia. 1994;39:587–590. [Google Scholar]
- 6.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–245. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 7.Mak P, Lejny YK, Tang WY, Harwood C, Ho SM. Apigenin suppresses cancer cell growth through Erb. Neoplasia. 2006;8:896–904. doi: 10.1593/neo.06538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.švehlíkovaá V, Repcaák M. Apigenin chemotypes of Matricaria chamomilla L. Biochem Syst Ecol. 2006;34:654–657. [Google Scholar]
- 9.Blumenthal M, Goldberg A, Brinckmann J. Austin: American Botanical Council; 1998. Herbal Medicine. Expanded Commission E monographs: Therapeutic guide to herbal medicines; pp. 57–61. [Google Scholar]
- 10.Newall CA, Anderson LA, Phillipson JD. London: Pharmaceutical Press; 1996. Herbal medicines: A guide for health-care professionals; pp. 69–71. [Google Scholar]
- 11.Raal A, Orav A, Pussa T, Valner C, Malmiste B, Arak E. Content of essential oil, terpenoids and polyphenols in commercial chamomile (Chamomilla recutita L. Rauschert) teas from different countries. Food Chem. 2012;131:632–638. [Google Scholar]
- 12.Carnat A, Carnat AP, Fraisse D, Ricoux L, Lamaison JL. The aromatic and polyphenolic composition of Roman camomile tea. Fitoterapia. 2004;75:32–38. doi: 10.1016/j.fitote.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Kováčcik J, Grúz J, Baúkor M, Tomko J, Strnad M, Repčák M. Phenolic compounds composition and physiological attributes of Matricaria chamomilla grown in copper excess. Environ Exp Bot. 2008;62:145–152. [Google Scholar]
- 14.Mulinacci M, Romani A, Pinelli P, Vincieri FF, Prucher D. Characterization of Matricaria recutita L. flower extract by HPLC-MS and HPLC-DAD. Chromatographia. 2000;51:301–307. [Google Scholar]
- 15.Pietta P. Simultaneous isocratic high-performance liquid chromatographic determination of flavones and coumarins in Matricaria chamomdla extracts. J Chromatogr. 1987;404:279–281. doi: 10.1016/s0021-9673(01)86861-8. [DOI] [PubMed] [Google Scholar]
- 16.Repcák M, Krausova T. Phenolic glucosides in the course of ligulate flower development in diploid and tetraploid Matricaria chamomilla. Food Chem. 2009;116:19–22. [Google Scholar]
- 17.Redaelli C, Formentini L, Santaniello E. Reversed-phase high-performance liquid chromatography analysis of apigenin and its glucosides in flowers of Matricaria chamomilla and chamomile extracts. Planta Med. 1981;42:288–292. doi: 10.1055/s-2007-971643. [DOI] [PubMed] [Google Scholar]
- 18.Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matrica chamomila. Biochem. Pharmacol. 2000;59:1387–1394. doi: 10.1016/s0006-2952(00)00264-1. [DOI] [PubMed] [Google Scholar]
- 19.Ayaz FA, Hayirlioglu-Ayaz S, Gruz J, Novak O, Strand M. Separation characterization, and quantitation of phenolic acids in a little-known blueberry (Vaccinium arctostaphylos L.) fruit by HPLC-MS. J Agric Food Chem. 2005;53:8116–8122. doi: 10.1021/jf058057y. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava JK, Gupta S. Extraction, Characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Mol Cell Pharmacol. 2009;1:138–147. doi: 10.4255/mcpharmacol.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber B, Herrmann M, Hartmann B, Joppe H, Schmidt CO, Bertram HJ. HPLC/MS and HPLC/NMR as hyphenated techniques for accelerated characterization of the main constituents in Chamomile (Chamomilla recutita L. Rauschert) Eur Food Res Technol. 2008;226:755–760. [Google Scholar]
- 22.Fonseca FN, Tavares MFM. Validation of a capillary electrophoresis method for the quantitative determination of free and total apigenin in extracts of Chamomilla recutita. Phytochem Anal. 2004;15:65–70. doi: 10.1002/pca.744. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca FN, Tavares MFM, Horváth C. Capillary electrochromatography of selected phenolic compounds of Chamomilla recutita. J Chromatogr. 2007;1154:390–399. doi: 10.1016/j.chroma.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 24.Repcˇak M, Krausova T. Phenolic glucosides in the course of ligulate flower development in diploid and tetraploid Matricaria chamomilla. Food chem. 2009;116:19–22. [Google Scholar]
- 25.London: 2010. British Pharmacopoeia. Introduction General Notices Monographs Medicinal and Pharmaceutical Substances; pp. 1236–1238. [Google Scholar]
- 26.Haghi G, Hatami A. Simultaneous quantification of flavonoids and phenolic acids in plant materials by a newly developed isocratic high-performance liquid chromatography approach. J Agric Food Chem. 2010;58:10812–10816. doi: 10.1021/jf102175x. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Simon JE, Aviles IF, He K, Zheng QY, Tadmore Y. Analysis of phenolic compounds in artichoke (Cynara scolymus L.) J Agric Food Chem. 2003;51:601–608. doi: 10.1021/jf020792b. [DOI] [PubMed] [Google Scholar]
- 28.Chang CC, Yang MU, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 29.Haghi G, Hatami A, Mehran M. UPLC and HPLC of caffeoyl esters in wild and cultivated Arctium lappa L. Food Chem. 2013;128:321–326. doi: 10.1016/j.foodchem.2012.10.040. [DOI] [PubMed] [Google Scholar]