Abstract
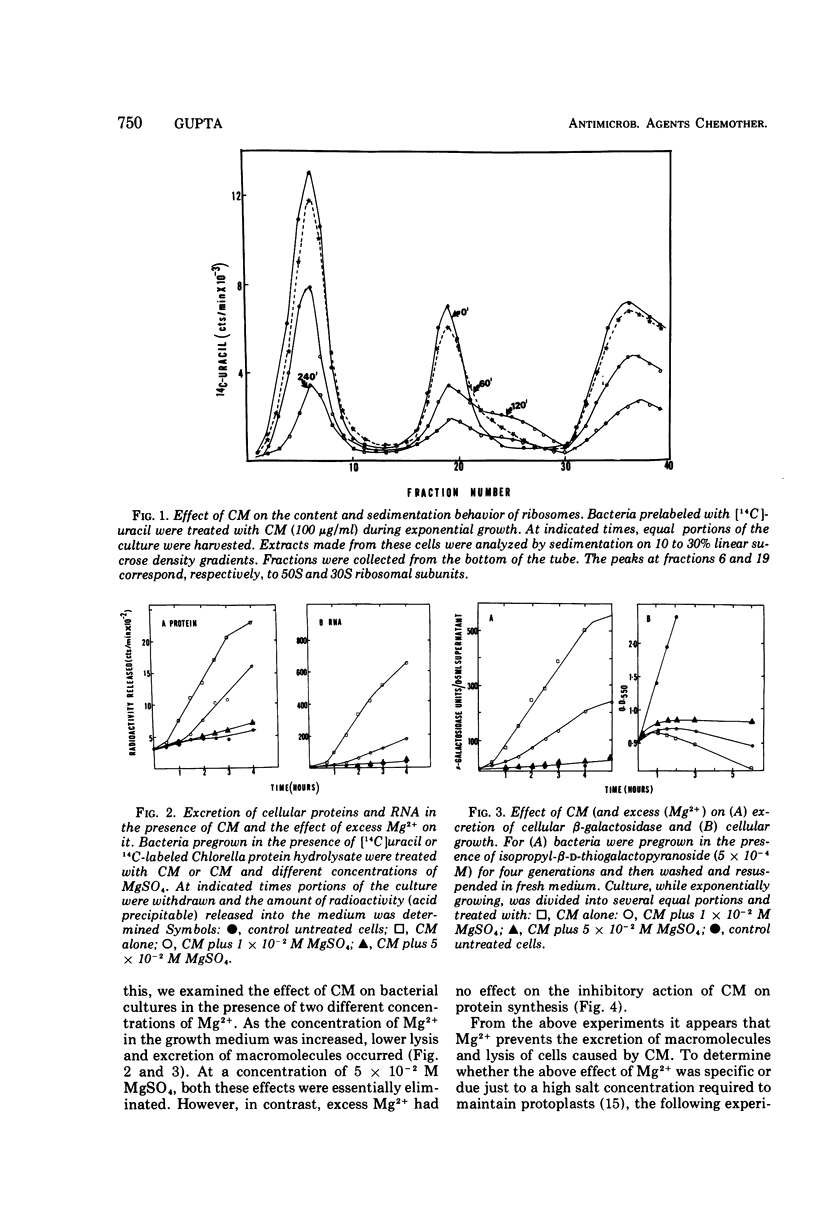
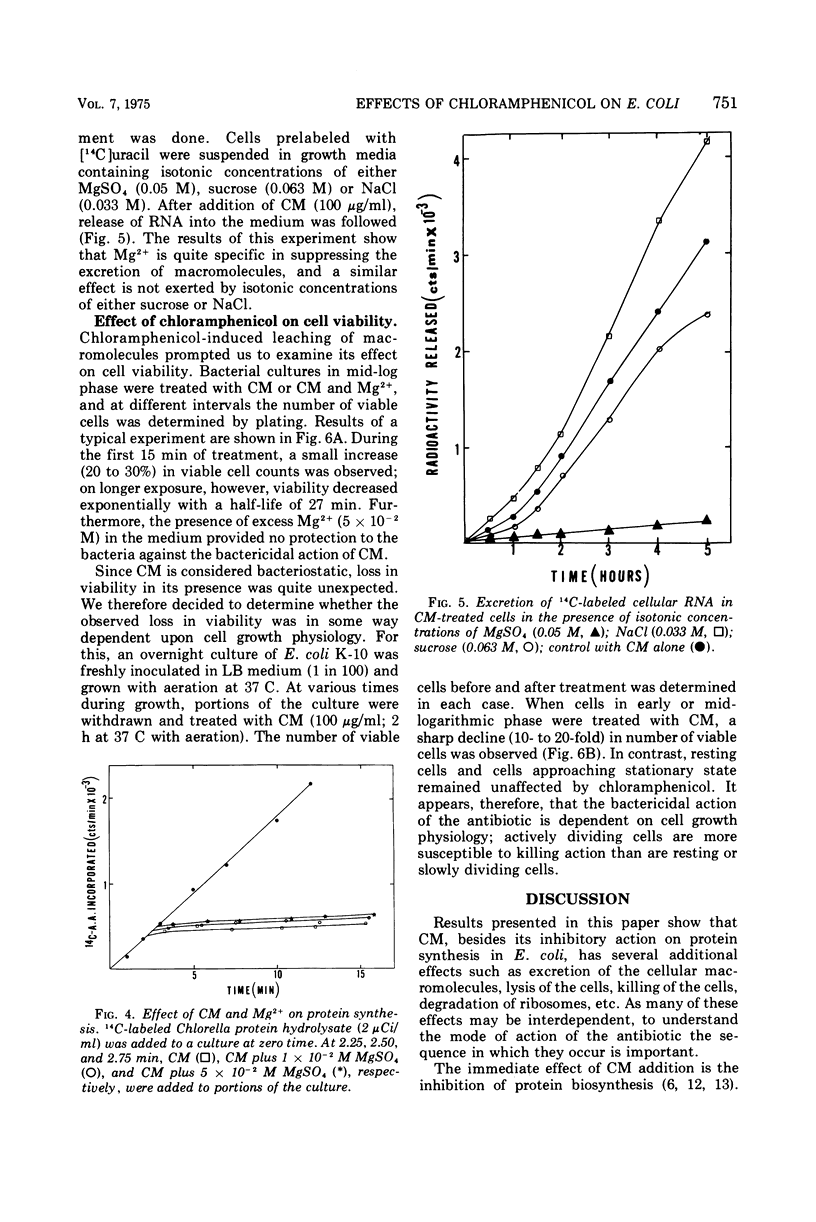
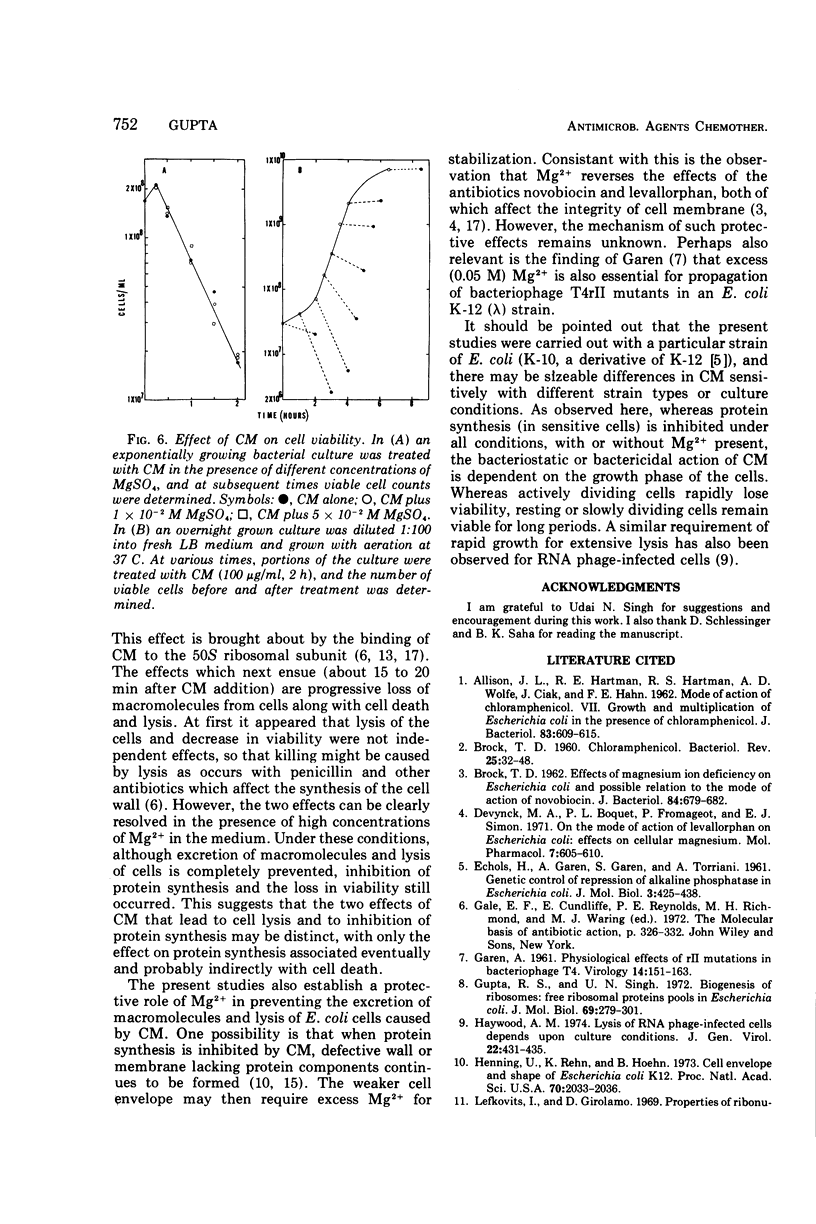
Treatment of Escherichia coli K-10 with 100 μg of chloramphenicol per ml for periods greater than 30 min leads to progressive lysis and killing of cells. The bactericidal action of the antibiotic is dependent on cell growth and physiology; only rapidly dividing cells are susceptible to killing; resting or slowly growing cells are not. The presence of excess Mg2+ in the growth medium specifically and competitively prevents excretion of macromolecules and cell lysis. However, inhibition of protein synthesis and killing of cells still occur even in the presence of added Mg2+. The possible relation of these effects to the mode of action of chloramphenicol is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON J. L., HARTMAN R. E., HARTMAN R. S., WOLFE A. D., CIAK J., HAHN