Abstract
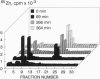
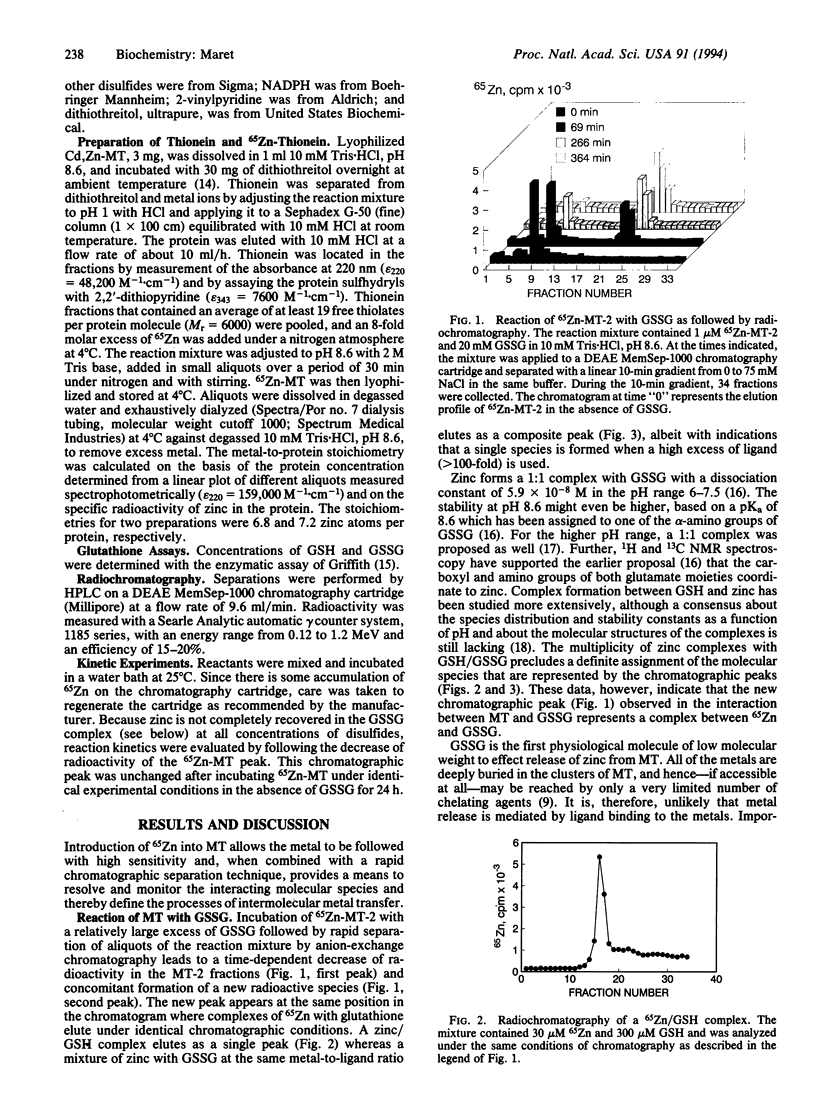
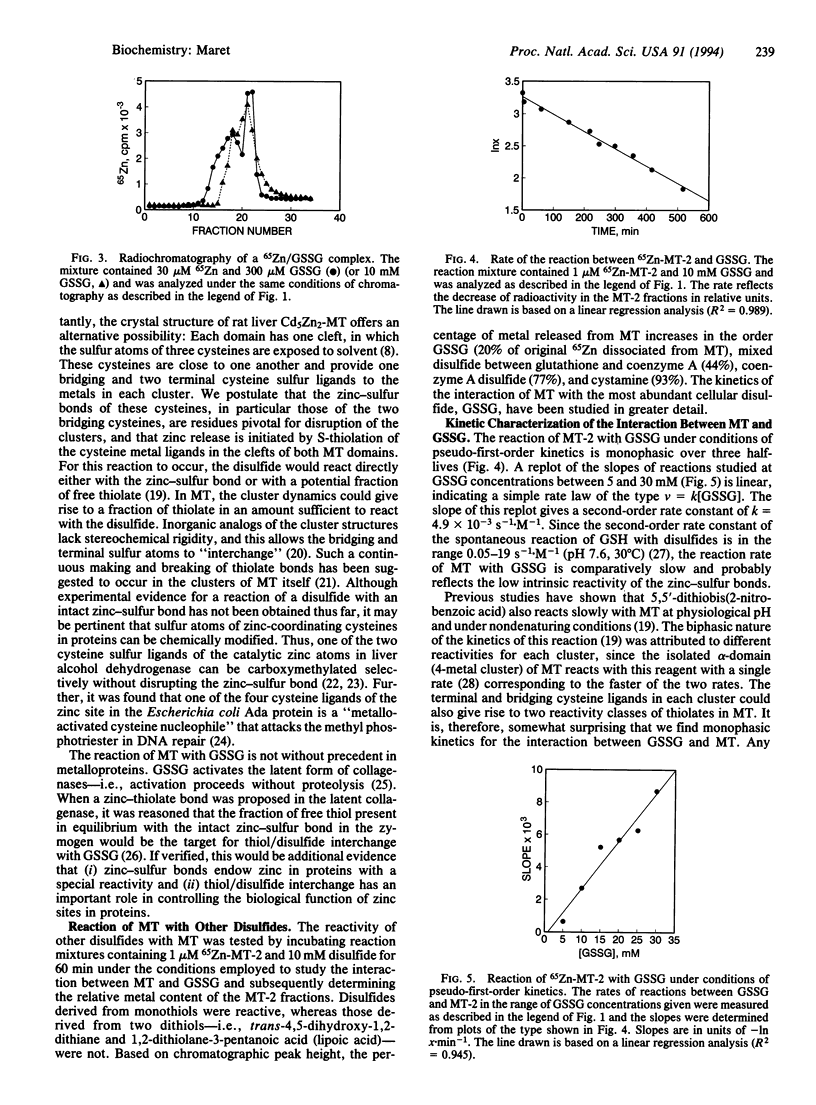
Mammalian metallothionein has been postulated to play a pivotal role in cellular zinc distribution. All seven of its metal atoms are bound with high thermodynamic stability in two clusters buried deeply in the molecule. If the protein is to function in metal delivery, there must be a biological mechanism to facilitate metal release. One means to achieve this would be a labilization of the clusters by interaction of metallothionein with an appropriate cellular ligand. To search for such a mediator, we have designed a rapid radiochromatographic method that can detect changes in the zinc content of 65Zn-labeled metallothionein in response to other biomolecules. Using this methodology, we have established that rabbit liver metallothionein 2 interacts with glutathione disulfide with concomitant release of zinc. Under conditions of pseudo-first-order kinetics, the monophasic reaction depends linearly on the concentration of glutathione disulfide in the range from 5 to 30 mM with a second-order rate constant k = 4.9 x 10(-3)s-1.M-1 (pH 8.6; 25 degrees C). Apparently, zinc release does not involve direct access of glutathione disulfide to the inner coordination sphere of the metals. Rather it appears that the solvent-accessible zinc-bound thiolates in two clefts of each domain of metallothionein [Robbins, A. H., McRee, D. E., Williamson, M., Collett, S. A., Xuong, N. H., Furey, W. F., Wang, B. C. & Stout, C. D. (1991) J. Mol. Biol. 221, 1269-1293] participate in a thiol/disulfide interchange with glutathione disulfide. This rate-limiting initial S-thiolation, which occurs with indistinguishable rates in both clusters, then causes the clusters to collapse and release their zinc. Such a mechanism of metal release would link the control of the metal content of metallothionein to the cellular glutathione redox status and raises important questions about the physiological implications of this observation with regard to a role of glutathione in zinc metabolism and in making zinc available for other biomolecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson K., Eriksson S., Mannervik B. Purification and characterization of cytoplasmic thioltransferase (glutathione:disulfide oxidoreductase) from rat liver. Biochemistry. 1978 Jul 25;17(15):2978–2984. doi: 10.1021/bi00608a006. [DOI] [PubMed] [Google Scholar]
