Abstract
Aiming to increase the potency of synthetic long peptide (SLP)-based cancer vaccines, the Toll-like receptor 2 (TLR2) ligand Pam3CSK4 was conjugated in a chemically defined fashion to SLPs harbouring both cytotoxic T lymphocyte (CTL) and T helper epitopes. We recently showed that these SLP-conjugates induce strong antitumor immunity in murine cancer models.
Keywords: conjugate, immunotherapy, lymphoma, melanoma, synthetic long peptides, SLP, Toll-like receptor, TLR2, Tumor, vaccination
Vaccines consisting of multiple overlapping synthetic long peptides (SLPs) constitute a promising immunization approach.1 SLP-based vaccines comprise all potential CD4- and CD8-reactive epitopes within an antigen of choice for presentation with a wide range of HLA-alleles. We have shown the efficacy of such a vaccine, consisting of SLPs of HPV16 E6 and E7, in treating patients with premalignant HPV16-induced vulvar intraepithelial neoplasia (VIN).2 In this Phase II trial, more than 50% of SLP vaccine-treated patients experienced a complete or partial regression of lesions. In contrast, patients with advanced HPV16-induced cervical lesions failed to exhibit clinically relevant responses to the SLP vaccine, although all patients displayed increased blood frequencies of HPV-specific T cells in peripheral blood, albeit at lower levels than the complete responders in the VIN study.3 Results from another trial in patients with cervical premalignant lesions indicated that the HPV16 SLP vaccine induced T helper type 2 (Th2) T cell responses long after vaccination,4 suggesting the vaccine may be improved by the addition of a T helper type 1 (Th1)-skewing adjuvant.
We have previously shown that covalent attachment of a synthetic Toll-like receptor ligand (TLR-L) to an SLP in a one-to-one stoichiometry improves the efficacy of SLP vaccination in several ways. Firstly, a TLR-L SLP conjugate has been shown to be efficiently targeted to TLR-expressing dendritic cells (DC) in vivo.5 Secondly, the synthetic triacylated lipoprotein and TLR2 ligand Pam3CSK4, and oligonucleotide and TLR9 ligand CpG, retained their DC maturation capacity upon covalent conjugation to a long peptide molecule, affecting expression of pro-inflammatory cytokines, up-regulation of co-stimulatory molecules and efficient cross-presentation by DCs.5 Furthermore, the ability of the TLR-L to mature DCs has been shown to be essential in TLR-L SLP conjugate-mediated priming of endogenous CD8+ T cells induced against a model antigen.6 Thirdly, we described the formation of an antigen storage depot within matured DCs upon uptake of conjugated SLP, which allows for prolonged cross-presentation of MHC Class I epitopes.7 Taken together, these properties explain the efficacy of TLR-L SLP conjugates in our recent vaccination study inducing antitumor immunity against melanoma and lymphoma in mice (see Fig. 1).
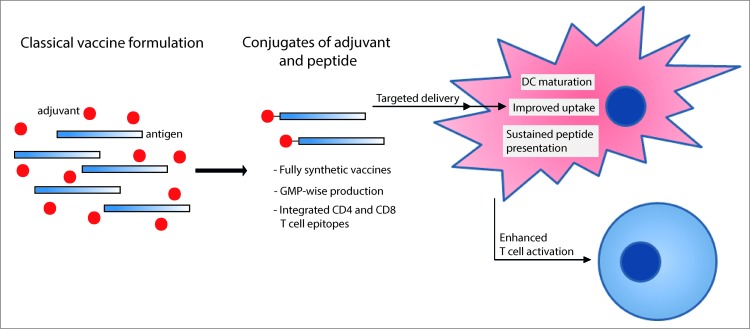
Figure 1.
Covalent attachment of adjuvants to long antigenic peptides results in improved immunological efficacy of fully synthetic vaccines. Biological and logistic advantages are indicated, including uptake, the ability to induce dendritic cell (DC) maturation and durability of presentation, all of which impact enhanced T cell activation.
In our recent report,1 we set out to determine the efficacy of different TLR2-L SLP conjugates harbouring either tumor-specific CTL or T helper epitopes in a subcutaneous vaccination approach in 2 aggressive murine tumor models. The conjugated SLPs used in this study were shown to induce robust T-cell activation in vitro and in vivo. T cells exposed to DCs loaded with a TLR2-L SLP conjugate in vitro produced more Th1-type cytokines. Importantly, vaccination with conjugated SLPs induced more effective antitumor immunity than the comparative mixture of SLPs and equimolar amounts of TLR2-L. It was estimated that the effective dose of conjugates was at least 50-fold lower than that of the equivalent mixtures. Besides, our data showed that the integration of CD4+ T cell epitopes in the vaccine were essential for tumor control. Control of the aggressive leukemia virus-induced RMA lymphoma8 by CD4+ T cell-dependent immunity, could even be achieved in the majority of mice with a single dose of TLR2-L SLP conjugate harbouring a CD4+ T cell epitope. This shows that TLR-L-SLP conjugates not only support CTL priming by effective processing through the MHC Class I cross-presentation route but also enhances T helper cell priming by effective MHC Class II peptide presentation.