F. E. Mode of action of chloramphenicol. VII. Growth and multiplication of Escherichia coli in the presence of chloramphenicol. J Bacteriol. 1962 Mar;83:609–615. doi: 10.1128/jb.83.3.609-615.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D. Effects of magnesium ion deficiency on Escherichia coli and possible relation to the mode of action of novobiocin. J Bacteriol. 1962 Oct;84:679–682. doi: 10.1128/jb.84.4.679-682.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. CHLORAMPHENICOL. Bacteriol Rev. 1961 Mar;25(1):32–48. doi: 10.1128/br.25.1.32-48.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devynck M. A., Boquet P. L., Fromageot P. On the mode of action of levallorphan on Escherichia coli: effects on cellular magnesium. Mol Pharmacol. 1971 Nov;7(6):605–610. [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Singh U. N. Biogenesis of ribosomes: free ribosomal protein pools in Escherichia coli. J Mol Biol. 1972 Aug 21;69(2):279–301. doi: 10.1016/0022-2836(72)90230-6. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkovits I., Di Girolamo M. Properties of ribonucleoprotein particles in chloramphenicol-treated cells of Escherichia coli B. Biochim Biophys Acta. 1969 Feb 18;174(2):561–565. doi: 10.1016/0005-2787(69)90285-8. [DOI] [PubMed] [Google Scholar]
- Malik V. S. Chloramphenicol. Adv Appl Microbiol. 1972;15:297–336. doi: 10.1016/s0065-2164(08)70095-9. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., LAMPEN J. O. Inhibition by antibiotics of the growth of bacterial and yeast protoplasts. J Bacteriol. 1962 Sep;84:508–512. doi: 10.1128/jb.84.3.508-512.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBB M. Effects of magnesium on cellular division in bacteria. Science. 1953 Nov 20;118(3073):607–611. doi: 10.1126/science.118.3073.607. [DOI] [PubMed] [Google Scholar]



