Abstract
Mucoadhesive drug delivery systems were developed to sustain drug delivery via various mucus membranes for either local or systemic delivery of poorly absorbed drugs such as peptides and proteins as well as drugs that are subjected to high first-pass metabolism. The present study was undertaken to use isolated Calendula mucilage as a mucoadhesive agent and to formulate controlled release buccoadhesive tablets with an intention to avoid hepatic first-pass metabolism as well as to enhance residence time of drug in the buccal cavity. The mucilage was isolated from the Calendula petals by aqueous extraction method and characterized for various physiochemical parameters as well as for its adhesive properties. By using direct compression technique, tablets were prepared containing dried mucilage and chlorpheniramine maleate (CPM) as a model drug. Three batches of tablets were prepared and evaluated containing three mucoadhesive components namely Methocel K4M, Carbopol 974P and isolated Calendula mucilage in 16.66%, 33.33 % and 50 % (1:2:3 ratio) resulting in 9 different formulations. FTIR studies between mucilage and CPM suggested the absence of a chemical interaction between CPM and Calendula mucilage. The results of the study showed that the isolated mucilage had good physicochemical and morphological characteristics and tablets conformed to the pharmacopoeial specifications. Also in vitro release studies showed controlled action of drug with increasing the concentration of the isolated Calendula mucilage as a mucoadhesive agent in the formulations. Permeability studies indicated that permeability behavior was not statistically different (P>0.05) by changing the mucoadhesive component. The formulated mucoadhesive tablets for buccal administration containing 75 mg Calendula mucilage showed controlled drug release. Thus, mucoadhesive natural Calendula mucilage based buccal tablets for controlled release were successfully formulated.
Keywords: Calendula, Natural polymers, Chlorpheniramine maleate, Controlled release, Mucoadhesive
INTRODUCTION
Free availability, relatively low cost, nontoxicity, ease of manufacturing, favorable in vivo performance, and versatility in controlling the release of drugs with a wide range of physicochemical properties are some of the imperative factors which may contribute to the successful and widespread use of polymeric systems. There is a continued need to develop new, safe, and effective polymers although a variety of polymeric substances are available to serve as mucoadhesive and controlled release component (1).
Natural polymers are easily available and when employed in controlled release drug delivery systems have some advantages such as bioacceptability, biocompatibility, bio-degradability and nontoxicity. Mucilages are most commonly used adjuvant in pharmaceutical preparations as binding, disintegrating, sus-pending, emulsifying and sustaining agents because of their low cost, ready availability, non-toxicity and non-irritancy (2,3). They consist of sugar and uronic acid units. They swell in water and form a gel (1).
Calendula officinalis L. (var. prolifera Hort) commonly known as pot marigold, is an aromatic annual plant that belongs to the Asteraceae (Compositae) family (4).
Calendula is native to the Mediterranean area (some believe it comes from Egypt) although it is widely spread throughout the world as an ornamental plant (5).
On chemical analysis, the Calendula flowers were found to contain a volatile oil, many bitter chemical principles, different types of carotenoids, a lot of mucilage, plant resin, all kinds of polysaccharides, plant acids, variety of alcoholic compounds, saponins as well as other glycosides and different kinds of sterols (6).
Mucoadhesive drug delivery systems were developed to sustain drug delivery via various mucus membranes for either local or systemic delivery of poorly absorbed drugs such as peptides and proteins (7,8,9) as well as drugs that are subjected to high first-pass metabolism (10,11,12). Target sites include various mucus membranes such as the gastrointestinal tract (13,14), eye (15), cervix (16), vagina (17), nasal cavities (18) and oral cavities (8,9,19,20). Mucoadhesive agents also increase residence time of the delivery system and provide intimate contact between the dosage form and the mucus membrane of interest, leading to increased drug transport. Such a method of drug delivery is less invasive and serves as an alternate to the parenteral administration.
