Abstract
Immune responses to tumor antigens have been reported in cancer patients. However, the relevance of such spontaneous immune responses to the clinical course has not been studied extensively. We showed that the overall survival of patients with antibodies against NY-ESO-1 or XAGE1 (GAGED2a) antigen was prolonged in gastric or lung cancer patients, respectively.
Keywords: cancer/testis (CT) antigen, NY-ESO-1, XAGE1 (GAGED2a), overall survival, spontaneous antibody response
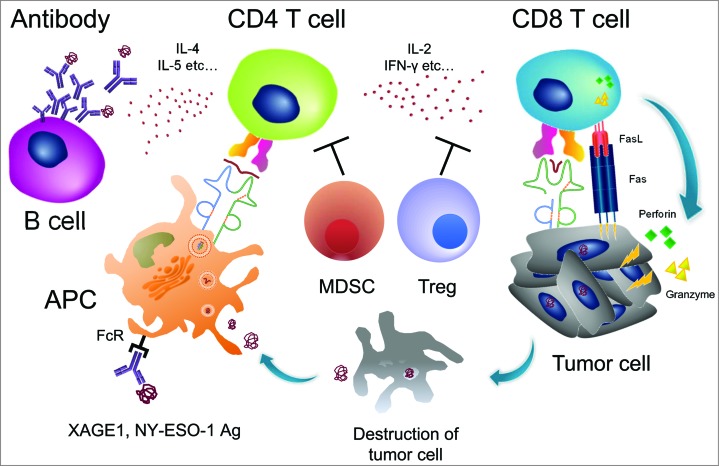
Cancer patients respond to their own tumors immunologically, and antibody responses, or CD4 and CD8 T-cell responses, against tumor cells or tumor cell products have been recognized (Fig. 1). Using such antibodies or T cells as probes, many tumor antigens have been identified in various human tumors over the past 2 decades. Those include mutated, differentiation, over-expressed and cancer/testis (CT) antigens. More recently, T-cell responses to various mutated peptides have been detected by whole exome analysis. The findings clearly indicate the presence of tumor antigens and the occurrence of immune responses to them in cancer patients. However, the relevance of such spontaneous immune responses to the clinical course has not been studied extensively. The reason for this appears to be the difficulty of detecting anti-tumor immune responses in patients because of the generally weak antigenicity of the tumor antigens together with the lack of a reliable methodology. However, some CT antigens, including NY-ESO-1 and XAGE1 (GAGED2a), have been shown to be highly immunogenic. Besides CT antigens, the tumor suppressor gene product p53 is also known to be strongly immunogenic. A previous study on spontaneous NY-ESO-1 immune responses in cancer patients revealed that antibody responses and CD4 and CD8 T-cell responses occur concomitantly as an integrated immune response.1 Because of its sensitivity and reproducibility as well as involving a simple assay procedure, an antibody response would be a useful immune biomarker to evaluate immune responsiveness in patients. Nevertheless, it should be noted that a split immune tolerance could take place depending on the antigens.2 NY-ESO-1 antigen readily elicits a CD8 T-cell response, but p53 has been reported as a weaker elicitor, irrespective of a similar efficiency of antibody induction with these antigens.
Figure 1.
Spontaneous immune responses to tumors in cancer patients. The cancer/testis (CT) antigens NY-ESO-1 and XAGE1 (GAGED2a) are strongly immunogenic, and an integrated immune response, consisting of an antibody response and CD4 and CD8 T-cell responses is frequently elicited spontaneously. The antibody response is a useful biomarker of immune responses because of its sensitivity and reproducibility, as well as involving a simple assay procedure. CD4 T-cell responses to CT antigens would be enhanced by the antigen-presenting cells (APC) that efficiently internalize the antigen/antibody complex via the Fc receptor (FcR) and promote the antibody response via IL-4 and IL-5 cytokines, and the CD8 T-cell response via IL-2 and IFNγ. CD8 T cells lyse tumor cells via Fas-FasL, perforin, and/or granzyme. Tregs and MDSCs suppress CD4 and CD8 T cells.
By the detection of antibody responses against CT antigens, we have investigated the clinical relevance of such spontaneous immune responses in cancer patients. In gastric cancer patients, we previously showed that an NY-ESO-1 antibody response was present at 3–4% in stage I and II, and 20–25% in stage III and IV patients, suggesting a higher antibody response rate in more advanced stage patients.3 Analysis of the overall survival in 310 gastric cancer patients with stages I to IV showed no difference between antibody-positive and -negative patients. However, in the 126 patients in stages III and IV, the overall survival of antibody positive patients was prolonged, although not significantly. It should be noted that NY-ESO-1 expression itself is tumorigenic, and patients with NY-ESO-1-antigen-positive tumors showed a shorter survival compared to patients with antigen-negative tumors in various cancer types.4 In our recent prospective study on advanced stage (stages IIIB and IV) lung adenocarcinoma patients, patients with antibody responses to XAGE1 (GEGED2a) showed a significantly prolonged overall survival compared to patients with no antibody responses.5 XAGE1 (GAGED2a) antigen expression was a worse predictor in patients with EGFR-mutated tumors. The XAGE1 (GAGED2a) antibody frequency in the advanced lung adenocarcinoma patients was similar (approximately 20%) to the frequency of NY-ESO-1 antibody responses in advanced gastric cancer patients. However, the patient cohort is more restricted at advanced stages in lung cancer compared to gastric cancer patients. Daudi, et al.6 recently reported that ovarian cancer patients with antibody responses to any of the MAGE family antigens showed a shorter survival compared to patients without antibody responses. In their study, patients with all stages were included, and so the shorter survival of advanced cancer patients should be carefully considered, as they discussed. A stage-controlled study is necessary to elucidate the effect of the antibody response on the clinical course. A similar overall survival-shortening effect of the antibody response was observed in patients with antibody against p53.7 Caution, as described in Daudi's report, should also be exercised when interpreting the results for p53.
