Abstract
Membrane fusion is carried out by core machinery that is conserved throughout eukaryotes. This is comprised of Rab GTPases and their effectors, and SNARE proteins, which together are sufficient to drive the fusion of reconstituted proteoliposomes. However, an outer layer of factors that are specific to individual trafficking pathways in vivo regulates the spatial and temporal occurrence of fusion. The homotypic fusion of Saccharomyces cerevisiae vacuolar lysosomes utilizes a growing set of factors to regulate the fusion machinery that include members of the ATP binding cassette (ABC) transporter family. Yeast vacuoles have five class C ABC transporters that are known to transport a variety of toxins into the vacuole lumen as part of detoxifying the cell. We have found that ABCC transporters can also regulate vacuole fusion through novel mechanisms. For instance Ybt1 serves as negative regulator of fusion through its effects on vacuolar Ca2+ homeostasis. Additional studies showed that Ycf1 acts as a positive regulator by affecting the efficient recruitment of the SNARE Vam7. Finally, we discuss the potential interface between the translocation of lipids across the membrane bilayer, also known as lipid flipping, and the efficiency of fusion.
Keywords: SNARE, PI3P, Vam7, Ycf1, Ybt1, Nft1, Vmr1, Bpt1, Ca2+ homeostasis, lipid flipping
Abbreviations: ABC, ATP binding cassette; DAG, diacylglycerol; HOPS, homotypic fusion and vacuole protein sorting complex; MDR, multidrug resistance; MSD, membrane spanning domain; NBD, nucleotide binding domain; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PI3P, phosphatidylinositol 3-phosphate; PI(3, 5)P2, phosphatidylinositol 3, 5-bisphosphate; PS, phosphatidylserine; PX, phox homology; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptors
Membrane Fusion
Eukaryotic cellular homeostasis requires the trafficking of membrane-bound cargo throughout the cell using mechanisms that are conserved from yeast to man as previously reviewed1-3 (Fig. 1). Each transport pathway culminates in the fusion of donor and acceptor membranes allowing the transfer of cargo. Membrane fusion has been dissected into experimentally defined stages that begin with the ATP-dependent disruption of inactive cis-SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) complexes on each membrane. The AAA+ protein NSF/Sec18 and its adaptor protein α-SNAP/Sec17 execute this function in what is termed the priming stage.4 Primed SNAREs from two membranes form parallel four helical bundles in trans through their SNARE motifs containing a critical central polar glutamine (Q), or arginine (R) that interact in the ionic zero-layer.5 Each SNARE bundle is composed of 1 R-SNARE and 3 Q-SNARE coils. Saccharomyces cerevisiae vacuole fusion depends on the R-SNARE Nyv1 and the Q-SNAREs Vam3, Vti1 and Vam7. Vam7 is the only vacuolar SNARE lacking a membrane anchor. It associates with the membrane via its N-terminal phox homology (PX) domain that interacts with the lipid phosphatidylinositol 3-phosphate (PI3P).6
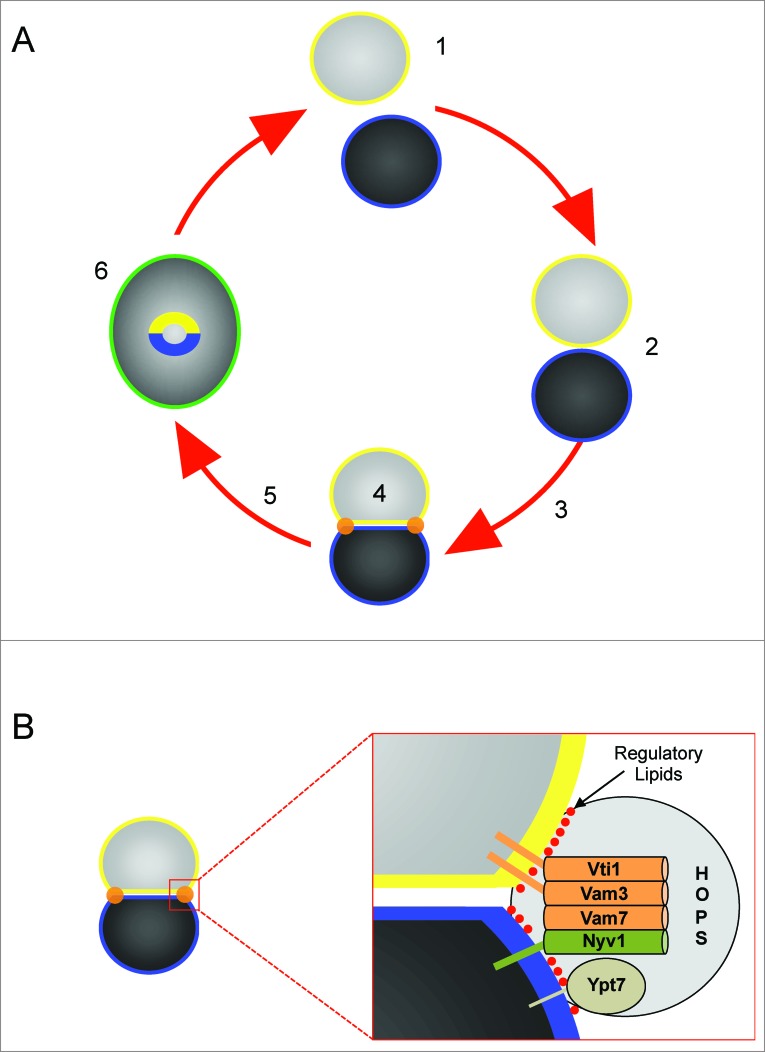
Figure 1.
Stages of vacuole fusion and formation of the vertex ring. (A) Vacuole fusion undergoes experimentally defined stages. 1. Priming–dispersed vacuoles harboring inactive cis-SNARE complexes are activated by the AAA+ ATPase Sec18 and its adaptor protein Sec17; 2. Tethering – association of vacuoles through the activity of Ypt7 and the HOPS complex; 3. Docking – Vacuoles become tightly apposed leading to the formation of the vertex ring (orange) and trans-SNARE paring; 5 – The docking to fusion transition may also go through a hemifusion intermediate where the outer leaflets mix while the inner leaflets remain intact and prevent content mixing. 6. – Fusion occurs at the vertex ring leading the merger of both membranes (green) and content mixing. Fusion also leads to the internalization of the boundary membrane (yellow and blue). (B) The vertex ring is enriched with the Q-SNAREs Vti1, Vam3, and Vam7, and the R-SNARE Nyv1. The vertex ring domain is also enriched with Ypt7, HOPS and regulatory lipids including PI3P, PI(4,5)P2, DAG and ergosterol. (Adapted from reference 2).