Most of the mucoadhesive materials are either synthetic or natural, hydrophilic or water-insoluble polymers and are capable of forming numerous hydrogen bonds because of the presence of carboxyl, sulfate, hydroxyl and amino functional groups. Formation of hydrogen bonds amongst the functional groups of the polymers and mucosal layer plays an important role. In general, stronger the hydrogen bonding, stronger is the adhesion. Various synthetic materials tested for mucoadhesion include Carbopol 934P, Carbopol 974P, sodium carboxymethyl cellulose (Sodium CMC), hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose (HPC), polymethyl methacrylates (PMMA), and polycarbophil, whereas natural materials tested for mucoadhesive properties include carageenan, xanthan gum, sodium alginate, gelatin, acacia, and tragacanth (21). All mucoadhesive materials interact with oligosaccharide molecules in the mucus layer that covers the mucosal epithelial surface.
The simplest dosage forms for delivery of drugs through the mucosa of the oral cavity are the conventional formulations like lozenges, troches, gels, oral rinses, or mouthwashes would be (22,23). However, these conventional dosage forms have two major disadvantages which consist of an initial burst of activity followed by a rapid decrease in concentration (24,25) and limited stability due to the constant flow of saliva and the mobility of the involved tissues. Buccal mucoadhesive formulations which control the drug release are expected to overcome these problems.
The buccal cavity has a very limited surface area of around 50 cm2 but the easy access to the site makes it a preferred location for delivering active agents. The site provides an opportunity to deliver pharmacologically active agents systemically by avoiding hepatic first-pass metabolism in addition to the local treatment of the oral lesions. The sublingual mucosa is relatively more permeable than the buccal mucosa (due to the presence of large number of smooth muscle and immobile mucosa), hence formulations for sublingual delivery are designed to release the active agent quickly while mucoadhesive formulation is of importance for the delivery of active agents to the buccal mucosa where the active agent has to be released in a controlled manner. This makes the buccal cavity more suitable for mucoadhesive drug delivery (26).
In the present study, an attempt was made to isolate and characterize the mucilage from Calendula flowers and also to evaluate the mucilage for its controlled release and mucoadhesive properties. Chlorpheniramine maleate was used as a model drug to evaluate the sustained-release potential of the mucilage. It has been used extensively as an antihistamine for symptomatic relief of the common cold and allergy (27).
MATERIALS AND METHODS
Materials
Chlorpheniramine maleate was received as gift sample from Alembic Ltd. Vadodara, Gujarat. Flowers were procured from local nursery of Vadodara. Ethyl alcohol, Methocel K4M, Carbopol 974P were procured form Loba Chem (Mumbai, India) and used as received. All other reagents used were of analytical grade.
Isolation of mucilage from Calendula flowers
The Calendula flowers and the petals were sun dried for 10 days. The dried petals were soaked in water for 5–6 h, boiled for 30 min and left to stand for 1 h to allow complete release of the mucilage into the water. The mucilage was extracted using a multi-layer muslin cloth bag to remove the marc from the solution. Ethanol (three times the volume of filtrate) was added to precipitate the mucilage. The mucilage was separated, dried in an oven at 35°C, collected, grounded, passed through a # 80 sieve and stored in a desiccator till use (28,29).
Characterization of isolated Calendula mucilage
The isolated dried mucilage was studied for percentage yield and characterized for various physiochemical parameters such as solubility, weight loss on drying (30), thin layer chromatography (31,32), viscosity, pH, swelling index and total carbohydrate content (33,34).
Compatibility study
Mixtures consisting of different ratios of CPM/Calendula mucilage, and either CPM or Calendula mucilage alone were subjected to FT-IR analysis using a model BRUKER ALPHA T FT-IR spectrophotometer (Bruker Optik GmbH, Germany).
Comparative mucoadhesive characterization of Calendula mucilage with Methocel K4M, Carbopol 974P as standard polymers; shear stress measurement
Different concentrations of the mucoadhesive agent solution, such as, 1, 2, and 3% w/v, using Methocel K4M, Carbopol 974P, and a natural isolated Calendula mucilage were prepared. Shear stress was calculated by self-fabricated apparatus made of wooden board with scale and two glass slides having two pans on the both sides mounted on a pulley. An excess of prepared solution was placed between two glass slides and 1000 g weight was placed on glass slide for 5 min to compress the sample to uniform thickness. Weight (250 g) was added to the pan. The weight required to separate two slides was taken as a measure of shear stress (35).
