Abstract
The tumor microenviroment and immune barrier are known to modulate malignant disease progression. We have recently identified Galectin-1 as a key player in a novel stromal regulatory reaction driving immune evasion in pancreatic tumors in vivo. These results suggest that Galectin-1 inhibition represents a potential therapeutic strategy for one of the most deadly types of cancer.
Keywords: Galectin-1, immune evasion, immunosurveillance, pancreatic cancer, stroma, tumor microenvironment
Pancreatic adenocarcinoma has a high mortality rate relative to other types of cancer, and to date, no treatment option has shown a significant long-term benefit in patients with unresectable tumors. Considering that the majority of cases fall into this category and to impact pancreatic cancer's dismal prognosis, the identification of new therapies is urgently needed. One of the major and quite unique hallmarks of pancreatic cancer is the abundant stroma surrounding epithelial cancer cells. This stroma is formed by a mixture of fibroblasts, endothelial cells, and immune cells embedded in a dense extracellular matrix. Emerging data have highlighted the important contribution of tumor stroma in pancreatic cancer maintenance, progression and chemotherapy resistance.1 In our recent article, “Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and Hedgehog signaling activation,”2 we define a novel role and molecular mechanism for Galectin-1 (Gal1) in pancreatic cancer epithelium and stroma crosstalk, and pinpoint this lectin as a potential molecular target for therapy.
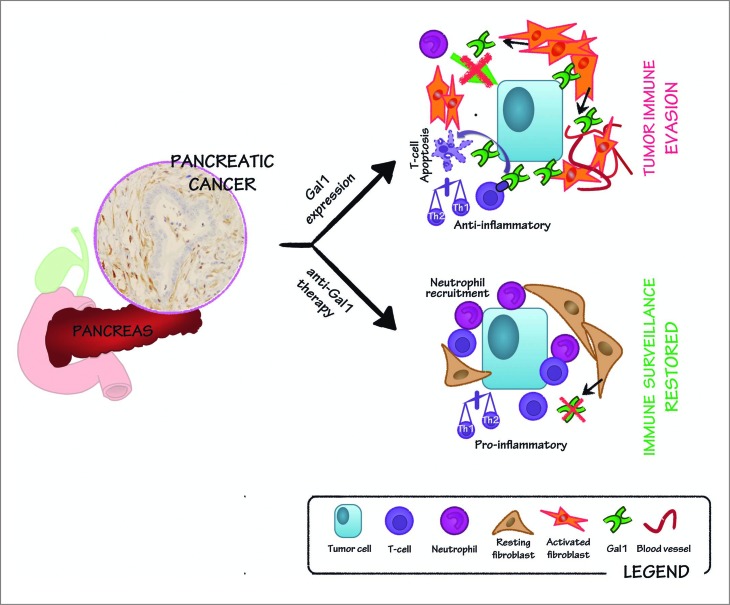
Gal1 is a glycan-binding protein that has been frequently found to be overexpressed in many cancers and, in particular, in human pancreatic tumor stroma,3 where its expression correlates with tumor stage and survival.4 In our recent publication,2 we used a transgenic mouse model overexpressing the oncogene Myc under the control of the elastase promoter (Ela-myc) to study the role of Gal1 during pancreatic tumor initiation and progression. Importantly, Ela-myc mice develop ductal tumors that express high levels of Gal1 in the stroma, mimicking human pancreatic cancer. Interestingly, crossing the Gal1 total knockout with the Ela-myc mouse, significantly increased animal survival, suggesting that this expression is functionally pro-tumorigenic. Histopathological analyses demonstrated that tumors lacking Gal1 show decreased tumor proliferation, angiogenesis as well as acinar-to-ductal metaplasia and stroma formation, a feature of the microenvironment crucial for tumor-initiation, maintenance and progression. Interestingly, Gal1 depletion also increased T-cell and neutrophil infiltrates in the tumors (Fig. 1).2 Moreover, analyses using xenografts after injection of wild-type or Gal1 depleted pancreatic carcinoma cells, showed no differences in tumor progression,2 suggesting a role for Gal1 in the modulation of the immune microenvironment.