The next stage of fusion is the tethering reaction driven by Rab GTPases and their effector molecules. Yeast vacuole tethering requires the Rab Ypt7 and the HOPS (homotypic fusion and vacuole protein sorting) complex.7,8 During the docking stage, trans-SNARE complexes form between vesicles and trigger the release of luminal Ca2+ stores.9,10 During docking the interacting membranes become deformed and can be divided into three morphologically distinct domains.11-13 Docked membranes become tightly apposed forming 2 flattened discs termed the boundary domain. The edge of the boundary where the two vacuoles come into contact is termed the vertex ring domain and is characterized by its sharp positive curvature where the membranes bend at the domain transition. The vertex ring is the site of fusion where it is enriched with SNAREs, Ypt7, HOPS and a group of regulatory lipids comprising of phosphoinositides (PI), diacylglycerol (DAG) and ergosterol (Fig. 1B). The remaining membrane domain termed the outer edge is free of contacts with other vesicles and retains its spherical shape. Fusion can occur through a hemifusion intermediate at the vertex ring where the outer leaflets of docked vacuoles fuse while the inner leaflets remain intact.14-17 Thus, the positive curvature of vertex domains during docking changes to negatively curved domains upon hemifusion that contributes to destabilization of the bilayers.18,19 Ultimately, the inner leaflets fuse and luminal contents mix.
The core fusion machinery has been identified and is sufficient to drive the fusion of reconstituted proteoliposomes.20,21 However, living biological systems require multiple layers of regulation to respond to changing conditions to prevent spurious fusion. Several regulators have been identified that lie outside of the core fusion machinery including the class-1 casein kinase Yck3,22-24 the phosphatidic acid (PA) phosphatase Pah1,25 the PI 3-kinase Vps34,26 the Na+/H+ exchanger Nhx1,27 phospholipase C,28 Rho GTPases,29 and actin.30,31
In the search for new regulators of vacuole fusion we have now turned to class C ABC transporters as inspired by the work of others. Recent work has demonstrated that ABC transporters, which are well characterized as proteins that detoxify the cell of metals, bile acids and other toxins, can also regulate vacuole homeostasis. For instance the yeast cadmium transporter Ycf1 physically interacts with vacuolar factors that regulate PI3P turnover as well as actin remodeling.32 As mentioned above, both PI3P production and actin dynamics are required for vacuole fusion. This served an impetus to examine the role of Ycf1 and the other class C ABC transporters on vacuole fusion. Here we review our findings of novel roles for ABCC transporters in regulating vacuole fusion.
ABC Transporters
ATP binding cassette (ABC) transporters operate in all living cells from bacteria to human and serve a variety of functions. Chiefly, they function as transporters that use the energy of ATP hydrolysis to move substrates across a membrane.33 However, ABC proteins can also function as ion channels, channel regulators, receptors, proteases, as well as environmental sensors.34 A variety of molecules are recognized as ABC substrates including ions, anticancer drugs, antibiotics, peptides, and phospholipids.35,36 ABC transporters can also function as importers and exporters. In bacteria the ABC pumps LmrA and HlyB export toxins out of the bacterium conferring resistance.37,38 As importers bacterial ABC transporters are employed to uptake nutrients as exemplified by the maltose importer MalFGK2 from Escherichia coli and the histidine permease HisQMP2 from Salmonella typhimurium.39,40
The ABC transporter family shares a common topology consisting of 2 membrane-spanning domains (MSD) and two nucleotide-binding domains (NBD).41 The subunits may be formed from separate polypeptides, as found in numerous bacterial transporters. Most eukaryotic ABC transporters are expressed as single polypeptides. Each MSD spans the membrane multiple times to form the substrate translocation channel. The class C transporters are further divided into the short and long subgroups with the latter containing a third MSD N-terminal extension.41 Some of the predicted membrane-spanning α-helices may not be crucial for substrate translocation but may serve other functions such as membrane insertion or transporter regulation.42
The dysfunction of ABC transporters is implicated in numerous human diseases such as cystic fibrosis, adrenoleukodystrophy, familial hyperinsulinemic hypoglycemia of infancy, and Stargardt disease.43,44 A separate class of ABC transporters, termed P-glycoproteins, is implicated in multidrug resistance (MDR) and overexpression can lead to the resistance of chemotherapeutic agents.45 Other important human ABC transporters are TAP1 and TAP2 (transporter associated with antigen processing), which translocate peptides from the cytosol to the endoplasmic reticulum for the presentation of antigens at the cell surface via MHC class-1 molecules.46
The S. cerevisiae genome contains 30 ABC protein genes that are implicated in a variety of cellular functions including drug resistance, pheromone secretion, stress response, and cellular detoxification.41,47,48 Based on phylogenetic analysis, yeast ABC proteins have been classified into six subfamilies: MDR, PDR; MRP/CFTR, ALDp, YEF3, and RLI subfamily47 and later reclassified as ABCA-ABCG to facilitate correlation of experimental findings between yeast and human ABC transporters.41 The human ABCA subfamily is absent in yeast and there are 2 genes that do not fit within the current classification system.
The best-characterized members of the multidrug resistance related protein (MRP/CFTR or ABCC) family are Yor1 and Ycf149,50 (Table 1). Overexpression of Yor1 confers resistance to oligomycin, reveromycin A and organic anions.51,52 Yor1 has also been implicated in the translocation of fluorescent phosphatidylethanolamine (PE) across the lipid bilayer.53 Ycf1 plays a role in the detoxification of the cytosol by transporting metal-containing peptides and metal ions such as cadmium and arsenic as glutathione-S-conjugates into the vacuole.50,54 Bpt1 was classified as a bilirubin translocator and also plays a similar role as Ycf1.55 Ybt1 was reported to translocate bile acids into vacuoles.56 The vacuole also contains the lesser-known ABC proteins Vmr1, Nft1 and the putative transporter YOL075c.41
Table 1.
Location and function of yeast ABCC transporters.
| Name | Location | Function | References |
| Yor1 | PM1 | Multidrug transporter | 51 49 |
| Ycf1 | V | Glutathione S-conjugate transporter; transports toxic metals including cadmium, mercury and aresentie; transports Ade2 pigment; transports bile pigments and free bilirubin; Vam7 recruitment to vacuoles; positive regulator of vacuole fusion | 50,54,55,83 |
| Ybt1 | V | Bile and Ade2 transport; phosphatidylcholine translocation; regulates calcium transport; negative regulator of vacuole fusion | 56-58,102 |
| Bpt1 | V | Glutathione S-conjugate transporter; transports cadmium and Ade2; positive regulator of vacuole fusion | 55,83,103 |
| Vmr1 | V | Multidrug resistance; Glutathione S-conjugate transporter; Resistance to cadmium and other toxic metals | 53,104 |
| Nft1 | V | Unknown | 105 |
1PM, plasma membrane; V, vacuole.
