Supplemental Digital Content is available in the text.
Abstract
Background:
Fibromodulin (FMOD) plays a critical role in the wound-healing process. Our previous studies revealed that FMOD deficiency led to marked alterations in adult wound healing characterized by delayed dermal cell migration, postponed wound closure, and increased scar formation, all accompanied by impeded angiogenesis. Therefore, the aim of this study was to reveal the effect of FMOD on angiogenesis during the wound-healing process.
Methods:
In vivo angiogenic effects of FMOD were assessed by a chick embryo chorioallantoic membrane assay, a Matrigel (BD Bioscience, Franklin Lakes, N.J.) plug implant assay, and rodent primary closure wound models. In vitro angiogenic effects of FMOD were recorded by cell invasion and dimensional and topological parameters of human umbilical vein endothelial cells.
Results:
We provided evidence that FMOD significantly enhanced vascularization: first, FMOD boosted blood vessel formation on the chorioallantoic membrane; second, FMOD markedly stimulated capillary infiltration into Matrigel plugs subcutaneously implanted in adult mice; and finally, FMOD robustly promoted angiogenesis in multiple adult rodent cutaneous wound models. Furthermore, FMOD administration restored the vascularity of fmod−/− mouse wounds. In support of this, FMOD endorsed an angiogenesis-favored microenvironment in adult rodent wounds not only by upregulating angiogenic genes but also by downregulating angiostatic genes. In addition, FMOD significantly enhanced human umbilical vein endothelial cell invasion and tube-like structure formation in vitro.
Conclusions:
Altogether, we demonstrated that in addition to reducing scar formation, FMOD also promotes angiogenesis. As blood vessels organize and regulate wound healing, its potent angiogenic properties will further expand the clinical application of FMOD for cutaneous healing of poorly vascularized wounds.
Cutaneous wound healing is a natural response involving a complex cascade of cellular events to generate resurfacing, reconstitution, and restoration of tensile strength of injured skin. Unfortunately, the reasoning behind the failure of some cutaneous wounds to heal is still poorly understood due to the fact that wound healing is a complex, multifaceted process.1,2 A fundamental problem of retarded wound healing is lack of a functional extracellular matrix (ECM) to stimulate, direct, and coordinate healing. For instance, deficiency of a single ECM molecule, fibromodulin (FMOD), in an adult mouse cutaneous wound model resulted in delayed dermal fibroblast migration, delayed granulation tissue formation, delayed wound closure, and subsequently increased scarring.3
FMOD is a broadly distributed small leucine-rich proteoglycan (SLRP), which regulates ECM assembly, organization, and degradation via binding with collagens.4–10 FMOD plays an essential role in cell fate determination and fetal scarless wound healing.5,11–14 In addition, our previous studies have demonstrated that FMOD controls significant aspects of adult cutaneous wound healing. Compared with their wild-type (WT) counterparts, FMOD-null (fmod−/−) mice have reduced fibronectin deposition, unorganized collagen architecture, altered transforming growth factor (Tgf)β signaling, and reduced dermal fibroblast infiltration followed by impeded angiogenesis.3,4,15 On the other hand, FMOD administration in both adenoviral and protein forms reduced scar formation in adult cutaneous wounds.16,17 Specifically, we have demonstrated that FMOD significantly promoted fibroblast migration into the wound area, aiding timely wound closure and reduced scar formation.3,15,18
Because newly generated blood vessels provide nutrients to support active cells, promote granulation tissue formation, and facilitate clearance of debris,19–21 wound healing cannot occur without angiogenesis, a process of neovascular formation by endothelial cells (ECs). Our previous studies revealed that retarded fmod−/− mouse wound healing is associated with markedly reduced blood vessel regeneration,3 suggesting a direct relationship between FMOD and angiogenesis. In this study, the effects of FMOD on angiogenesis under both uninjured and wounded scenarios were investigated.
MATERIALS AND METHODS
Ethics Statement
All animal surgeries were performed under institutional approved protocols provided by Chancellor’s Animal Research Committee at University of California, Los Angeles (protocol number: 2000-058).
