Abstract
Serine phosphorylation has generally been considered indispensable for full transcriptional activity of STAT proteins. Recent data indicate that CDK8-mediated phosphorylation of signal transducer and activator of transcription 1 (STAT1) on S727 inhibits natural killer (NK) cell cytotoxicity and restrains tumor surveillance. These findings implicate CDK8 as a promising target for immunotherapy.
Keywords: CDK8, immunotherapy, NK cells, STAT1, tumor immune surveillance
Abbreviations: CDK, cyclin-dependent kinase; CTLA-4, cytotoxic T lymphocyte antigen 4; FDA, food and drug administration; KLRG1, killer cell lectin-like receptor subfamily G member 1; MHC, major histocompatibility complex; NK, natural killer; PD-1, programmed cell death 1; STAT1, signal transducer and activator of transcription 1
Main Text
The phenomenon of tumor immunosurveillance has been controversial for much of the past century. Thanks to the unwavering dedication of a few key researchers and physicians, there is now conclusive proof that the host immune system has an important role in the fight against cancer. Recent reports have convinced even the skeptics and Science Magazine chose cancer immunotherapy as breakthrough of the year in 2013.1
Immune cells pose a constant challenge to nascent neoplasms. Thus, malignant cells need strategies to evade immunosurveillance or to suppress antitumor immune responses. Novel therapies frequently aim to overcome T-cell paralysis by targeting cytotoxic T lymphocyte associated protein 4 (CTLA-4) or programmed cell death 1 (PD-1), thereby reducing the threshold for attack on malignant cells. Additionally, natural killer (NK) cells are prominent players in the battle against cancer, particularly in response to leukemia, melanoma and renal cell carcinoma. NK cells are highly cytotoxic and need no prior sensitization. Although NK cell-based antitumor immunotherapy holds great promise,2 clinical responses to date have been modest at best. There is thus a clear need for a more detailed understanding of the molecular players involved in NK cell-dependent antitumor immunity.
Our recent study focused on a particular phosphorylation site of the signal transducer and activator of transcription protein 1 (STAT1-S727) in NK cells. We previously showed that STAT1 acts as a tumor promoter in leukemia.3 Loss of STAT1 precludes expression of MHC Class I molecules on tumor cells, providing a strong trigger for NK cell-mediated tumor clearance. However, STAT1 is required for a full blown cytotoxic response in NK cells, so loss or inactivation of STAT1 should lessen the ability of NK cells to mount a full response. The overall outcome of these 2 opposing effects is that Stat1-deficient mice are more resistant to the induction of leukemia. We wondered whether it is possible to separate the opposing functions of STAT1 on NK cell-dependent tumor surveillance.
Contrary to indications in the literature, we found a significant level of phosphorylated STAT1-S727 in resting NK cells in vivo. Surprisingly, this was not accompanied by tyrosine phosphorylation, which is considered a prerequisite for the phosphorylation of S727. Further experiments revealed that STAT1-S727 phosphorylation in NK cells is important for cytotoxicity and for NK cell antitumor function in vivo.4 The situation is complex and STAT1-S727 phosphorylation has been shown to impart both activating and inhibitory effects on NK cells in vitro. It is required for full interferon-γ (IFNγ) induction but it antagonizes the production of granzyme B and perforin. As perforin exhibits a gene-dosage effect, we reasoned that higher levels of perforin cause the increased cytotoxicity observed in murine NK cells, in which S727 has been mutated to alanine (Stat1-S727A). The reduction of expression of the inhibitory receptor killer cell lectin-like receptor subfamily G member 1 (KLRG1), the increase in expression of NKG2A/C/E and the elevated amount of granzyme B might also contribute to the effect. The significance of the other molecular alterations in Stat1-S727A NK cells, such as decreased levels of miRNA-30e and −378, is unclear. However, the net result of all changes caused by the mutation of S727 to alanine in NK cells is that the cells acquire enhanced potency against tumors. Under our experimental conditions, Stat1-S727A mice are considerably more resistant to B16F10 melanoma and to v-abl + leukemic cells and are entirely resistant to 4T1 breast cancer metastasis.