- Bali P. K., Aisen P. Receptor-modulated iron release from transferrin: differential effects on N- and C-terminal sites. Biochemistry. 1991 Oct 15;30(41):9947–9952. doi: 10.1021/bi00105a019. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Vairetti M., Stivala L., Mirabelli F., Richelmi P., Orrenius S. Demonstration of nuclear compartmentalization of glutathione in hepatocytes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4412–4416. doi: 10.1073/pnas.89.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramini M., Lerch K. Copper transfer between Neurospora copper metallothionein and type 3 copper apoproteins. FEBS Lett. 1982 Jun 7;142(2):219–222. doi: 10.1016/0014-5793(82)80138-5. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Brouwer-Hoexum T. Glutathione-mediated transfer of copper(I) into American lobster apohemocyanin. Biochemistry. 1992 Apr 28;31(16):4096–4102. doi: 10.1021/bi00131a028. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Hoexum-Brouwer T., Cashon R. E. A putative glutathione-binding site in CdZn-metallothionein identified by equilibrium binding and molecular-modelling studies. Biochem J. 1993 Aug 15;294(Pt 1):219–225. doi: 10.1042/bj2940219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A. M., Ciriolo M. R., Marcocci L., Rotilio G. Copper(I) transfer into metallothionein mediated by glutathione. Biochem J. 1993 Jun 15;292(Pt 3):673–676. doi: 10.1042/bj2920673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss H., Ménard M. Hypochlorous acid-induced mobilization of zinc from metalloproteins. Arch Biochem Biophys. 1991 May 15;287(1):175–179. doi: 10.1016/0003-9861(91)90403-6. [DOI] [PubMed] [Google Scholar]
- Fliss H., Ménard M. Oxidant-induced mobilization of zinc from metallothionein. Arch Biochem Biophys. 1992 Feb 14;293(1):195–199. doi: 10.1016/0003-9861(92)90384-9. [DOI] [PubMed] [Google Scholar]
- Freedman J. H., Ciriolo M. R., Peisach J. The role of glutathione in copper metabolism and toxicity. J Biol Chem. 1989 Apr 5;264(10):5598–5605. [PubMed] [Google Scholar]
- Gilbert H. F. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Shapiro B. M. The respiratory burst oxidase of fertilization. A physiological target for regulation by protein kinase C. J Biol Chem. 1992 Apr 25;267(12):7959–7962. [PubMed] [Google Scholar]
- Hwang C., Sinskey A. J., Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992 Sep 11;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Krezoski S. K., Villalobos J., Shaw C. F., 3rd, Petering D. H. Kinetic lability of zinc bound to metallothionein in Ehrlich cells. Biochem J. 1988 Oct 15;255(2):483–491. [PMC free article] [PubMed] [Google Scholar]
- Kägi J. H., Schäffer A. Biochemistry of metallothionein. Biochemistry. 1988 Nov 15;27(23):8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- LI T. K., VALLEE B. L. Selective carboxymethylation of functional sulfhydryl groups at the active center of horse liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1963 Jul 10;12:44–49. doi: 10.1016/0006-291x(63)90411-x. [DOI] [PubMed] [Google Scholar]