Thus, in order to evaluate the clinical significance of the spontaneous antibody response in cancer patients, the clinical relevance of antigen expression should be carefully examined. The CT antigen expression itself generally worsens survival. Furthermore, it should be noted that the antibody response rate generally increases according to the stage. Therefore, the clinical benefit of the spontaneous antibody response should be evaluated in a patient cohort with restricted stages and antigen-positive tumors.
The presence of circulating NY-ESO-1-specific T-cells has been shown to be correlated with a favorable prognosis in melanoma patients.8 Conversely, the presence of MDSCs has been shown to be correlated with a poor prognosis.8 The clinical relevance of Foxp3-positive Treg infiltration is controversial, being correlated with poor survival in some cancers, but with better survival in others.9 In the latter, the contribution of nonspecific inflammation associated with Treg infiltration to the favorable prognosis has been suggested. Although such findings suggest an association of the immune phenotype with the clinical course in cancer patients, findings linking spontaneous immune responses to the clinical benefit are still limited. Our study, showing a link between NY-ESO-1 and XAGE1 (GAGED2a) antibody responses and prolonged overall survival, sheds light on the role of naturally occurring immune responses in cancer patients. Furthermore, we observed that genetic variants of immunoglobulin γ and κ chains influence the XAGE1 (GAGED2a) antibody response.10 The present findings should be confirmed with different patient cohorts and extended to other antigens in other cancers to establish a firm basis for immunotherapy.
References
- 1. Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res 2006; 95:1-30; PMID:; http://dx.doi.org/ 10.1016/S0065-230X(06)95001-5 [DOI] [PubMed] [Google Scholar]
- 2. Tsuji T, Matsuzaki J, Ritter E, Miliotto A, Ritter G, Odunsi K, Old LJ, Gnjatic S. Split T cell tolerance against a self/tumor antigen: spontaneous CD4+ but not CD8+ T cell responses against p53 in cancer patients and healthy donors. PLoS One 2011; 6:e23651; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0023651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujiwara S, Wada H, Kawada J, Kawabata R, Takahashi T, Fujita J, Hirao T, Shibata K, Makari Y, Iijima S, et al. NY-ESO-1 antibody as a novel tumor marker of gastric cancer. Br J Cancer 2013; 108:1119-1125, PMID:; http://dx.doi.org/ 10.1038/bjc.2013.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res 2005; 11:8055-8062, PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1203 [DOI] [PubMed] [Google Scholar]
- 5. Ohue Y, Kurose K, Mizote Y, Matsumoto H, Nishio Y, Isobe M, Fukuda M, Uenaka A, Oka M, Nakayama E. Prolongation of overall survival in advanced lung adenocarcinoma patients with the XAGE1 (GAGED2a) antibody. Clin Cancer Res 2014; pii: clincanres.0742. [Epub ahead of print]; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0742 [DOI] [PubMed] [Google Scholar]
- 6. Daudi S, Eng KH, Mhawech-Fauceglia P, Morrison C, Miliotto A, Beck A, Matsuzaki J, Tsuji T, Groman A, Gnjatic S, et al. Expression and immune responses to MAGE antigens predict survival in epithelial ovarian cancer. PLoS One 2014; 9:e104099, PMID:; http://dx.doi.org/ 10.1371/journal.pone.0104099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Müller M, Meyer M, Schilling T, Ulsperger E, Lehnert T, Zentgraf H, Stremmel W, Volkmann M, Galle PR. Testing for anti-p53 antibodies increases the diagnostic sensitivity of conventional tumor markers. Int J Oncol 2006; 29:973-980, PMID:; http://dx.doi.org/ 10.3892/ijo.29.4.973 [DOI] [PubMed] [Google Scholar]
- 8. Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res 2014; 20:1601-1609, PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2508 [DOI] [PubMed] [Google Scholar]
- 9. Tanchot C, Terme M, Pere H, Tran T, Benhamouda N, Strioga M, Banissi C, Galluzzi L, Kroemer G, Tartour E. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron 2013; 6:147-157, PMID:; http://dx.doi.org/ 10.1007/s12307-012-0122-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pandey JP, Namboodiri AM, Ohue Y, Oka M, Nakayama E. Genetic variants of immunoglobulin γ and κ chains influence humoral immunity to the cancer-testis antigen XAGE-1b (GAGED2a) in patients with non-small cell lung cancer. Clin Exp Immunol 2014; 176:78-83, PMID:; http://dx.doi.org/ 10.1111/cei.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]