Ybt1 Acts as a Negative Regulator of Membrane Fusion
The role of Ybt1 was originally described as transporting bile acids from the cytoplasm to the lumen of yeast vacuoles.56 It was later revealed Ybt1 translocates the lipid phosphatidylcholine (PC) across the membrane bilayer as part of choline recycling.57 However, its role in vacuole fusion had not been explored. Deletion of YBT1 leads to a marked increase in vacuole homotypic fusion.58 Vacuole fusion can be augmented by multiple means including deleting the type 1 casein kinase Yck3,59 altering osmolarity,60 and increasing the number of SNAREs per vacuole.17 Vacuoles purified from ybt1Δ yeast contained wild type levels of SNAREs suggesting that the augmented fusion was not caused by increased copies of SNAREs. It remains unclear whether deleting YBT1 affects Yck3 function or osmoregulation. The increased fusion was also not due to changes in any of the key fusion regulators including Ypt7 and HOPS. The changes in fusion did not alter sensitivities to characterized fusion inhibitors (e.g. antibodies against SNAREs) demonstrating that the increased fusogenicity was on pathway. Together these data suggest that the difference in fusion might be due to changes in the efficacy of the fusion machinery. Further experiments showed that the formation of vertex ring microdomains and trans-SNARE complexes were indistinguishable between ybt1Δ and wild type vacuoles, indicating that the alteration occurred after docking. The formation of trans-SNARE complexes triggers the release of vacuolar Ca2+ stores.10 In the absence of Ybt1 the kinetics of Ca2+ release was strikingly delayed relative to wild type vacuoles, suggesting that Ybt1 normally attenuates fusion by affecting the release of Ca2+ after docking. This is in accord with a report showing that elevated concentrations of extraluminal Ca2+ potently inhibited vacuole fusion.61
Others have shown that lysis can occur during the fusion process, which could contribute to the detected release of Ca2+.62 In their study, the authors overexpressed soluble GFP in the lumen of yeast vacuoles that lacked the protease Pep4 but contained the inactive alkaline phosphatase zymogen pro-Pho8. These were incubated with vacuole containing Pep4 but lacking pro-Pho8. Upon fusion, Pep4 activates Pho8 and alkaline phosphatase activity serves as a measure of fusion. Interestingly, they detected the release of GFP from the vacuole lumen when fusion was inhibited by various means. Released GFP was measured in the membrane and supernatant fractions after fractionation by centrifugation. They detected a substantial release of GFP in all conditions including those that potently inhibited fusion, as detected by Pho8 activity. Consequently they concluded that lysis readily occurs during incubation. Thus, this could contribute to the detection of extraluminal Ca2+. However, we do not believe that spurious lysis is the source of the detected Ca2+ signal. In the lysis experiments, released GFP was detected when fusion was inhibited by numerous reagents including the Ypt7 GAP Gyp1 as well as antibodies against SNAREs and the priming machinery. In sharp contrast, Ca2+ release is not seen when fusion is inhibited by Gyp1 or antibodies targeting Sec17 and SNAREs. This is in keeping with work by Merz and Wickner showing the link between SNARE pair formation and Ca2+ efflux.10
S. cerevisiae contains multiple Ca2+ transporters including the channels Yvc1, Cch1 and Mid1, the pump Pmc1, and the exchanger Vcx1.63 The release of vacuolar Ca2+ during osmotic shock occurs through Yvc1 and requires the production of PI(3,5)P2 by the PI3P 5-kinase Fab1.64 However, the channel responsible for Ca2+ efflux upon trans-SNARE pairing remains unidentified.10 The role of Ybt1 in Ca2+ efflux is unclear. Ybt1 interacts with the Ca2+ ATPase Pmc1, which transports the cation into the vacuole lumen.65 When inactive, resting Pmc1 is found in complex with free Nyv1. The Nyv1-Pmc1 interaction is subsequently disrupted when Nyv1 is competed away by its cognate Q-SNAREs.66 The dissociation of Nyv1-Pmc1 interactions leads to Pmc1 activation, thus linking the fusion machinery to Ca2+ homeostasis. To date it is unknown whether Ybt1 interacts with the Nyv1-Pmc1 complex or with Pmc1 alone. It is possible that the absence of Ybt1 from a putative trimeric complex could destabilize the Pmc1-Nyv1 interaction and lead to facile trans-SNARE pairing and more efficient fusion. Others have found that Pmc1 and Yvc1 are both required for the uptake of extracellular Ca2+ during glucose induced Ca2+ signaling,67 suggesting that the intake and efflux of vacuolar Ca2+ is interdependent. Yvc1 is a homolog of the TRPC family, which can be regulated through mechanical and osmotic stress.68,69 Thus, changes in curvature and lipid composition during vertex ring formation could change the physical environment in which Yvc1 functions. As stated above, the formation of trans-SNARE complexes during vacuole docking leads to pronounced changes in membrane curvature and the lipid make up of the vertex ring microdomain. This coincides with the release of luminal Ca2+. Because Ybt1 is a PC flippase, it is possible that ybt1Δ vacuoles accumulate PC on the outer leaflet altering membrane curvature and mechanical signaling to Yvc1 or other unidentified Ca2+ channels. Thus, the augmented fusion of ybt1Δ vacuoles could be due to inefficient channel activation due to the changes in the physical properties of the vacuole membrane.
There are several possible mechanisms by which Ca2+ efflux might contribute to the regulation of vacuole fusion. As previously mentioned, the docking stage of the fusion pathway requires the formation of the vertex microdomain that is enriched in the proteins and lipids that drive fusion and disrupting the formation or stability of vertex microdomains alters fusion.11-13 It is likely that altering the spatiotemporal release of Ca2+ could neutralize the electrostatic repulsion of tightly packed anionic lipids at the site of fusion, therefore facilitating the tight apposition of the two vesicles and altering the energy barrier for fusion.70,71 Others have shown that Ca2+ can affect the rate of fusion. Using reconstituted proteoliposomes Diao et al. found that fusion could occur through a hemifusion intermediate during slow fusion, or fuse both leaflets simultaneously during fast fusion.16 Additionally hemifusion could occur in the absence of added Ca2+ and that its addition increased the amount of hemifused vesicles and underwent slow fusion. Interestingly, vesicles that only had point contacts (without hemifusion) rapidly fused when Ca2+ was added. It must be noted that these experiments were performed with synaptic SNAREs and the Ca2+ responsive regulator synaptotagmin 1. The yeast vacuole lacks a known synaptotagmin homologue making it difficult to draw direct comparisons between the 2 sets of data. That said it remains possible that delaying the Ca2+ efflux by ybt1Δ vacuoles could allow for a build-up in the number of hemifused and point-contacted vesicles relative to wild type conditions leading to an overall increase in fusion. The mechanisms for the differential effects of Ca2+ on docked and hemifused vesicles remain unclear. Furthermore, the physical requirements that determine slow and fast fusion are unknown. Yet, one could speculate that changes in local microviscosity and membrane tension may effect the final path of fusion. The concentration of extraluminal Ca2+ could control the level of electrostatic repulsions between membranes. Thus, the early release of Ca2+ could stabilize the interactions between membranes and promote the hemifusion path. In contrast the delay in Ca2+ efflux would result in a greater tension and trigger the fast fusion path. Clearly, much more work will be required to further investigate these possibilities.
In addition to effecting clustering of anionic lipids, Ca2+ can also effect the interactions of proteins with the membrane and interactions between proteins including SNAREs. Zilly et al. showed that sub-micromolar Ca2+ concentrations induce the clustering of plasma membrane SNAREs by neutralizing negatively charged amino acid side chains.72 Albeit, the role of charged amino acids in clustering was not directly tested. Moreover, they did not take into account the role of Ca2+ on the lipid bilayer. It was also found that ≥10 μM Ca2+ inhibits SNARE pairing. This illustrates that the spatial and temporal flux of Ca2+ could act as a positive and negative regulator of membrane fusion. This is consistent with data showing that vacuole fusion can be inhibited by either Ca2+ removal or an excess of extraluminal Ca2+.61 Thus, it is not unlikely that low levels of extraluminal Ca2+ promotes the stable interactions of SNARE proteins. This could promote full zippering of the trans-SNARE complexes, triggering the release Ca2+ that may reach inhibitory local concentrations and stop the fusion of lagging vacuoles. While the in vitro detection of Ca2+ efflux only measures global concentrations, it is possible that local concentrations of Ca2+ at the site of efflux may reach micromolar levels thus inhibiting fusion. Therefore, a delay in Ca2+ efflux in ybt1Δ vacuoles could promote the build-up of fusion-ready membranes relative to wild type vesicles.