In Ovo Chick Embryo Chorioallantoic Membrane Assay
The in ovo chorioallantoic membrane (CAM) assay was performed as previously described.22,23 Fertilized chicken eggs (Charles River Labs, North Franklin, Conn.) were incubated at 37°C and 60% relative humidity in an egg incubator. On day 3, 5-ml albumin was withdrawn from the pointed end of the egg. Rectangle windows were cut into the shell as a portal of access for the CAM. On day 10, 2.0 mg/ml FMOD in 30 μl 1:3-diluted growth-factor-reduced Matrigel (BD Bioscience, Franklin Lakes, N.J.) was loaded on an autoclaved 5 × 5-mm polyester mesh layer (grid size: 530 μm; Component Supply Company, Fort Meade, Fla.) and incubated for 45 minutes at 37°C for gel formation before transplantation onto the CAM. A non-FMOD phosphate buffered saline (PBS) control was transplanted onto the same CAM with a 1-cm distance. On day 13, CAMs were excised and photographed. The capillary area density directly under the mesh was measured by ImageJ (National Institutes of Health, Bethesda, Md.).24
Matrigel Plug Assay
Four hundred μl of growth-factor-reduced Matrigel containing 0 or 4.0 mg/ml FMOD was subcutaneously injected into the abdomen of adult 129/sv male mice, which were harvested with the overlying skin 14 days post injection.25
Wound Generation
Four (per adult male 129/sv mouse) or 6 (per adult male Sprague-Dawley rat) full thickness, 10 mm × 3 mm skin ellipses with the underlying panniculus carnosus muscle were excised from each animal. All wounds were separated by at least 2 cm to minimize adjacent wound effects. Each open wound edge was injected with 25 μl PBS or 0.4 mg/ml recombinant human FMOD in PBS (25 μl × 2 edges = 50 μl/wound) before being primarily closed. Sutures were removed at day 7 post injury, and wounds were harvested at 14 days post injury. Tissues were bisected centrally for histology or gene expression analysis.3,4,14,15
Histology and Immunohistochemistry Staining
After fixation in 4% paraformaldehyde, samples were dehydrated, paraffin-embedded, and sectioned at 5-μm increments for hematoxylin and eosin (H&E), picrosirius red (PSR), and histology and immunohistochemistry staining.3,4 PSR-coupled polarized light microscopy (PSR) was used to identify the wound area.3 Blood vessels were identified and quantitated by von Willebrand factor (Abcam Inc., Cambridge, Mass.).
Gene Expression Assay
RNA was isolated using RNeasy Mini Kit with DNase treatment (Qiagen, Valencia, Calif.),3,15 and 1.0 μg mouse RNA was used for reverse transcription with iScript Reverse Transcription Supermix for real time-quantitative polymerase chain reaction (Bio-Rad Laboratories, Hercules, Calif.). Quantitative real time polymerase chain reaction (qRT-PCR) was performed with TaqMan Gene Expression Assays (Life Technologies, Grand Island, N.Y.) and SsoFast Probes Supermix with ROX (Bio-Rad Laboratories) on a 7300 Real-Time PCR system (Life Technologies). Meanwhile, 2.5 μg RNA isolated from adult rat wounds was injected into RT2 First Strand Kit (Qiagen) for reverse transcription. qRT-PCR was performed in a 96-well format of rat wound-healing RT2 PCR Array (Qiagen) according to the manufacturer’s protocol. Three different cDNA templates were tested. Concomitant glyceraldehyde 3-phosphate dehydrogenase (gapdh) was used as a housekeeping standard. Data analysis was achieved by the manufacturer’s online services (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php).
Cell Culture
Passages 3–6 human umbilical vein endothelial cells (HUVECs) were cultured in Medium 200PRF supplied with Low Serum Growth Supplement according to manufacturer instruction (Life Technologies).