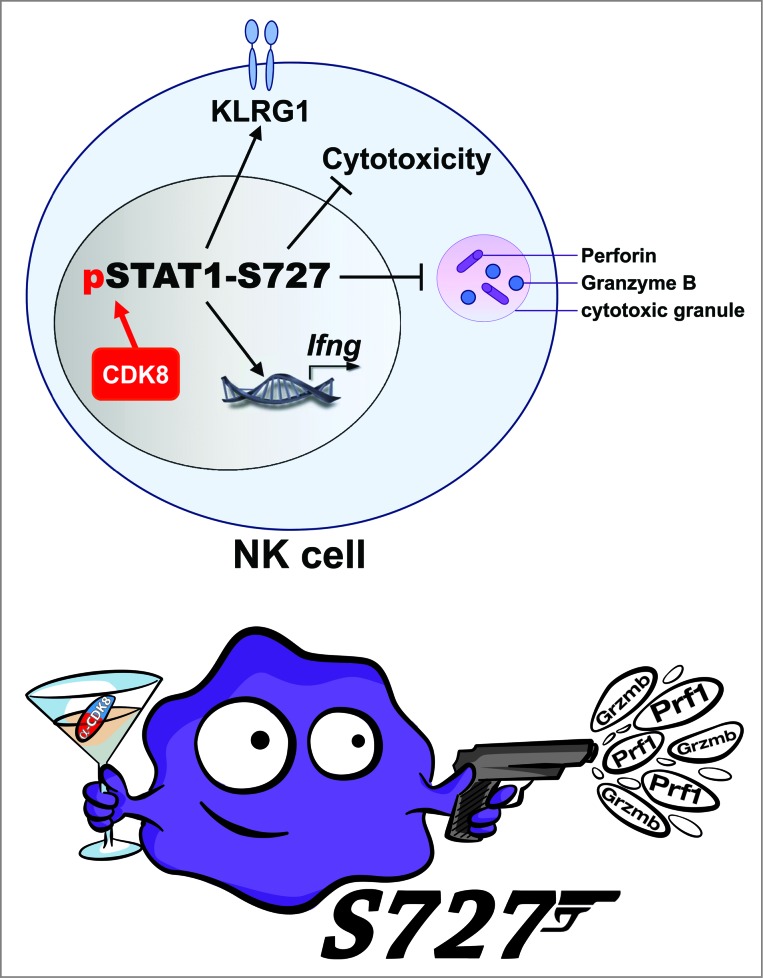
The identification of the serine/threonine kinase that phosphorylates STAT1-S727 in resting NK cells represents an additional twist to the tale. Several lines of evidence implicate cyclin-dependent kinase 8 (CDK8) in mediating this function. We thus postulate that inhibiting CDK8 in cancer patients might block STAT1-S727 phosphorylation and enhance NK cell cytotoxicity, as observed in a murine NK cell line (Fig. 1). Of course, targeting CDK8 might interfere with a plethora of other downstream targets. Although our data suggest that there is a window of opportunity for targeting CDK8 in cancer patients, considerable further experiments are required before CDK8 inhibitors can be recommended for clinical trials: the side-effects of blocking CDK8 in patients are likely to be wide-ranging and hard to predict.
Figure 1.
CDK8-mediated STAT1-S727 phosphorylation restrains NK cell cytotoxicity and tumor surveillance. Natural killer (NK) cells display a pronounced basal level of phosphorylation of signal transducer and activator of transcription 1 serine 727 (STAT1-S727), which is mediated by the serine/threonine kinase CDK8. STAT1-S727 phosphorylation attenuates NK cell cytotoxicity by restraining the production of perforin and granzyme B. Inhibiting STAT1-S727 phosphorylation via targeting CDK8 might thus represent a powerful tool to enhance NK cell cytotoxicity against cancer.