Formulation and evaluation of buccal tablets containing Calendula mucilage, Methocel K4 M and Carbopol 974 P
Three batches of tablets each containing 8 mg of CPM as model drug were prepared changing the quantity of mucoadhesive component (16.66%, 33.33% and 50%) by direct compression method using flat face 6-mm punch (Rimek Mini Press-I machine), resulting in 9 different formulations (CF1, CF2, CF3 for Carbopol 974P; CMF1, CMF2, CMF3 for Calendula mucilage; HF1, HF2, HF3 for Methocel K4M). The tablet weight was adjusted to 150 mg. (Table 4). The prepared tablets were evaluated for average thickness, hardness, friability test, weight variation test and mucoadhesive strength measurement (36,37).
Table 4.
Evaluation of tablets

Dissolution testing
Dissolution studies were performed using a USP dissolution apparatus 2 (paddle method) at 50 rpm. The dissolution medium consisted of 900 ml phosphate buffer (pH 6.8) at 37°C. Samples (sampling interval 60 min) were analyzed for CPM by UV spectrophotometer at 262 nm. Tablets were tested and the experiments were performed in triplicate. For each formulation, the time to reach 90% of CPM release (t90%) was calculated from the mean dissolution data according to the respective dissolution curve.
The tablet was designed to absorb water and swell, changing into a gelling mass that would release a high percentage of the drug before disintegration occur. Therefore the drug release from a tablet can be considered as release from a swelling matrix rather than a release from a disintegrating matrix. The release kinetics of each tablet can be assessed by inserting the experimental data in the semi-empirical equation Mt/M∞=Ktn where Mt/M∞ is the fractional amount of the drug at the time t, K is a kinetic constant of the system which indicates rate of the release and the n is the release exponent, indicative of the mechanism of release. Values for n and K for each system were obtained from the logarithmic plot of the fractional release against the time, considering data between the first withdrawal at 30 min and the one corresponding to the release of 60% of the dose (38). The slope of the line is n while log K is the intercept. The values of n and K were calculated by regression analysis and the statistical parameter R2 was established to evaluate the fitting of the semi-empirical equation to the release kinetics.
Mucoadhesion studies
The aim of this study was to quantitate the force of detachment (mucoadhesive strength) of CPM buccal tablets applied to freshly excised goat buccal mucosa as a model membrane. The force of detachment was measured in grams by using self fabricated apparatus (modified physical balance.
In Vitro drug permeation
The in vitro buccal drug permeation studies of CPM through the goat buccal mucosa were done by using modified Franz diffusion cell at 37 °C ± 0.5 °C (diameter of 1.5 cm with a diffusional area of 1.76 cm2). Fresh goat buccal mucosa was mounted between the donor and receptor compartments. The buccal tablet was placed in donor compartment with the core facing the mucosa and the compartments clamped together. The receptor compartment 15 ml capacity) was filled with phosphate buffer of pH 6.8. The temperature of media was maintained at 37 ± 0.5° C with the help of temperature controlled water jacket and the hydrodynamics in the receptor compartment was maintained by stirring with a magnetic bead at 100 rpm. A 2 ml sample was withdrawn at predetermined time intervals and analyzed for drug content at 262 nm using a UV spectrophotometer. The volume of release media was maintained by adding equal volume of the fresh media after every sampling.
A test on the reference suspension was carried out by placing 2 ml of the suspension in the donor compartment. The suspension was obtained by adding an excess of drug in purified water at room temperature. The system was heated up to 50°C in order to dissolve the drug and then equilibrated at 37° C ± 0.5° C for 24 h (40,41).
Permeation through the membrane can be considered as a passive diffusion process and can be described by Fick's law equation:
Js= dQr/ Adt
where, Js is the steady-state buccal mucosa flux in mcg/cm2 per h, dQr is the change in quantity of material passing through the membrane into the receptor compartment expressed in mcg, A is the active diffusion area in cm2, and dt is the change in time in hours. The steady state flux of CPM through the goat buccal mucosa was calculated from the slope of the linear portion of the cumulative amount permeated through the membrane per unit area versus time plot. For the CPM suspension the permeability coefficient was calculated using the equation:
Kp= Js/ Cd
where, Kp is the permeability coefficient, Js is the flux calculated at the steady-time and Cd is the donor concentration (42).