Tube-like Structure Formation Analysis
Technologies Endothelial Tube Formation Assay protocol provided by Life Technologies (http://www.lifetechnologies.com/us/en/home/references/protocols/cell-and-tissue-analysis/cell-profilteration-assay-protocols/angiogenesis-protocols/endothelial-cell-tube-formation-assay.html) was used to assay tube-like structure (TLS) in vitro. Briefly, a 24-well plate was coated with 100 μl/well reduced growth factor basement membrane matrix for 1 hour at 37°C before being seeded with 2.5 × 104 HUVECs in Medium 200PRF supplied with different doses of FMOD. Five images per well and 4 wells per treatment were documented after 4 hours by using an Olympus fluorescent microscope (Center Valley, Pa.). Images were assessed by recording dimensional and topological analyses with ImageJ (http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ.html&lang=en#outil_sommaire_0).
Cell Invasion Assay
Cell invasion assay was performed in 24-well tissue culture plates using HTS Fluoroblok inserts with 8-μm pore size fluorescence blocking Poly(ethylene terephthalate) track-etched membranes (BD Bioscience). The upper surfaces of the inserts were coated with 100 μl 2 mg/ml reduced growth factor basement membrane matrix (Geltrex; Life Technologies) and placed into 24-well tissue culture plates containing 750 μl medium; 2.5 × 104 HUVECs in 500 μl medium with different doses of FMOD were added to each insert chamber and allowed to invade toward the underside of the membrane for 24 hours. Noninvading cells were removed by wiping the upper side of the membrane with a cotton swab. Invaded cells were fixed and stained with 0.4 mg/ml 4′,6-diamino-2-phenlindole (Sigma-Aldrich, St. Louis, Mo.) before counting.3
Statistical Analysis
Statistical significance was performed by OriginPro 8 (Origin Lab Corp., Northampton, Mass.), including 1-way analysis of variance, paired t test, 2-sample t test, and Mann-Whitney analyses. P values less than 0.05 were considered statistically significant.
RESULTS
FMOD Promotes Vascularization in Uninjured Scenarios
FMOD-administrated CAMs showed a 1.5 times greater proportion of blood vessels with large diameters than the PBS control (Fig. 1), confirming that FMOD promotes vasculogenesis during development. As angiogenesis in adults may differ in important ways from the process during development,26 a predocumented Matrigel plug assay25 was used to confirm the proangiogenic action of FMOD in vivo. FMOD markedly elevated angiogenesis in Matrigel plugs subcutaneously implanted in adult mice, whose capillary densities were 4-fold that of non-FMOD plugs. (See figure, Supplemental Digital Content 1, which displays Matrigel plugs subcutaneously injected into the abdomen of adult 129/sv male mouse, http://links.lww.com/PRSGO/A77.) Thus, FMOD is a proangiogenic factor in uninjured scenarios.