Despite their key roles in regulating growth and gene transcription, inhibitors of individual cyclin-dependent kinases have entered clinical trials. As such, CDK4/6 inhibitors represent a success story and were selected as “breakthrough therapy of the year” by the FDA. Inhibitors of CDK4/6 have fairly specific effects exerted on tumor cells and have been associated with only mild or tolerable side effects, so targeting CDKs may well provide a useful concept.
The development of an inhibitor targeting CDK8 may be of even greater clinical significance. Even when chemotherapy succeeds in eradicating all visible signs of cancer, it fails to target dormant cancer stem cells. Such stem cells can cause cancer relapse, which limits the success of the treatment. In leukemia, there is an increasing body of evidence that NK cells are able to eradicate leukemic stem cells and control minimal residual disease.5 Recent approaches have combined conventional therapy with immunotherapy, aiming to ensure sustained long-term success. There is a great demand for pharmaceuticals that can effect both tumor cell destruction and immune cell activation. CDK8 has recently been implicated as a tumor promoter in colorectal cancer,6 breast cancer7 and melanoma8 and seems to foster tumor cell dedifferentiation.9 CDK8 thus offers a promising molecular target to attack and directly destroy cancer cells. Our study also suggests that targeting CDK8 may enhance antitumor immunity via altering STAT-mediated NK cell activation. Taken together, CDK8 inhibitors may effectively kill 2 birds with one stone, attenuating bulk tumor cells while licensing NK cells to target cancer (stem) cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342:1432-3; PMID:; http://dx.doi.org/10.1126/science.342.6165.1432 [DOI] [PubMed] [Google Scholar]
- 2. Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10:230-52; PMID:; http://dx.doi.org/10.1038/cmi.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovacic B, Stoiber D, Moriggl R, Weisz E, Ott RG, Kreibich R, Levy DE, Beug H, Freissmuth M, Sexl V. STAT1 acts as a tumor promoter for leukemia development. Cancer Cell 2006; 10:77-87; PMID:; http://dx.doi.org/10.1016/j.ccr.2006.05.025 [DOI] [PubMed] [Google Scholar]
- 4. Putz EM, Gotthardt D, Hoermann G, Csiszar A, Wirth S, Berger A, Straka E, Rigler D, Wallner B, Jamieson AM, et al. CDK8-mediated STAT1-S727 phosphorylation restrains NK cell cytotoxicity and tumor surveillance. Cell Rep 2013; 4:437-44; PMID:; http://dx.doi.org/10.1016/j.celrep.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jewett A, Tseng H-C, Arasteh A, Saadat S, Christensen RE, Cacalano NA. Natural killer cells preferentially target cancer stem cells; role of monocytes in protection against NK cell mediated lysis of cancer stem cells. Curr Drug Deliv 2012; 9:5-16; PMID:; http://dx.doi.org/10.2174/156720112798375989 [DOI] [PubMed] [Google Scholar]
- 6. Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature 2008; 455:547-51; PMID:; http://dx.doi.org/10.1038/nature07179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li X-Y, Luo Q-F, Wei C-K, Li D-F, Fang L. siRNA-mediated silencing of CDK8 inhibits proliferation and growth in breast cancer cells. Int J Clin Exp Pathol 2013; 7:92-100; PMID: [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Kapoor A, Goldberg MS, Cumberland LK, Ratnakumar K, Segura MF, Emanuel PO, Menendez S, Vardabasso C, Leroy G, Vidal CI, et al. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature 2010; 468:1105-9; PMID:; http://dx.doi.org/10.1038/nature09590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adler AS, McCleland ML, Truong T, Lau S, Modrusan Z, Soukup TM, Roose-Girma M, Blackwood EM, Firestein R. CDK8 maintains tumor dedifferentiation and embryonic stem cell pluripotency. Cancer Res 2012; 72:2129-39; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-11-3886 [DOI] [PubMed] [Google Scholar]