RESULTS
The mucilage was isolated and characterized for various physicochemical properties. The results are shown in Table 1.
Table 1.
Physicochemical parameters of Calendula flower mucilage

Fig. 1 depicts the results of compatibility study. The FT-IR spectrum of Calendula mucilage showed broad characteristics peak at 2424.98 cm-1 due to O-H intramolecular hydrogen bonding, sharp peaks at 1621.94 cm-1 C-C bond stretching vibrations, 1202 cm-1 is present due to C-N aliphatic vibrations and 616.28 cm-1 is due to C=C bending vibrations. The FT-IR spectrum of CPM showed characteristic bands at 3018 cm-1 aromatic C-H stretching vibrations, 2453 cm-1 due to C-H stretching of alkane, 1704 cm-1 due to C=O stretch, 1620 due to C=C stretching, 1587 cm-1 due to C=N stretching, 1480 cm-1 due to C-H stretching, 1335 cm-1 due to C-H bending vibrations. A sharp band can be observed at 866 and 834 due to C-C stretching vibration of maleate and 760 cm-1 due to C-Cl stretching vibration.
Fig. 1.
FT-IR spectra of CPM-Calendula mucilage (CM).
While demonstrating shear stress measurement Calendula mucilage was found to possess comparable and remarkable adhesiveness to that of Methocel K4M and less adhesiveness than Carbopol 974P within 60 min. The results of shear stress measurement are shown in Table 2.
Table 2.
Shear stress measurement

Three batches of tablets each containing 8 mg of CPM as model drug were prepared with different quantity of mucoadhesive component (16.66 %, 33.33 % and 50 %) by direct compression method, resulting in 9 different formulations (Table 3). The results of evaluation of tablets are shown in Table 4. The thickness, hardness, average weight and drug content percent of all formulations CF1, CF2, CF3, CMF1, CMF2, CMF3, HF1, HF2, and HF3 was in the range of 2.20 ± 0.3 to 2.45 ± 0.05 mm, 4 to 6 Kg/Cm2, 148 ± 0.81 to 151 ± 0.86, 97.5 ± 0.5 to 99.7 ± 0.6 respectively and friability percentage in all these formulations was found to be less than 0.1%.
Table 3.
Composition of tablets

The results for mucoadhesion studies are shown in Table 5. The mucoadhesive strength of Calendula mucilage was comparable to that of Methocel K4M.
Table 5.
Mucoadhesive strength determination

The percentage of drug released from tablets containing the different mucoadhesive agents at different concentrations is depicted in Figs. 2, 3, and 4. The values of n, K and R2 of these release rates are represented in Table 6.
Fig. 2.

Release profile of tablets containing Methocel K4M in 16.66 %, 33.33 % and 50 % quantity.
Fig. 3.

Release profile of tablets containing Carbopol 974P in 16.66 %, 33.33 % and 50 % quantity.
Fig. 4.

Release profile of tablets containing Calendula mucilage in 16.66 %, 33.33 % and 50 % quantity.
Table 6.
n and k values of the different tablets

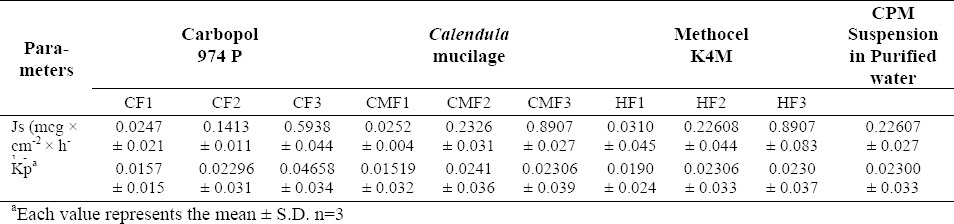
Fig. 5 depicts the comparative drug release between tablets containing the three mucoadhesive polymers, at highest concentration (formulation CF3, CMF3, HF3). The permeation profile of the CPM suspension in water is shown in Fig. 6, while Figs. 7, 8, and 9 show permeation profiles of tablets. Permeation tests from the CPM suspension showed a Kp value of 8.99 × 10-2 corresponding to a flux of 0.1799 mcg × cm-2 × h-1. The fluxes and Kp values in these profiles are reported in Table 7 that also shows the values for the CPM suspension in water.