Fig. 1.
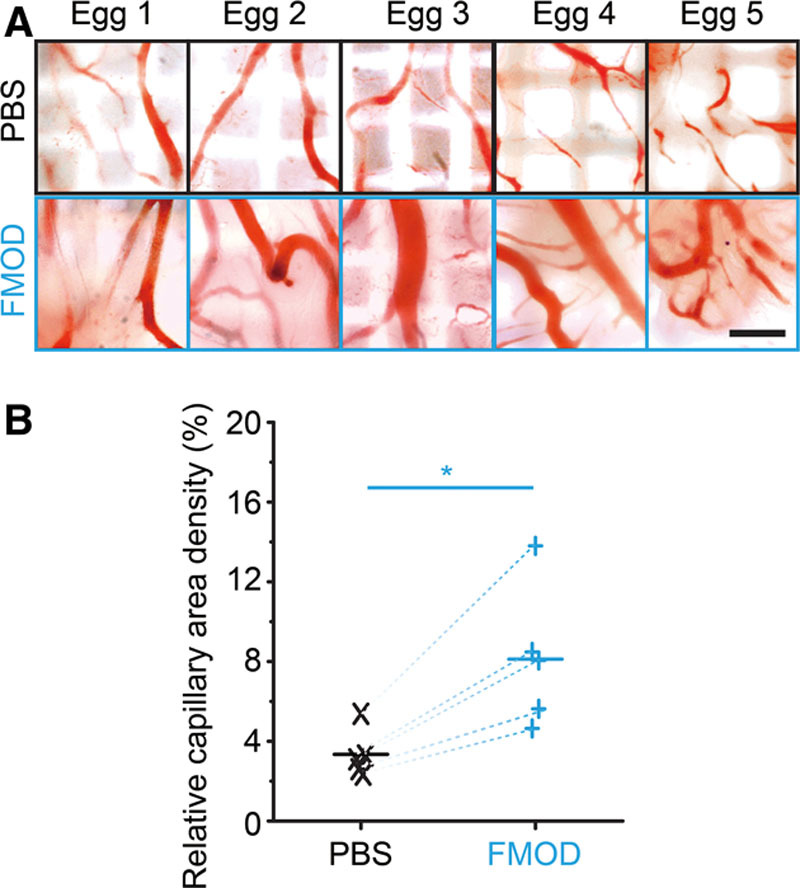
Effects of FMOD on vascularization assessed by in ovo CAM assay. Macroscopic photographs (A) and computerized quantitation (B) showed significantly increased more capillary generation on 30 μl 2.0 mg/ml FMOD-treated CAMs than on PBS-control groups. Significant differences compared by paired t test (P < 0.05) are marked with asterisks (n = 5). Bar = 500 μm.
FMOD Is Important for Angiogenesis during Wound Healing
In agreement with our previous studies at day 7 post injury,3 vascular generation in adult fmod−/− mouse skin wounds at day 14 post injury was diminished by approximately 50% as compared with the age-matched WT wounds (Fig. 2). [See Supplemental Digital Content 2, which displays H&E staining and a PSR-coupled polarized light microscopy demonstration of adult mouse cutaneous wounds (outlined by dashed lines) at day 14 post injury, http://links.lww.com/PRSGO/A78.] On the contrary, exogenous FMOD administration restored vascularity of fmod−/− wounds to the same level as that of FMOD-treated WT wounds, further signifying that FMOD deficiency was responsible for the reduced angiogenesis in fmod−/− mouse wounds (Fig. 2). Additionally, capillary density of FMOD-treated adult WT mouse skin wounds was approximately 2.6 times greater than that of PBS-control groups (Fig. 2). This is in agreement with the finding that FMOD administration into an established adult rat cutaneous wound model causes a significant increase in wound vascularity (Fig. 3). (See figure, Supplemental Digital Content 3, which displays H&E staining of adult rat cutaneous wounds at day 14 post injury, http://links.lww.com/PRSGO/A79.) Therefore, these results strongly endorse our hypothesis that FMOD is angiogenic in both uninjured and wounded scenarios.
Fig. 2.
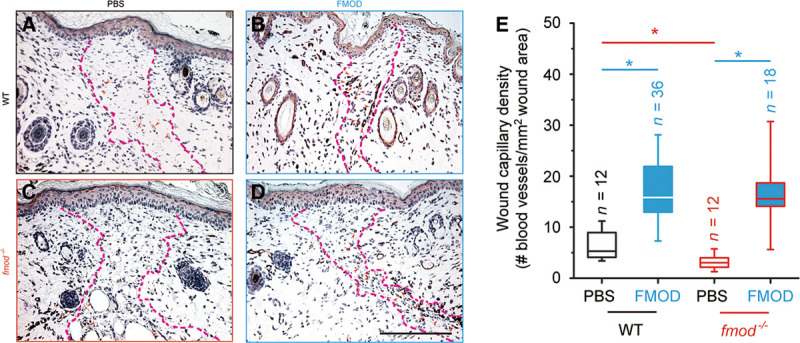
von Willebrand factor staining of adult mouse cutaneous wounds. Sections of PBS-treated WT (A), FMOD-treated WT (B), PBS-treated fmod−/− (C), FMOD-treated fmod−/− (D) wounded mouse skin at day 14 post injury, whose wound capillary density was quantitated (E). Wound areas are outlined by dashed lines and blood vessels are indicated by red arrowheads. (See Supplemental Digital Content 2, which displays H&E staining and a PSR-coupled polarized light microscopy demonstration of adult mouse cutaneous wounds (outlined by dashed lines) at day 14 post injury, http://links.lww.com/PRSGO/A78.] FMOD: 0.4 mg/ml × 50 μl/wounds. Significant differences compared by Mann-Whitney analysis (P < 0.05) are marked with asterisks: red asterisk indicates significance resulting from fmod knockout, and blue asterisks indicate significance resulting from FMOD administration. Bar = 200 μm.