Ycf1 and Fusion
The best-characterized yeast ABCC family member is Ycf1, which regulates the transport of cadmium, mercury and other toxins as glutathione conjugates into the vacuole lumen to detoxify the cytoplasm.50,54,73 Paumi et al. used an integrated split-ubiquitin membrane yeast two-hybrid (iMYTH) analysis to discover novel Ycf1 binding partners.32 Of interest, Ycf1 was found to physically interact with the PI3P 5-kinase Fab1, which phosphorylates PI3P to produce PI(3,5)P2.74 This suggested that Ycf1 might play a critical role in vacuole homeostasis through binding Fab1. PI3P is an essential factor in the endolysosomal pathway where it interacts with various proteins to support vesicular transport.75 On vacuoles PI3P interacts with the soluble SNARE Vam7 via its PX domain as well as the HOPS complex and the Ypt7 nucleotide exchange factor Mon1-Ccz1.6,24,76,77 The functional role of PI3P can be inactivated by either specific phosphatases such as Ymr1/MTM family members or the kinase activity of Fab1/PIKfyve.78-81 Fab1 activity leads to the production of PI(3,5)P2, which itself can be modified by the phosphatase activity of (Fig. 4) to regenerate PI3P.82
Deletion of Ycf1 caused a significant reduction in fusion efficiency that was linked to the exclusion of Vam7 from isolated ycf1Δ vacuoles, whereas other SNAREs, HOPS subunits and Ypt7 were uneffected.83 The attenuated fusion was restored to wild type levels by the addition of recombinant Vam7, indicating that it was able to readily interact with other SNAREs, HOPS and lipids on the membrane to stimulate fusion. Because Vam7 associates with PI3P and Ycf1 might effect the PI3P levels during the fusion reaction we next asked whether the effect of deleting YCF1 on Vam7 recruitment was due to altered PI3P levels. When examining the levels of PI3P on wild type and ycf1Δ vacuoles, we found that the total levels of the lipid were identical in both strains. Together, these data suggested that the physical interactions between Ycf1 and Fab1 are not linked to the steady state levels of PI3P during fusion or the recruitment of Vam7 to vacuoles. Interestingly, deleting YCF1 has a significant effect on the localization of PI3P. In the absence of Ycf1, PI3P localization to vertex microdomains was elevated relative to wild type vacuoles. It remains unclear if the increase in PI3P enrichment at vertices could alter fusion or the recruitment of Vam7 to the membrane. It is possible that the increase in vertex localized PI3P is a side effect of an unidentified negative effects of deleting YCF1.
One possibility for the change in PI3P localization on ycf1Δ vacuoles could be linked to actin dynamics. Previous studies have shown that vacuolar actin undergoes remodeling during the fusion reaction.30,31 Early in the pathway filamentous actin depolymerizes which could facilitate the lateral movement of proteins to the vertex microdomain. Late in the pathway globular actin polymerizes, where it could potentially serve as a molecular fence to stabilize protein complexes into small domains such as the vertex ring.84,85 These possibilities are mirrored by the distribution of PI3P and actin dynamics. Using latrunculin to depolymerize actin leads to the accumulation of PI3P at vertex sites.13 Conversely, the use of jasplakinolide to stabilize actin filaments sharply reduces PI3P localization at vertices. Jasplakinolide has also been shown to block the vertex enrichment of SNAREs and HOPS.12 Together, these data illustrate that the state of actin polymerization effects the lateral movement of PI3P, SNAREs and HOPS and their accumulation at the vertex microdomain.
The polymerization of actin requires numerous factors including the activity of the Rho1 GTPase.86,87 Rho1 interacts with Ycf1 to protect cells from oxidative stress.88 Rho1 is activated by its nucleotide exchange factor Tus1,89 which physically interacts with Ycf1.32 This suggests that deleting YCF1 could indirectly effect actin dynamics by excluding Tus1 from the vacuole. The increase in vertex localized PI3P on ycf1Δ vacuoles is consistent with the notion that actin polymerization might be attenuated on ycf1Δ vacuoles.
Separately we found that the ATPase activity of Ycf1 was important for vacuole fusion. This was discovered using a point mutation in the NBD1 of Ycf1 that abolished ATPase activity. Complementation of ycf1Δ cells with Ycf1K669M did not rescue the fusion defect, yet Vam7 levels were not reduced, indicating that Ycf1 performs a secondary ATPase-dependent mechanism to support vacuole fusion. This might appear to be inconsistent with data showing that the in vitro fusion of ycf1Δ vacuole was rescued by adding recombinant Vam7. However, it should be noted that adding recombinant Vam7 to in vitro fusion reactions can bypass blocks in fusion that occur prior to docking and trans-SNARE pairing.10,17,90,91 Thus, it is possible that Ycf1 serves a second function during fusion that is linked to transport activity prior to docking. Because the in vitro fusion assay is devoid of toxic levels of Cd2+ and other soluble substrates, or glutathione S-transferase for that matter, we must suppose that Ycf1 carries out a novel ATPase dependent function. As mentioned above, ABC transporters can have many functions including the translocation of phospholipids across the membrane bilayer. This activity would likely alter the membrane asymmetry and curvature needed for optimal fusion efficiency. This in not unprecedented for a vacuolar ABCC as Ybt1 is known to translocate PC across the vacuole bilayer.57,92 Therefore, it is possible that Ycf1 translocates a different lipid across the bilayer as part of regulating fusion efficiency.
ABCC Transporters and Membrane Asymmetry
During vesicular trafficking, membranes can be stretched, compressed, made to bulge out during budding, or invaginate to form intraluminal vesicles.93 The curvature of membranes is altered where shape changes occur and might require adjusting the number of lipids on either side of the bilayer to accommodate protein and lipid packing. Membrane distortion can either dissipate compression or focus tension where it is needed in order to regulate mechanisms such as activating mechanosensitive proteins or triggering fusion and fission events.64,94 Some of these adjustments are mediated by the translocation of lipids between leaflets by proteins termed flippases and floppases with specificities for different lipid classes.92,95,96 The combined function of multiple flip-/floppases can result in establishing an asymmetric membrane with each leaflet containing a unique lipid profile as observed at the plasma membrane. The cytoplasmic surface of the eukaryotic plasma membrane is enriched in PI, PA, PE, and phosphatidylserine (PS) while the extracytoplasmic leaflet contains primarily PC and sphingomyelin.97,98 The thermodynamic barrier prevents passive movement of phospholipids between leaflets, thus any changes must occur through the action of energy-dependent carriers.99 Importantly, the translocation of lipids can cause changes in membrane shape that effects fission or fusion.94 Some of the described flip/floppases belong to the ABC transporter family. For example the human multi-drug resistance associated protein (hMRP1) has been shown to transport PS analogs in human erythrocytes.100,101 In yeast the ABCC transporter Yor1 translocates PE at the plasma membrane.53 Together this indicates a potential role for other ABC transporters in maintaining lipid asymmetry and effecting membrane curvature.