Fig. 5.
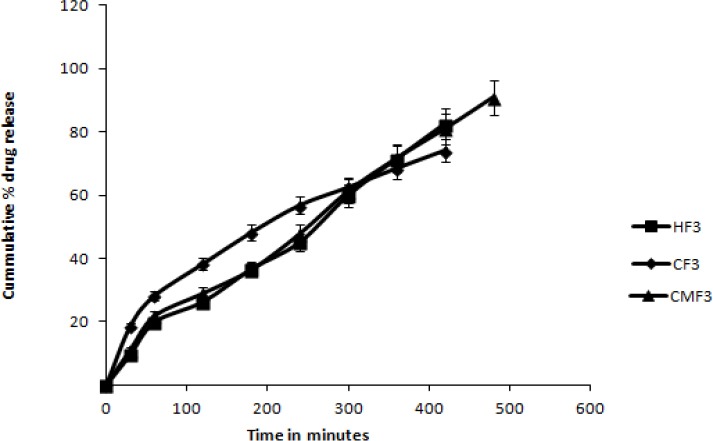
Comparison between release profile of tablets containing Methocel K4M, Carbopol 974P and Calendula mucilage at higher quantity.
Fig. 6.
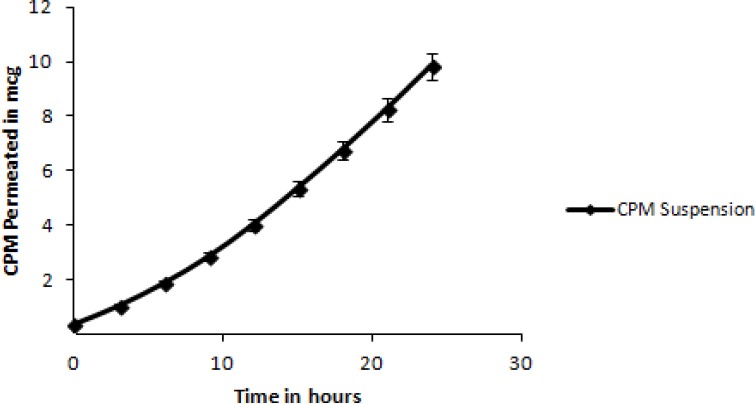
Cumulative amount of permeated CPM from a saturated solution in purified water.
Fig. 7.

Cumulative amount of permeated CPM from the tablets (CF1, CMF1 and HF1) containing Carbopol 974P, Calendula mucilage and Methocel K4M.
Fig. 8.

Cumulative amount of permeated CPM from the tablets (CF2, CMF2 and HF2) containing Carbopol 974P, Calendula mucilage and Methocel K4M.
Fig. 9.

Cumulative amount of permeated CPM from the tablets (CF3, CMF3 and HF3) containing Carbopol 974P, Calendula mucilage and Methocel K4M.
Table 7.
Flux (Js) and Kp values of CPM from tablets and from the suspension in purified water
DISCUSSION
The main aim of this work was to isolate and evaluate mucilage from Calendula flowers for its controlled release and mucoadhesive properties in buccal tablets. Carbopol 974P, Methocel K4M and natural Calendula mucilage were selected as buccoadhesive polymers. Calendula flowers yielded 10-11% w/w mucilage using alcohol as mucilage precipitating solvent. The isolated mucilage was characterized for various physicochemical properties and specifications were set as per the Pharmacopoeial guidelines (34). The mucilage was found to be water miscible and it formed colloidal solution in water. The mucilage was tested for presence of carbohydrates and a positive result was obtained. Total carbohydrates content was found to be 79.08%. The pH was found 6.3, indicating that the Calendula mucilage might not irritate the epithelium and mucus membrane of oral cavity (30,31,32,33,34). All other physicochemical parameters conferred to the pharmacopoeial guidelines.