Fig. 3.
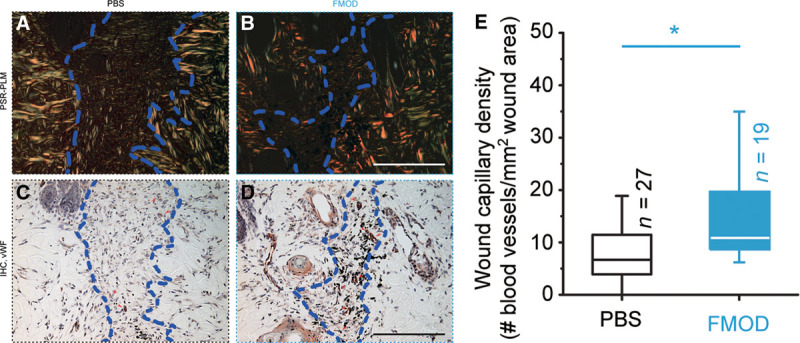
von Willebrand factor (vWF) staining of rat mouse cutaneous wounds at day 14 post injury. PSR-coupled polarized light microscopy demonstrated the wound area (A, B; outlined by dashed lines), whereas the blood vessels were identified by histology and immunohistochemistry staining against vWF (C, D; red arrowheads) and were quantitated (E). H&E staining of the identical wounds were shown in Supplemental Digital Content 3 (which displays H&E staining of adult rat cutaneous wounds at day 14 post injury, http://links.lww.com/PRSGO/A79). FMOD: 0.4 mg/ml × 50 μl/wounds. Significant differences compared by Mann-Whitney analysis (P < 0.05) are marked with asterisks. Bar = 200 μm.
FMOD Broadly Enhances the Transcription of Angiogenic Genes and Impedes the Expression of Angiostatic Genes
Double-transgenic mice overexpressing vascular endothelial growth factor (Vegf) and angiopoietin 1 (Angpt1) in skin showed a greater quantity and size of blood vessels.27 Vegf is massively produced by the epidermis during wound healing and has strong stimulating effects on angiogenesis via enhancement of microvascular permeability and stimulation of EC proliferation and migration.28–32 There was no meaningful difference in Vegf expression between adult WT and fmod−/− mouse unwounded skin tissues; however, vegf levels in WT wounds significantly increased at day 7 and 14 post injury (Fig. 4A). In contrast, vegf expression stayed at consistently low levels in fmod−/− wounds throughout the entire 14-day wound-healing period (Fig. 4A). Meanwhile, FMOD significantly stimulated vegf expression in both WT and fmod−/− adult mouse wounds (Fig. 4A). Like Vegf, Angpt1 is highly specific for vascular endothelium. Secreted by pericytes, Angpt1 is required for EC survival and proliferation and for vessel maturation.27,31,33 Although no considerable difference in angpt1 expression in unwounded skin tissues was observed between adult WT and fmod−/− mice, transcription levels of angpt1 were significantly lower in fmod−/− wounds after wound closure compared with that of age-matched WT mouse wounds (Fig. 4B). Interestingly, FMOD-treated WT and fmod−/− adult mouse wounds had similar vegf and angpt1 levels at day 14 post injury (Fig. 4), which was correlated to their similar wound capillary densities (Fig. 2). Considering the fact that fmod−/− wounds have decreased vascularity which can be rescued by exogenous FMOD administration, these data are highly associated with Vegf’s critical angiogenic function during granulation tissue formation and Angpt1’s important mediation of vessel remodeling and maturation.27,33–35
Fig. 4.