TLR-ligands have received considerable attention to be incorporated into vaccination regimens as they bridge innate signaling and adaptive immune responses.9 Up to now, the TLR7-L imiquimod, the bacillus Calmette-Guerin (BCG) and the vaccine adjuvant monophosphoryl lipid A (MPL) are the only TLR-ligands that are approved by the Food and Drug Administration for use as either standalone therapies or as a vaccine adjuvant.10 We initiated studies on different types of TLR-L SLP conjugates as potential vaccines. In this setting, we found that a small-molecule based TLR7-L loses its potency to trigger its receptor upon conjugation to an SLP.6 Contrary to this, the TLR9-L CpG was successfully conjugated to SLP with intact biological activity, although it did not outperform SLP conjugated to the TLR2-L Pam3CSK4.5 Besides, TLR9 is only selectively expressed on human DC types and is therefore less suitable for clinical translation. In view of manufacturing, conjugating nucleotide-based TLR-ligands is less favorable than Pam3CSK4, which can be N-terminally conjugated to the SLP produced by ‘in-line’ automatic peptide synthesis. For future clinical studies we have successfully synthesized TLR2-L SLP conjugates of HPV16 E6 by this procedure of good manufacturing procedure (GMP) quality. These conjugates were able to activate HPV16-E6 specific patient-derived T cell clones (unpublished data). In addition, we have further optimized the potency of TLR2-L Pam3CSK4 by chemical modification,11 a variant that we will further explore for clinical applications.
In the coming years, a Phase I/II clinical trial is planned to assess safety and immunogenicity of optimized TLR2-L SLP conjugates in patients with HPV-induced cancer. A plethora of clinical applications of these synthetic adjuvants and therapeutic cancer vaccines can be envisaged to further improve immunotherapy regimens. These may include combination treatment with immunomodulatory drugs, such as ipilimumab and nivolumab, application along with Treg-depleting therapies, or together with chemo- or radiotherapy. Recently, we have observed decreased levels of myeloid derived suppressor cells in advanced stage cervical cancer patients after chemotherapy, favoring SLP-vaccine induced T-cell responses (unpublished observations).
The TLR-L conjugated SLP platform by itself is very versatile and is amenable to any antigen. Therefore, this novel approach constitutes a step forward in personalized treatment, as SLPs of cancer- and patient-specific epitopes and neoantigens can readily be synthesized.
Funding Statement
This work has been funded by Netherlands Organization for Scientific Research (NWO) as a part of the ‘From Molecule to Cell’ program (to DVF, GAM, and FO), a Dr Mildred Scheel-Grant from the Deutsche Krebshilfe (CMB), and the framework of project D1-101 within of the Dutch Top Institute Pharma (to CM, FO, HO and SK) and a Dutch Cancer Society (KWF) grant (GGZ).
Disclosure of Potential Conflicts of Interest
CJ Melief is chief scientific officer of the biotech company ISA (Immune System Activation), aiming to develop synthetic peptide–based cancer vaccines, including conjugates between a proprietary TLR ligand and synthetic long peptides. As CSO, CJ Melief receives a salary from ISA and is in possession of stock appreciation rights.
References
- 1.Zom GG, Khan S, Britten CM, Sommandas V, Camps MGM, Loof NM, Budden CF, Meeuwenoord NJ, Filippov DV, van der Marel GA, et al. Efficient induction of antitumor immunity by synthetic Toll-like receptor ligand-peptide conjugates. Cancer Immunol Res 2014; PMID: [DOI] [PubMed] [Google Scholar]
- 2.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009; 361:1838-47; PMID:; http://dx.doi.org/10.1056/NEJMoa0810097 [DOI] [PubMed] [Google Scholar]
- 3.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 2008; 14:178-87; PMID:; http://dx.doi.org/10.1158/1078-0432.CCR-07-1880 [DOI] [PubMed] [Google Scholar]
- 4.de Vos van Steenwijk PJ, van Poelgeest MI, Ramwadhdoebe TH, Lowik MJ, Berends-van der Meer DM, van der Minne CE, Loof NM, Stynenbosch LF, Fathers LM, Valentijn AR, et al. The long-term immune response after HPV16 peptide vaccination in women with low-grade pre-malignant disorders of the uterine cervix: a placebo-controlled phase II study. Cancer Immunol Immunother 2014; 63:147-60; PMID:; http://dx.doi.org/10.1007/s00262-013-1499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan S, Bijker MS, Weterings JJ, Tanke HJ, Adema GJ, van HT, Drijfhout JW, Melief CJ, Overkleeft HS, van der Marel GA, et al. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J Biol Chem 2007; 282:21145-59; PMID:; http://dx.doi.org/10.1074/jbc.M701705200 [DOI] [PubMed] [Google Scholar]
- 6.Zom GG, Khan S, Filippov DV, Ossendorp F. TLR ligand-peptide conjugate vaccines: toward clinical application. Adv Immunol 2012; 114:177-201; PMID:; http://dx.doi.org/10.1016/B978-0-12-396548-6.00007-X [DOI] [PubMed] [Google Scholar]
- 7.van Montfoort N, Camps MG, Khan S, Filippov DV, Weterings JJ, Griffith JM, Geuze HJ, van Hall T, Verbeek JS, Melief CJ, et al. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc Natl Acad Sci U S A 2009; 106:6730-5; PMID:; http://dx.doi.org/10.1073/pnas.0900969106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med 1998; 187:693-702; PMID:; http://dx.doi.org/10.1084/jem.187.5.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 2005; 115:739-46; PMID:; http://dx.doi.org/10.1172/JCI23373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H. TLR Agonists for cancer immunotherapy: tipping the balance between the immune stimulatory and inhibitory effects. Front Immunol 2014; 5:83; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willems MM, Zom GG, Khan S, Meeuwenoord N, Melief CJ, van der Stelt M, et al. N-Tetradecylcarbamyl Lipopeptides as Novel Agonists for Toll-like Receptor 2. J Med Chem 2014; 57:6873-8. [DOI] [PubMed] [Google Scholar]