Results of FT-IR spectroscopy studies suggested the absence of a chemical interaction between CPM and Calendula mucilage.
All the formulations pass test for weight variation, hardness, content uniformity and show acceptable results with respect to drug content (99.7 ± 0.6) and friability percent.
The mucoadhesive characteristics were affected by ratio of mucoadhesive agents. Due to a higher concentration of the isolated Calendula mucilage in formulation CMF3, it showed more mucoadhesive strength than CMF1 and CMF2. The highest mucoadhesive strength may be due to possibility of proper hydration and erosion of natural polymer adhered to mucosal surface with strong bond which have been supported by maximum mucoadhesive force.
From the R2 values, it is observed that the semi-empirical equation described by Ritger and Peppas is able to fit the release from tablets containing all the three polymers. The polymers showed a better modulation capacity, with a release on average, of 37 and 39% respectively during two h. This aspect is shown by the comparison between tablets containing three mucoadhesive polymers, at highest concentration (formulations CF3, CMF3, HF3) in Fig. 4. Tablets containing Methocel K4M and Calendula mucilage showed a controlled release, characterized by an exponent n that changed according to the type of mucoadhesive polymer. For tablets containing Methocel K4M, n was between 0.4759 ± 0.01 and 0.4827 ± 0.07 and for Calendula mucilage between 0.4880 ± 0.01 and 0.4787 ± 0.03. An “n” value of 0.5 indicates a Fickian process that describes release of a drug from a matrix governed by diffusion.
Tablet permeation profiles are lower than those obtained from the suspension (tablet fluxes as a whole fell under 0.0575 ± 0.002 and 0.1789 ± 0.033 mcg × cm-2 × h-1) which can be explained when considering that CPM present in tablets must be dissolved and released before permeation occurs. From the comparison of profiles of different tablets, it is observed that changing the mucoadhesive component, permeability behavior was not statistically different (P>0.05). The higher fluxes shown by Methocel K4M and Calendula mucilage can be explained by its rapid deaggregation. On the other hand, in all tablets, the cumulative amount of permeated CPM increased in respect to the concentration of the mucoadhesive polymer, probably because an increase in the mucoadhesive component allowed a closer contact between the tablet and the mucosa.
CONCLUSION
FTIR studies showed that there was no interaction between the CPM and the Calendula mucilage. The CPM release kinetics showed that tablets containing Calendula mucilage were the better formulations because they showed a prolonged drug release with linear kinetics, comparable to Methocel K4M. Permeability tests indicated that all tablets showed a satisfactory drug permeability flux, compared with the flux from a saturated solution of drug in water. The permeability behavior was not statistically different (P>0.05) on changing the mucoadhesive component.
In conclusion, the developed mucoadhesive tablets for buccal administration containing Calendula mucilage (CMF3) showed controlled drug release.
Our future studies will be directed at determining the bioavailability of CPM from the prototype Calendula mucilage based buccal tablets following application to the buccal mucosa of rabbits.
ACKNOWLEDGMENT
The authors are thankful to Dr. Pradip Wahile (The Alembic Ltd. Vadodara) for the kind supply of CPM as gift sample and Dr. Devanshu Patel (Managing Trustee, Parul Trust) for providing the facilities to carry out the research work.