Thus far Ybt1 has been demonstrated to translocate PC across the vacuole bilayer.57 As described above, the deletion of Ybt1 caused an increase in fusion that was linked to a delay in Ca2+ efflux. Although it remains uncertain whether the flipping of PC is related to Ca2+ transport, it is appealing to think that changing the asymmetry and curvature of the bilayer could have direct physical effects on the vacuolar Ca2+ channel activated by trans-SNARE complex formation. The presence of up to 5 additional ABCC transporters on the vacuole opens up the possibility that multiple lipids could be translocated under various conditions to maintain vacuole homeostasis. Regarding fusion, changes in lipid asymmetry could effect the assembly and dynamics of the vertex microdomain. It is already known that disruption of the vertex microdomain by binding or modifying various regulatory lipids inhibits the organization and interaction of SNAREs as well as the binding of HOPS, Mon1 and Vam7 to the vacuole membrane.13,24,76 Although it remains to be seen if lipid flippases directly effect the fusion machinery there is no doubt that ABCC proteins effect various aspects of vacuole function.
Concluding Remarks and Future Directions
Vacuole fusion is carried out by core machinery composed of a Rab GTP, 4 SNAREs, and the HOPS tethering complex. Like all membrane fusion events, the homotypic fusion of vacuoles relies on a complex circuitry of regulators be they kinases, phosphatases, lipases or ion transporters. While exploring the outer shells of fusion regulation we can now include members of the ABCC family. In addition to their critical roles in the detoxification of the cell cadmium and other toxic metals, we now know that Ycf1 can also regulate vacuole fusion through the recruitment of the soluble SNARE Vam7 to the vacuole. We have yet to discover how this occurs thus future studies will investigate whether the 2 proteins directly interact, or whether it occurs via an indirect mechanism. Similarly we now know that the function of Ybt1 is not limited to the transport of bile, but includes the regulation of Ca2+ transport during fusion. Future studies will focus on dissecting the interactions of Ybt1 with the other regulators of membrane fusion and how those interactions might be linked to Ca2+ efflux. In addition to elucidating the mechanisms of Ycf1 and Ybt1 we will continue in examining the potential roles of the other ABCC proteins in the regulation of this vacuole homeostasis. Discovering how this unexpected family of transporters influences vacuole fusion and homeostasis will be a challenge for years to come.
Acknowledgments
We thank members of the Fratti Lab for the critical reading of the manuscript.
Funding Statement
This work was supported by a grant from the National Institutes of Health (GM101132) to RAF.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem 1999; 68:863-911; PMID: ; http://dx.doi.org/ 10.1146/annurev.biochem.68.1.863 [DOI] [PubMed] [Google Scholar]
- 2.Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol 2010; 26:115-36; PMID: ; http://dx.doi.org/ 10.1146/annurev-cellbio-100109-104131 [DOI] [PubMed] [Google Scholar]
- 3.Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol 2006; 7:631-43; PMID: ; http://dx.doi.org/ 10.1038/nrm2002 [DOI] [PubMed] [Google Scholar]
- 4.Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell 1996; 85:83-94; PMID: ; http://dx.doi.org/ 10.1016/S0092-8674(00)81084-3 [DOI] [PubMed] [Google Scholar]
- 5.Fasshauer D, Eliason WK, Brunger AT, Jahn R. Identification of a minimal core of the synaptic SNARE complex sufficient for reversible assembly and disassembly. Biochemistry 1998; 37:10354-62; PMID: ; http://dx.doi.org/ 10.1021/bi980542h [DOI] [PubMed] [Google Scholar]
- 6.Cheever ML, Sato TK, de Beer T, Kutateladze TG, Emr SD, Overduin M. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat Cell Biol 2001; 3:613-8; PMID: ; http://dx.doi.org/ 10.1038/35083000 [DOI] [PubMed] [Google Scholar]
- 7.Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. Embo J 1995; 14:5258-70; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF). J Cell Biol 1997; 136:307-17; PMID: ; http://dx.doi.org/ 10.1083/jcb.136.2.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature 1998; 396:543-8; PMID: ; http://dx.doi.org/ 10.1038/25069 [DOI] [PubMed] [Google Scholar]
- 10.Merz AJ, Wickner W. Trans-SNARE interactions elicit Ca2+ efflux from the yeast vacuole lumen. J Cell Biol 2004; 164:195-206; PMID: ; http://dx.doi.org/ 10.1083/jcb.200310105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell 2002; 108:357-69; PMID: ; http://dx.doi.org/ 10.1016/S0092-8674(02)00632-3 [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Merz AJ, Collins KM, Wickner W. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J Cell Biol 2003; 160:365-74; PMID: ; http://dx.doi.org/ 10.1083/jcb.200209095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol 2004; 167:1087-98; PMID: ; http://dx.doi.org/ 10.1083/jcb.200409068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese C, Heise F, Mayer A. Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature 2005; 436:410-4; PMID: [DOI] [PubMed] [Google Scholar]
- 15.Reese C, Mayer A. Transition from hemifusion to pore opening is rate limiting for vacuole membrane fusion. J Cell Biol 2005; 171:981-90; PMID: ; http://dx.doi.org/ 10.1083/jcb.200510018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diao J, Grob P, Cipriano DJ, Kyoung M, Zhang Y, Shah S, Nguyen A, Padolina M, Srivastava A, Vrljic M, et al. Synaptic proteins promote calcium-triggered fast transition from point contact to full fusion. Elife 2012; 1:e00109; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karunakaran S, Fratti R. The lipid composition and physical properties of the yeast vacuole affect the hemifusion-fusion transition. Traffic 2013; 14:650-62; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das S, Rand RP. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem Biophys Res Commun 1984; 124:491-6; PMID: [DOI] [PubMed] [Google Scholar]
- 19.Seddon JM.An inverse face-centered cubic phase formed by diacylglycerol-phosphatidylcholine mixtures. Biochemistry 1990; 29:7997-8002; PMID: [DOI] [PubMed] [Google Scholar]