Figure 1.
Role of Gal1 in stroma remodeling and immune escape in pancreatic cancer: implications in anti-Gal1 blockade therapy. Galectin-1 (Gal1) is highly expressed in the stroma of pancreatic tumors (see immunohistochemistry on the left). Gal1 promotes angiogenesis and is involved in pancreatic stellate cell activation and proliferation. Thus, Gal1 functionally contributes to the intense stromal reaction commonly occurring in pancreatic tumors (right, upper panel). Moreover, Gal1 impairs neutrophil recruitment and induces T-cell apoptosis, and favors a T helper type 2 (Th2) anti-inflammatory environment, thus resulting in tumor immune evasion (right, upper panel). Blocking Gal1 in pancreatic tumors hampers tumor progression by reducing angiogenesis, stromal activation and restoring immunosurveillance, leading to reduced tumor growth (right, lower panel).
Gal1 has been reported to build an immunological barrier in progressing cancer by inhibiting full T-cell activation and proliferation, as well as by promoting apoptosis of T cells.5 Besides, Gal1 avoids the tumor immune attack by negatively regulating T helper type 1 (Th1) and pro-inflammatory cytokine secretion while favoring a T helper type 2 (Th2) anti-inflammatory environment, which has also been observed in pancreatic stellate cells in vitro.6 Gal1 is able to modulate its effects on T cells by binding to known cell surface receptors5 and, further, it has been reported that these receptors can be more or less exposed to the lectin depending on their glycosylation profile. Although the functional mechanism is much more well characterized for T cells, evidence suggests that Gal1 affects other immune cell types also, resulting in a profound immunosuppressive microenvironment. For instance, in accordance with our findings in Ela-myc pancreatic tumors, Gal1 has been reported to inhibit neutrophil chemotaxis and migration in vitro.7 In our study, we provide for the first time, in vivo data proving that the absence of Gal1 is sufficient to restore, at least in part, immunosurveillance in pancreatic cancer.2
Because of the lack of effective treatments for pancreatic ductal adenocarcinoma, immune-based therapy has recently emerged as an alternative.8 Most of the current cancer immunotherapies are focused on the induction of the immune system response toward antigens preferentially expressed by cancer cells. These cancer vaccines – for example, the use of mutated K-RAS, mucin-1 or mesothelin, among others– are in clinical or preclinical studies for pancreatic cancer.8 Nevertheless, the strong immunosuppressive environment found in pancreatic tumors may preclude the efficiency of such strategies. Indeed, pancreatic cancer patients with the worst prognosis display lower numbers of tumor-infiltrating T cells and increased anti-inflammatory Th2 responses.9 Therefore, there is an increasing interest in developing new treatments targeting pancreatic cancer's ability to evade immune attack. In this regard, our data reporting increased number of T cells and neutrophils upon Gal1 depletion in pancreatic cancer in vivo shows that targeting Gal1 may be considered a new treatment to restore the cancer immunological barrier. Interestingly, several Galectin inhibitors and monoclonal antibodies have already been reported to show anti-proliferative and anti-angiogenic effects in different tumor types,10 opening the door for using these inhibitory strategies to block Gal1-mediated immunosuppression in pancreatic cancer. Moreover, the fact that Gal1 knockout mice are viable and fertile strengthens the hypothesis that Gal1 blockade could be a safe cancer therapy.