- Li T. Y., Minkel D. T., Shaw C. F., 3rd, Petering D. H. On the reactivity of metallothioneins with 5,5'-dithiobis-(2-nitrobenzoic acid). Biochem J. 1981 Feb 1;193(2):441–446. doi: 10.1042/bj1930441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macartney H. W., Tschesche H. Latent and active human polymorphonuclear leukocyte collagenases. Isolation, purification and characterisation. Eur J Biochem. 1983 Jan 17;130(1):71–78. doi: 10.1111/j.1432-1033.1983.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Myers L. C., Terranova M. P., Ferentz A. E., Wagner G., Verdine G. L. Repair of DNA methylphosphotriesters through a metalloactivated cysteine nucleophile. Science. 1993 Aug 27;261(5125):1164–1167. doi: 10.1126/science.8395079. [DOI] [PubMed] [Google Scholar]
- Nettesheim D. G., Engeseth H. R., Otvos J. D. Products of metal exchange reactions of metallothionein. Biochemistry. 1985 Nov 19;24(24):6744–6751. doi: 10.1021/bi00345a003. [DOI] [PubMed] [Google Scholar]
- O'Halloran T. V. Transition metals in control of gene expression. Science. 1993 Aug 6;261(5122):715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Engeseth H. R., Nettesheim D. G., Hilt C. R. Interprotein metal exchange reactions of metallothionein. Experientia Suppl. 1987;52:171–178. doi: 10.1007/978-3-0348-6784-9_10. [DOI] [PubMed] [Google Scholar]
- Postal W. S., Vogel E. J., Young C. M., Greenaway F. T. The binding of copper(II) and zinc(II) to oxidized glutathione. J Inorg Biochem. 1985 Sep;25(1):25–33. doi: 10.1016/0162-0134(85)83004-x. [DOI] [PubMed] [Google Scholar]
- Robbins A. H., McRee D. E., Williamson M., Collett S. A., Xuong N. H., Furey W. F., Wang B. C., Stout C. D. Refined crystal structure of Cd, Zn metallothionein at 2.0 A resolution. J Mol Biol. 1991 Oct 20;221(4):1269–1293. [PubMed] [Google Scholar]
- Springman E. B., Angleton E. L., Birkedal-Hansen H., Van Wart H. E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a "cysteine switch" mechanism for activation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Cocatalytic zinc motifs in enzyme catalysis. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2715–2718. doi: 10.1073/pnas.90.7.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Coleman J. E., Auld D. S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L. Implications and inferences of metallothionein structure. Experientia Suppl. 1987;52:5–16. doi: 10.1007/978-3-0348-6784-9_1. [DOI] [PubMed] [Google Scholar]
- Vasák M. Dynamic metal-thiolate cluster structure of metallothioneins. Environ Health Perspect. 1986 Mar;65:193–197. doi: 10.1289/ehp.65-1474703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeppezauer E., Jörnvall H., Ohlsson I. Carboxymethylation of horse-liver alcohol dehydrogenase in the crystalline state. The active-site zinc region and general anion-binding site of the enzyme correlated in primary and teritiary structures. Eur J Biochem. 1975 Oct 1;58(1):95–104. doi: 10.1111/j.1432-1033.1975.tb02353.x. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]