- 20.Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. Embo J 2008; 27:2031-42; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mima J, Wickner W. Complex lipid requirements for SNARE-and SNARE chaperone dependent membrane fusion. J Biol Chem 2009; 284:27114-22; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabrera M, Ostrowicz CW, Mari M, LaGrassa TJ, Reggiori F, Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell 2009; 20:1937-48; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brett CL, Plemel RL, Lobingier BT, Vignali M, Fields S, Merz AJ. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J Cell Biol 2008; 182:1141-51; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence G, Brown CC, Flood BA, Karunakaran S, Cabrera M, Nordmann M, Ungermann C, Fratti RA. Dynamic association of the PI3P-interacting Mon1-Ccz1 GEF with vacuoles is controlled through its phosphorylation by the type-1 casein kinase Yck3. Mol Biol Cell 2014; 25:1608-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasser T, Qiu QS, Karunakaran S, Padolina M, Reyes A, Flood B, Smith S, Gonzales C, Fratti RA. Yeast lipin 1 orthologue pah1p regulates vacuole homeostasis and membrane fusion. J Biol Chem 2012; 287:2221-36; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 1993; 260:88-91; PMID: [DOI] [PubMed] [Google Scholar]
- 27.Qiu QS, Fratti RA. The Na+/H+ exchanger Nhx1p regulates the initiation of Saccharomyces cerevisiae vacuole fusion. J Cell Sci 2010; 123:3266-75; PMID: [DOI] [PubMed] [Google Scholar]
- 28.Jun Y, Fratti RA, Wickner W. Diacylglycerol and its formation by phospholipase C regulate Rab- and SNARE-dependent yeast vacuole fusion. J Biol Chem 2004; 279:53186-95; PMID: [DOI] [PubMed] [Google Scholar]
- 29.Eitzen G, Thorngren N, Wickner W. Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking. Embo J 2001; 20:5650-6; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eitzen G, Wang L, Thorngren N, Wickner W. Vacuole-bound actin regulates homotypic membrane fusion. J Cell Biol 2002; 158:669-79; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karunakaran S, Sasser T, Rajalekshmi S, Fratti RA. SNAREs, HOPS, and regulatory lipids control the dynamics of vacuolar actin during homotypic fusion. J Cell Sci 2012; 14:650-62 [DOI] [PubMed] [Google Scholar]
- 32.Paumi CM, Menendez J, Arnoldo A, Engels K, Iyer KR, Thaminy S, Georgiev O, Barral Y, Michaelis S, Stagljar I. Mapping protein-protein interactions for the yeast ABC transporter Ycf1p by integrated split-ubiquitin membrane yeast two-hybrid analysis. Mol Cell 2007; 26:15-25; PMID: ; http://dx.doi.org/ 10.1016/j.molcel.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 33.Higgins CF.ABC transporters: from microorganisms to man. Annu Rev Cell Biol 1992; 8:67-113; PMID: ; http://dx.doi.org/ 10.1146/annurev.cb.08.110192.000435 [DOI] [PubMed] [Google Scholar]
- 34.Higgins CF.The ABC of channel regulation. Cell 1995; 82:693-6; PMID: ; http://dx.doi.org/ 10.1016/0092-8674(95)90465-4 [DOI] [PubMed] [Google Scholar]
- 35.Dean M, Allikmets R. Evolution of ATP-binding cassette transporter genes. Curr Opin Genet Dev 1995; 5:779-85; PMID: ; http://dx.doi.org/ 10.1016/0959-437X(95)80011-S [DOI] [PubMed] [Google Scholar]
- 36.Kuchler K, Thorner J. Functional expression of human mdr1 in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 1992; 89:2302-6; PMID: ; http://dx.doi.org/ 10.1073/pnas.89.6.2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blight MA, Menichi B, Holland IB. Evidence for post-transcriptional regulation of the synthesis of the Escherichia coli HlyB haemolysin translocator and production of polyclonal anti-HlyB antibody. Mol Gen Genet 1995; 247:73-85; PMID: ; http://dx.doi.org/ 10.1007/BF00425823 [DOI] [PubMed] [Google Scholar]
- 38.van Veen HW, Higgins CF, Konings WN. Multidrug transport by ATP binding cassette transporters: a proposed two-cylinder engine mechanism. Res Microbiol 2001; 152:365-74; PMID: ; http://dx.doi.org/ 10.1016/S0923-2508(01)01208-6 [DOI] [PubMed] [Google Scholar]
- 39.Ames GF, Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A 1970; 66:1096-103; PMID: ; http://dx.doi.org/ 10.1073/pnas.66.4.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bavoil P, Hofnung M, Nikaido H. Identification of a cytoplasmic membrane-associated component of the maltose transport system of Escherichia coli. J Biol Chem 1980; 255:8366-9; PMID: [PubMed] [Google Scholar]
- 41.Paumi CM, Chuk M, Snider J, Stagljar I, Michaelis S. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol Mol Biol Rev 2009; 73:577-93; PMID: ; http://dx.doi.org/ 10.1128/MMBR.00020-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins CF. ABC transporters: physiology, structure and mechanism–an overview. Res Microbiol 2001; 152:205-10; PMID: ; http://dx.doi.org/ 10.1016/S0923-2508(01)01193-7 [DOI] [PubMed] [Google Scholar]
- 43.Dean M, Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr 2001; 33:475-9; PMID: ; http://dx.doi.org/ 10.1023/A:1012823120935 [DOI] [PubMed] [Google Scholar]
- 44.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 2001; 11:1156-66; PMID: ; http://dx.doi.org/ 10.1101/gr.GR-1649R [DOI] [PubMed] [Google Scholar]
- 45.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 1993; 62:385-427; PMID: ; http://dx.doi.org/ 10.1146/annurev.bi.62.070193.002125 [DOI] [PubMed] [Google Scholar]
- 46.Lankat-Buttgereit B, Tampe R. The transporter associated with antigen processing: function and implications in human diseases. Physiol Rev 2002; 82:187-204; PMID: [DOI] [PubMed] [Google Scholar]
- 47.Decottignies A, Goffeau A. Complete inventory of the yeast ABC proteins. Nat Genet 1997; 15:137-45; PMID: ; http://dx.doi.org/ 10.1038/ng0297-137 [DOI] [PubMed] [Google Scholar]
- 48.Taglicht D, Michaelis S. Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol 1998; 292:130-62; PMID: ; http://dx.doi.org/ 10.1016/S0076-6879(98)92012-2 [DOI] [PubMed] [Google Scholar]
- 49.Katzmann DJ, Epping EA, Moye-Rowley WS. Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol Cell Biol 1999; 19:2998-3009; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szczypka MS, Wemmie JA, Moye-Rowley WS, Thiele DJ. A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem 1994; 269:22853-7; PMID: [PubMed] [Google Scholar]
- 51.Cui Z, Hirata D, Tsuchiya E, Osada H, Miyakawa T. The multidrug resistance-associated protein (MRP) subfamily (Yrs1/Yor1) of Saccharomyces cerevisiae is important for the tolerance to a broad range of organic anions. J Biol Chem 1996; 271:14712-6; PMID: ; http://dx.doi.org/ 10.1074/jbc.271.25.14712 [DOI] [PubMed] [Google Scholar]
- 52.Li ZS, Szczypka M, Lu YP, Thiele DJ, Rea PA. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem 1996; 271:6509-17; PMID: ; http://dx.doi.org/ 10.1074/jbc.271.11.6509 [DOI] [PubMed] [Google Scholar]