REFERENCES
- 1.Trease GE, Evans MC. 15th ed. London: Balliere Tindall; 2002. Textbook of Pharmacognosy; pp. 206–212. [Google Scholar]
- 2.Baveja SK, Rao KV, Arora J. Examination of natural gums and mucilages as sustaining materials in tablet dosage forms, Part 2. Indian J Pharm Sci. 1989;51:115–118. [Google Scholar]
- 3.Ahsan SK, Tariq M, Ageel AM, Al-yahya MA, Shah AH. Effect of Trigonella foenum graecum and Ammi majus on calcium oxalate urolithiasis in rats. J Ethnopharmacol. 1989;26:249–254. doi: 10.1016/0378-8741(89)90097-4. [DOI] [PubMed] [Google Scholar]
- 4.Cetkovic GS, Djilas SM, Canadanovic-Brunet JM, Tumbas VT. Antioxidant properties of marigold extracts. Food Res Int. 2004;37:643–650. [Google Scholar]
- 5.Loggia RD, Tubaro A, Sosa1 S, Becker H, Saar St, Isaac O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Plant Med. 1994;60:516–520. doi: 10.1055/s-2006-959562. [DOI] [PubMed] [Google Scholar]
- 6.Garcia D, Sanchez E, Crespo M, Carballo C. Estudio farmacognóstico de caléndula. rev. Cubana Plant Med. 1996;1:21–25. [Google Scholar]
- 7.Dowty ME, Knuth KE, Irons BK, Robinson JR. Transport of thyrotropin releasing hormone in rabbit buccal mucosa in vitro. Pharm Res. 1992;9:1113–1122. doi: 10.1023/a:1015883217858. [DOI] [PubMed] [Google Scholar]
- 8.Li CR, Koch L, Raul VA, Bhatt PP, Johnston TP. Absorption of thyrotropin-releasing hormone in rats using a mucoadhesive buccal patch. Drug Dev Ind Pharm. 1997;23:239–246. [Google Scholar]
- 9.Li C, Bhatt PP, Johnston TP. Transmucosal delivery of oxytocin to rabbits using a mucoadhesive buccal patch. Pharm Dev Technol. 1997;2:265–274. doi: 10.3109/10837459709031446. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda K, Murata K, Kobayashi M, Noda K. Enhancement of bioavailability of dopamine via nasal route in beagle dogs. Chem Pharm Bull. 1992;40:2155–2158. doi: 10.1248/cpb.40.2155. [DOI] [PubMed] [Google Scholar]
- 11.Konda S, Sugimoto I. Moment analysis of intravenous, intraduodenal, buccal, rectal, and percutaneous nifedipine in rats. J Pharmacobio Dyn. 1987;10:462–469. doi: 10.1248/bpb1978.10.462. [DOI] [PubMed] [Google Scholar]
- 12.Hoskin PJ, Hanks GW, Aherne GW, Chapman D, Littleton P, Filshie J. The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. Br J Clin Pharmacol. 1989;27:499–505. doi: 10.1111/j.1365-2125.1989.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ch’ng HS, Park H, Kelly P, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery II: Synthesis and evaluation of some swelling, water insoluble bioadhesive polymers. J Pharm Sci. 1985;74:399–405. doi: 10.1002/jps.2600740407. [DOI] [PubMed] [Google Scholar]
- 14.Harris D, Fell JT, Sharma H, Taylor DC, Linch J. Studies on potential bioadhesive systems for oral drug delivery. STP Pharma. 1989;5:852–856. [Google Scholar]
- 15.Robinson JR. Ocular drug delivery: Mechanisms of corneal transport and mucoadhesive delivery systems. STP Pharma. 1989;5:838–846. [Google Scholar]
- 16.Nagai T. Topical mucosal adhesive dosage forms. Med Res Rev. 1986;6:227–242. doi: 10.1002/med.2610060205. [DOI] [PubMed] [Google Scholar]
- 17.Gorsoy KI, Sohtorik N, Uyanik, Peppas NA. Bioadhesive controlled release systems for vaginal delivery. STP Pharma. 1989;5:886–892. [Google Scholar]
- 18.Nagai T, Nishimoto Y, Nambu N, Suzuki Y, Skeine K. Powder dosage forms of insulin for nasal administration. J Controlled Release. 1984;1:15–22. [Google Scholar]
- 19.Nagai T, Konishi R. Buccal/gingival drug delivery systems. J Controlled Release. 1987;6:353–360. [Google Scholar]
- 20.Li C, Bhatt PP, Johnston TP. In vitro release and permeation of oxytocin from a mucoadhesive buccal patch. Pharm Dev Technol. 1996;1:357–364. doi: 10.3109/10837459609031430. [DOI] [PubMed] [Google Scholar]