Nevertheless, despite these promising preclinical data, several aspects deserve attention before anticipating clinical applications of Gal1 inhibition. First, galectins’ inherent complexity requires researchers to be cautious when designing agents targeting the lectin, especially with chemical inhibitors, which typically are not specific for a single galectin. Many factors could confound Gal1-inhibitory effects, including: Gal1 reported pleiotropic functions, protein conformation, oxidation state, the dynamic glycan repertoire of its receptors, subcellular localization, cell type, and cell status, among others. Secondly, we must be aware that our results may not directly translate from mouse to humans, particularly considering differences in lectin display and specificity, as well as the distinct glycomic and immunologic profiles found in both species. However, considering the high expression of Gal1 in human pancreatic cancer and our recently published data showing that Gal1 depletion affects multiple anticancer battle front lines–boosting the immune response, decreasing tumor cell proliferation and remodeling tumor stroma (Fig. 1)2–is tempting to propose Gal1 as a strong candidate for pancreatic cancer therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work was supported by research grants from MICINN ISCII-FEDER (PI11/01562) and Generalitat de Catalunya (2009SGR1409 and 2014 SGR143) to PN. NMB was supported by a grant from Fundación Ramón Areces.
References
- 1.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21:418-29; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Bosch N, Martinez-Bosch MG, Moreno M, Ortiz-Zapater E, Munne-Collado J, Iglesias M, André S, Gabius HJ, Hwang RF, Poirier F, et al. Galectin-1 Drives pancreatic carcinogenesis through stroma remodeling and hedgehog signaling activation. Cancer Res 2014; 74:3512-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roda O, Ortiz-Zapater E, Martinez-Bosch N, Gutierrez-Gallego R, Vila-Perello M, Ampurdanes C, Gabius HJ, Andre S, Andreu D, Real FX, et al. Galectin-1 is a novel functional receptor for tissue plasminogen activator in pancreatic cancer. Gastroenterology 2009;136:1379-5; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2008.12.039 [DOI] [PubMed] [Google Scholar]
- 4.Chen R, Pan S, Ottenhof NA, de Wilde RF, Wolfgang CL, Lane Z, Post J, Bronner MP, Willmann JK, Maitra A, et al. Stromal galectin-1 expression is associated with long-term survival in resectable pancreatic ductal adenocarcinoma. Cancer Biol Ther 2012; 13:899-907; PMID:; http://dx.doi.org/ 10.4161/cbt.20842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K, Stannard K, Gabutero E, Clark AM, Neo SY, Onturk S, Blanchard H, Ralph SJ. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment. Cancer Metastasis Rev 2012; 31:763-8; PMID:; http://dx.doi.org/ 10.1007/s10555-012-9388-2 [DOI] [PubMed] [Google Scholar]
- 6.Tang D, Yuan Z, Xue X, Lu Z, Zhang Y, Wang H, Chen M, An Y, Wei J, Zhu Y, et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer 2012; 130:2337-48; PMID:; http://dx.doi.org/ 10.1002/ijc.26290 [DOI] [PubMed] [Google Scholar]
- 7.La M, Cao TV, Cerchiaro G, Chilton K, Hirabayashi J, Kasai K, Oliani SM, Chernajovsky Y, Perretti M. A novel biological activity for galectin-1: inhibition of leukocyte-endothelial cell interactions in experimental inflammation. Am J Pathol 2003; 163:1505-15; PMID:; http://dx.doi.org/ 10.1016/S0002-9440(10)63507-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sideras K, Braat H, Kwekkeboom J, van Eijck CH, Peppelenbosch MP, Sleijfer S, Bruno M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev 2014; 40:513-22; PMID:; http://dx.doi.org/ 10.1016/j.ctrv.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Wormann SM, Diakopoulos KN, Lesina M, Algul H. The immune network in pancreatic cancer development and progression. Oncogene 2014; 33:2956-67. [DOI] [PubMed] [Google Scholar]
- 10.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, Serova M, Neuzillet C, Albert S, Raymond E, Faivre S. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat Rev 2014; 40:307-19; PMID:; http://dx.doi.org/ 10.1016/j.ctrv.2013.07.007 [DOI] [PubMed] [Google Scholar]