- 53.Decottignies A, Grant AM, Nichols JW, de Wet H, McIntosh DB, Goffeau A. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem 1998; 273:12612-22; PMID: ; http://dx.doi.org/ 10.1074/jbc.273.20.12612 [DOI] [PubMed] [Google Scholar]
- 54.Li ZS, Szczypka M, Lu YP, Thiele DJ, Rea PA. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem 1996; 271:6509-17; PMID: ; http://dx.doi.org/ 10.1074/jbc.271.11.6509 [DOI] [PubMed] [Google Scholar]
- 55.Petrovic S, Pascolo L, Gallo R, Cupelli F, Ostrow JD, Goffeau A, Tiribelli C, Bruschi CV. The products of YCF1 and YLL015w (BPT1) cooperate for the ATP-dependent vacuolar transport of unconjugated bilirubin in Saccharomyces cerevisiae. Yeast 2000; 16:561-71; PMID: ; http://dx.doi.org/ 10.1002/(SICI)1097-0061(200004)16:6%3c561::AID-YEA551%3e3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 56.Ortiz DF, St Pierre MV, Abdulmessih A, Arias IM. A yeast ATP-binding cassette-type protein mediating ATP-dependent bile acid transport. J Biol Chem 1997; 272:15358-65; PMID: ; http://dx.doi.org/ 10.1074/jbc.272.24.15358 [DOI] [PubMed] [Google Scholar]
- 57.Gulshan K, Moye-Rowley WS. Vacuolar import of phosphatidylcholine requires the ATP-binding cassette transporter Ybt1. Traffic 2011; 12:1257-68; PMID: ; http://dx.doi.org/ 10.1111/j.1600-0854.2011.01228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasser TL, Padolina M, Fratti RA. The yeast nacuolar ABC transporter Ybt1p regulates membrane fusion through Ca2+ transport modulation. Biochem J 2012; 448:365-72; PMID: ; http://dx.doi.org/ 10.1042/BJ20120847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaGrassa TJ, Ungermann C. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J Cell Biol 2005; 168:401-14; PMID: ; http://dx.doi.org/ 10.1083/jcb.200407141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brett CL, Merz AJ. Osmotic regulation of Rab-mediated organelle docking. Curr Biol 2008; 18:1072-7; PMID: ; http://dx.doi.org/ 10.1016/j.cub.2008.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ungermann C, Wickner W, Xu Z. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc Natl Acad Sci U S A 1999; 96:11194; PMID: ; http://dx.doi.org/ 10.1073/pnas.96.20.11194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Starai VJ, Jun Y, Wickner W. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc Natl Acad Sci U S A 2007; 104:13551-8; PMID: ; http://dx.doi.org/ 10.1073/pnas.0704741104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cunningham KW.Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium 2011; 50:129-38; PMID: ; http://dx.doi.org/ 10.1016/j.ceca.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 2010; 1:38; PMID: ; http://dx.doi.org/ 10.1038/ncomms1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tarassov K, Messier V, Landry CR, Radinovic S, Serna Molina MM, Shames I, Malitskaya Y, Vogel J, Bussey H, Michnick SW. An in vivo map of the yeast protein interactome. Science 2008; 320:1465-70; PMID: ; http://dx.doi.org/ 10.1126/science.1153878 [DOI] [PubMed] [Google Scholar]
- 66.Takita Y, Engstrom L, Ungermann C, Cunningham KW. Inhibition of the Ca(2+)-ATPase Pmc1p by the v-SNARE protein Nyv1p. J Biol Chem 2001; 276:6200-6; PMID: ; http://dx.doi.org/ 10.1074/jbc.M009191200 [DOI] [PubMed] [Google Scholar]
- 67.Bouillet LE, Cardoso AS, Perovano E, Pereira RR, Ribeiro EM, Tropia MJ, Fietto LG, Tisi R, Martegani E, Castro IM, et al. The involvement of calcium carriers and of the vacuole in the glucose-induced calcium signaling and activation of the plasma membrane H(+)-ATPase in Saccharomyces cerevisiae cells. Cell Calcium 2012; 51:72-81; PMID: ; http://dx.doi.org/ 10.1016/j.ceca.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 68.Denis V, Cyert MS. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol 2002; 156:29-34; PMID: ; http://dx.doi.org/ 10.1083/jcb.200111004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su Z, Anishkin A, Kung C, Saimi Y. The core domain as the force sensor of the yeast mechanosensitive TRP channel. J Gen Physiol 2011; 138:627-40; PMID: ; http://dx.doi.org/ 10.1085/jgp.201110693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boettcher JM, Davis-Harrison RL, Clay MC, Nieuwkoop AJ, Ohkubo YZ, Tajkhorshid E, Morrissey JH, Rienstra CM. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry 2011; 50:2264-73; PMID: ; http://dx.doi.org/ 10.1021/bi1013694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ellenbroek WG, Wang YH, Christian DA, Discher DE, Janmey PA, Liu AJ. Divalent cation-dependent formation of electrostatic PIP2 clusters in lipid monolayers. Biophys J 2011; 101:2178-84; PMID: ; http://dx.doi.org/ 10.1016/j.bpj.2011.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zilly FE, Halemani ND, Walrafen D, Spitta L, Schreiber A, Jahn R, Lang T. Ca2+ induces clustering of membrane proteins in the plasma membrane via electrostatic interactions. EMBO J 2011; 30:1209-20; PMID: ; http://dx.doi.org/ 10.1038/emboj.2011.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gueldry O, Lazard M, Delort F, Dauplais M, Grigoras I, Blanquet S, Plateau P. Ycf1p-dependent Hg(II) detoxification in Saccharomyces cerevisiae. Eur J Biochem 2003; 270:2486-96; PMID: ; http://dx.doi.org/ 10.1046/j.1432-1033.2003.03620.x [DOI] [PubMed] [Google Scholar]
- 74.Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol 1998; 143:65-79; PMID: ; http://dx.doi.org/ 10.1083/jcb.143.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kutateladze TG.Translation of the phosphoinositide code by PI effectors. Nat Chem Biol 2010; 6:507-13; PMID: ; http://dx.doi.org/ 10.1038/nchembio.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. Embo J 2006; 25:1579-89; PMID: ; http://dx.doi.org/ 10.1038/sj.emboj.7601051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cabrera M, Nordmann M, Perz A, Schmedt D, Gerondopoulos A, Barr F, Piehler J, Engelbrecht-Vandre S, Ungermann C. The Mon1-Ccz1 GEF activates the Rab7 GTPase Ypt7 via a longin fold-Rab interface and association with PI-3-P-positive membranes. J Cell Sci 2014; 127:1043-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell 1995; 6:525-39; PMID: ; http://dx.doi.org/ 10.1091/mbc.6.5.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 1998; 95:847-58; PMID: ; http://dx.doi.org/ 10.1016/S0092-8674(00)81707-9 [DOI] [PubMed] [Google Scholar]