- 21.Smart JD, Kellaway IM, Worthington HEC. An in vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. J Pharm Pharmacol. 1984;36:295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- 22.Harris D, Robinson JR. Drug delivery via the mucous membranes of the oral cavity. J Pharm Sci. 1992;81:1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- 23.Zegarelli DJ. Mouthwashes in the treatment of oral disease. Drugs. 1991;42:171–173. doi: 10.2165/00003495-199142020-00001. [DOI] [PubMed] [Google Scholar]
- 24.Needleman P, Lang S, Johnson EM. Organic nitrates, relationship between biotrasformation and rational angina pectoris therapy. J Pharmacol Exp Ther. 1972;181:489–497. [PubMed] [Google Scholar]
- 25.Niitani HH, Takano TT, Tanaka KK, Hiramori KK, Kimata SS, Ikeda MM. Effect of isosorbide dinitrate tape (TY-0081) on congestive heart failure: results of multicenter study. Kokyu To Junkan. 1984;32:841–847. [PubMed] [Google Scholar]
- 26.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: A Review. J Pharma Sci. 1998;1:15–30. [PubMed] [Google Scholar]
- 27.Rumore MM. Clinical pharmacokinetics of chlorpheniramine. Drug Intell Clin Pharmacy. 1984;18:701–707. doi: 10.1177/106002808401800905. [DOI] [PubMed] [Google Scholar]
- 28.Baveja SK, Rao KV, Arora J. Examination of natural gums and mucilages as sustaining materials in tablet dosage forms. Indian J Pharm Sci. 1988;50:89–92. [Google Scholar]
- 29.Girish K Jani, Dhiren P Shah. Gum and mucilages: versatile excipients for pharmaceutical formulations. Asian journal of pharmaceutical science. 2009;4:309–323. [Google Scholar]
- 30.4th ed. New Delhi: Controller of Publications; 1996. Indian Pharmacopoeia; pp. A53–A54. [Google Scholar]
- 31.Vogel AI. 4th ed. New York: Prentice Hall; 1978. A textbook of practical organic chemistry; pp. 207–210. [Google Scholar]
- 32.Nekrasov VV. 1st ed. Moscow: Mir Publisher; 1978. Practical organic chemistry: a basic course; p. 204. [Google Scholar]
- 33.Scott JE. Methods in carbohydrate chemistry. In: Whistler RL, editor. V. New York: Academic Press; 1965. pp. 38–44. [Google Scholar]
- 34.British Pharmacopoeia. I & II. London, UK: British Pharmacopoeia Commission; 2003. pp. 44–45. [Google Scholar]
- 35.Sabale VP, Sabale PM, Lakhotiya CL. Comparative evaluation of rice bran wax as ointment base with standard base. Indian J Pharm Sci. 2009;71:77–79. doi: 10.4103/0250-474X.51965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vol. 4. London, UK: Her majesty's stationary office for the department of health; 2009. British Pharmacopoeia; pp. A313–314. [Google Scholar]
- 37.Ceschel GC, Maffei P, Lombardi BS. Design and evaluation of buccal adhesive hydrocortisone acetate hca tablets. Drug Delivery. 2001;8:161–171. doi: 10.1080/107175401316906937. [DOI] [PubMed] [Google Scholar]
- 38.Philip L, Ritger Peppas NA. A simple equation for description of solute release ii. Fickian and anomalous release from swellable devices. J Controlled Release. 1987;5:37–42. [Google Scholar]
- 39.Chandrasekara MJN, Maheshkumara S, Manikandanb D, Nanjanb MJ. Isolation and evaluation of a polysaccharide from Prunus amygdalus as acarrier for transbuccosal delivery of losartan potassium. Int J Biol Macromol. 2011;48:773–778. doi: 10.1016/j.ijbiomac.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Santoyo S, Arellano A, Ygartua P, Martin C. Penetration enhancer effects on the in vitro percutaneous absorption of piroxicam through rat skin. Int J Pharm. 1995;117:219–224. [Google Scholar]
- 41.Bronaugh RL, Maibach HI. In vitro percutaneous absorption: principles, fundamentals and applications. In: Williams DF, editor. Boca Raton, Florida: CRC Press; 1991. p. 146. [Google Scholar]
- 42.Zhang H, Robinson JR. In vitro methods for measuring permeability of the oral mucosa. In: Rathbone MJ, editor. Oral Mucosa Drug Delivery. New York: Marcel Dekker; 1996. pp. 85–100. [Google Scholar]