- 80.Parrish WR, Stefan CJ, Emr SD. Essential role for the myotubularin-related phosphatase Ymr1p and the synaptojanin-like phosphatases Sjl2p and Sjl3p in regulation of phosphatidylinositol 3-phosphate in yeast. Mol Biol Cell 2004; 15:3567-79; PMID: ; http://dx.doi.org/ 10.1091/mbc.E04-03-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor GS, Maehama T, Dixon JE. Inaugural article: myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc Natl Acad Sci U S A 2000; 97:8910-5; PMID: ; http://dx.doi.org/ 10.1073/pnas.160255697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gary JD, Sato TK, Stefan CJ, Bonangelino CJ, Weisman LS, Emr SD. Regulation of fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by vac7 protein and fig4, a polyphosphoinositide phosphatase family member. Mol Biol Cell 2002; 13:1238-51; PMID: ; http://dx.doi.org/ 10.1091/mbc.01-10-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sasser TL, Lawrence G, Karunakaran S, Brown C, Fratti RA. The yeast ABC transporter Ycf1p enhances the recruitment of the soluble SNARE Vam7p to vacuoles for efficient membrane fusion. J Biol Chem 2013; 288:18300-10; PMID: ; http://dx.doi.org/ 10.1074/jbc.M112.441089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kusumi A, Suzuki K, Koyasako K. Mobility and cytoskeletal interactions of cell adhesion receptors. Curr Opin Cell Biol 1999; 11:582-90; PMID: ; http://dx.doi.org/ 10.1016/S0955-0674(99)00020-4 [DOI] [PubMed] [Google Scholar]
- 85.Tamkun MM, O'Connell K M, Rolig AS. A cytoskeletal-based perimeter fence selectively corrals a sub-population of cell surface Kv2.1 channels. J Cell Sci 2007; 120:2413-23; PMID: ; http://dx.doi.org/ 10.1242/jcs.007351 [DOI] [PubMed] [Google Scholar]
- 86.Marelli M, Smith JJ, Jung S, Yi E, Nesvizhskii AI, Christmas RH, Saleem RA, Tam YY, Fagarasanu A, Goodlett DR, Aebersold R, et al. Quantitative mass spectrometry reveals a role for the GTPase Rho1p in actin organization on the peroxisome membrane. J Cell Biol 2004; 167:1099-112; PMID: ; http://dx.doi.org/ 10.1083/jcb.200404119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tolliday N, VerPlank L, Li R. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr Biol 2002; 12:1864-70; PMID: ; http://dx.doi.org/ 10.1016/S0960-9822(02)01238-1 [DOI] [PubMed] [Google Scholar]
- 88.Lee ME, Singh K, Snider J, Shenoy A, Paumi CM, Stagljar I, Park HO. The Rho1 GTPase acts together with a vacuolar glutathione S-conjugate transporter to protect yeast cells from oxidative stress. Genetics 2011; 188:859-70; PMID: ; http://dx.doi.org/ 10.1534/genetics.111.130724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmelzle T, Helliwell SB, Hall MN. Yeast protein kinases and the RHO1 exchange factor TUS1 are novel components of the cell integrity pathway in yeast. Mol Cell Biol 2002; 22:1329-39; PMID: ; http://dx.doi.org/ 10.1128/MCB.22.5.1329-1339.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thorngren N, Collins KM, Fratti RA, Wickner W, Merz AJ. A soluble SNARE drives rapid docking, bypassing ATP and Sec17/18p for vacuole fusion. Embo J 2004; 23:2765-76; PMID: ; http://dx.doi.org/ 10.1038/sj.emboj.7600286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fratti RA, Collins KM, Hickey CM, Wickner W. Stringent 3Q: 1R composition of the SNARE 0-layer can be bypassed for fusion by compensatory SNARE mutation or by lipid bilayer modification. J Biol Chem 2007; 282:14861-7; PMID: ; http://dx.doi.org/ 10.1074/jbc.M700971200 [DOI] [PubMed] [Google Scholar]
- 92.Daleke DL.Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res 2003; 44:233-42; PMID: ; http://dx.doi.org/ 10.1194/jlr.R200019-JLR200 [DOI] [PubMed] [Google Scholar]
- 93.Andersen OS, Koeppe RE 2nd. Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 2007; 36:107-30; PMID: ; http://dx.doi.org/ 10.1146/annurev.biophys.36.040306.132643 [DOI] [PubMed] [Google Scholar]
- 94.Papadopulos A, Vehring S, Lopez-Montero I, Kutschenko L, Stockl M, Devaux PF, Kozlov M, Pomorski T, Herrmann A. Flippase activity detected with unlabeled lipids by shape changes of giant unilamellar vesicles. J Biol Chem 2007; 282:15559-68; PMID: ; http://dx.doi.org/ 10.1074/jbc.M604740200 [DOI] [PubMed] [Google Scholar]
- 95.Rauch C, Farge E. Endocytosis switch controlled by transmembrane osmotic pressure and phospholipid number asymmetry. Biophys J 2000; 78:3036-47; PMID: ; http://dx.doi.org/ 10.1016/S0006-3495(00)76842-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008; 9:112-24; PMID: ; http://dx.doi.org/ 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bretscher MS. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol 1972; 236:11-2; PMID: ; http://dx.doi.org/ 10.1038/newbio236011a0 [DOI] [PubMed] [Google Scholar]
- 98.Op den Kamp JA. Lipid asymmetry in membranes. Annu Rev Biochem 1979; 48:47-71; PMID: ; http://dx.doi.org/ 10.1146/annurev.bi.48.070179.000403 [DOI] [PubMed] [Google Scholar]
- 99.Kornberg RD, McConnell HM. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry 1971; 10:1111-20; PMID: ; http://dx.doi.org/ 10.1021/bi00783a003 [DOI] [PubMed] [Google Scholar]
- 100.Huang Z, Chang X, Riordan JR, Huang Y. Fluorescent modified phosphatidylcholine floppase activity of reconstituted multidrug resistance-associated protein MRP1. Biochim Biophys Acta 2004; 1660:155-63; PMID: ; http://dx.doi.org/ 10.1016/j.bbamem.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 101.Smriti, Nemergut EC, Daleke DL. ATP-dependent transport of phosphatidylserine analogues in human erythrocytes. Biochemistry 2007; 46:2249-59; PMID: ; http://dx.doi.org/ 10.1021/bi061333x [DOI] [PubMed] [Google Scholar]
- 102.Sharma KG, Kaur R, Bachhawat AK. The glutathione-mediated detoxification pathway in yeast: an analysis using the red pigment that accumulates in certain adenine biosynthetic mutants of yeasts reveals the involvement of novel genes. Arch Microbiol 2003; 180:108-17; PMID: ; http://dx.doi.org/ 10.1007/s00203-003-0566-z [DOI] [PubMed] [Google Scholar]
- 103.Klein M, Mamnun YM, Eggmann T, Schuller C, Wolfger H, Martinoia E, Kuchler K. The ATP-binding cassette (ABC) transporter Bpt1p mediates vacuolar sequestration of glutathione conjugates in yeast. FEBS Lett 2002; 520:63-7; PMID: ; http://dx.doi.org/ 10.1016/S0014-5793(02)02767-9 [DOI] [PubMed] [Google Scholar]
- 104.Wawrzycka D, Sobczak I, Bartosz G, Bocer T, Ulaszewski S, Goffeau A. Vmr 1p is a novel vacuolar multidrug resistance ABC transporter in Saccharomyces cerevisiae. FEMS Yeast Res 2010; 10:828-38; PMID: ; http://dx.doi.org/ 10.1111/j.1567-1364.2010.00673.x [DOI] [PubMed] [Google Scholar]
- 105.Mason DL, Mallampalli MP, Huyer G, Michaelis S. A region within a lumenal loop of Saccharomyces cerevisiae Ycf1p directs proteolytic processing and substrate specificity. Eukaryot Cell 2003; 2:588-98; PMID: ; http://dx.doi.org/ 10.1128/EC.2.3.588-598.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]