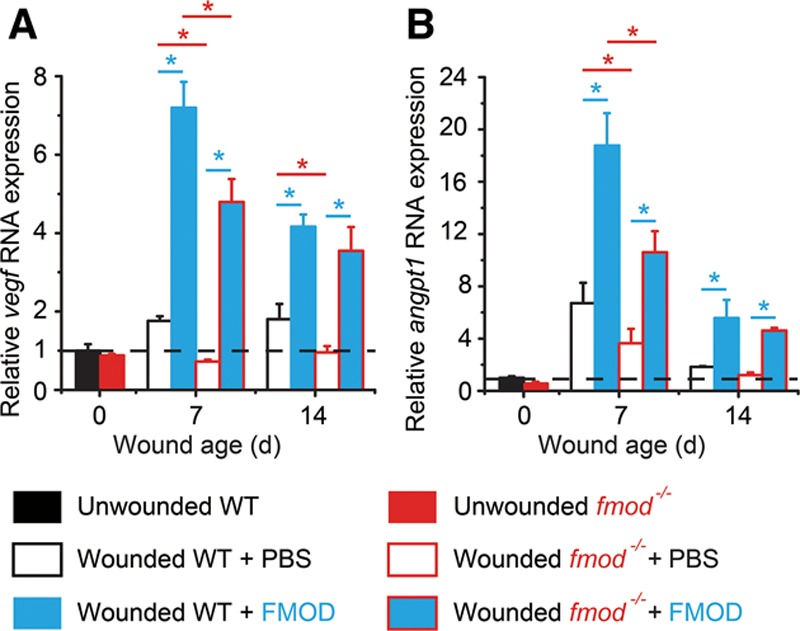
Gene expression in adult WT and fmod−/− mouse cutaneous wounds. Expression levels of vegf (A) and angpt1 (B) were measured by real-time PCR and were normalized to uninjured adult WT skin tissue (dashed lines). FMOD: 0.4 mg/ml × 50 μl/wounds. Data are presented as mean ± SD (n = 3 different cDNA templates, each template underwent reverse transcription from an RNA pool of 3 wounds harvested from 3 different animals, a total of 9 wounds from 9 animals per treatment were used). Significant differences compared by 2-sample t test (P < 0.05) are marked with asterisks: red asterisks indicate the significance from fmod knockout, and blue asterisks indicate the significance that resulted from exogenous FMOD administration.
Numerous angiogenic and angiostatic factors have been identified in the past.36,37 To further enrich our knowledge of how FMOD affects angiogenesis-related genes during wound healing, a RT2 PCR Array for rat wound healing (Qiagen) was employed for high-throughput gene expression analysis in an adult rat cutaneous wound model. As seen in the adult mouse data shown above, FMOD administration elevated both angpt1 and vegf expression (Fig. 5). Moreover, FMOD not only upregulated the expression of angpt1 and vegf but also upregulated expression of other angiogenic genes, such as tgfα (which stimulates chemotactic response, proliferation, and Vegf expression of ECs),38–40 fibroblast growth factor (fgf)2 (which induces EC proliferation, migration, and Vegf secretion),31,41 platelet-derived growth factor (pdgf)α [which escorts connective tissue cells (such as fibroblasts and mast cells) into the wound area to produce angiogenic factors and enhances angiogenic effects of Vegf and Fgf2],42–45 and colony stimulation factor (csf)3 (which recruits monocytes to trigger the synthesis of angiogenic cytokines)32 (Fig. 5). On the other hand, FMOD reduced the levels of angiostatic genes including interferon (ifn)γ (which inhibits EC growth and Vegf expression46–48 and blocks capillary growth induced by Fgf and Pdgf),49 tgfβ1 (which hinders activation of differentiated ECs for sprouting and thus maintains endothelial quiescence),50 and plasminogen (plg; which inhibits EC proliferation51 and their response to Fgf and Vegf)52 after wound closure (Fig. 5). Therefore, FMOD endorsed an angiogenesis-favoring gene expression network in adult rodent wound models.
Fig. 5.
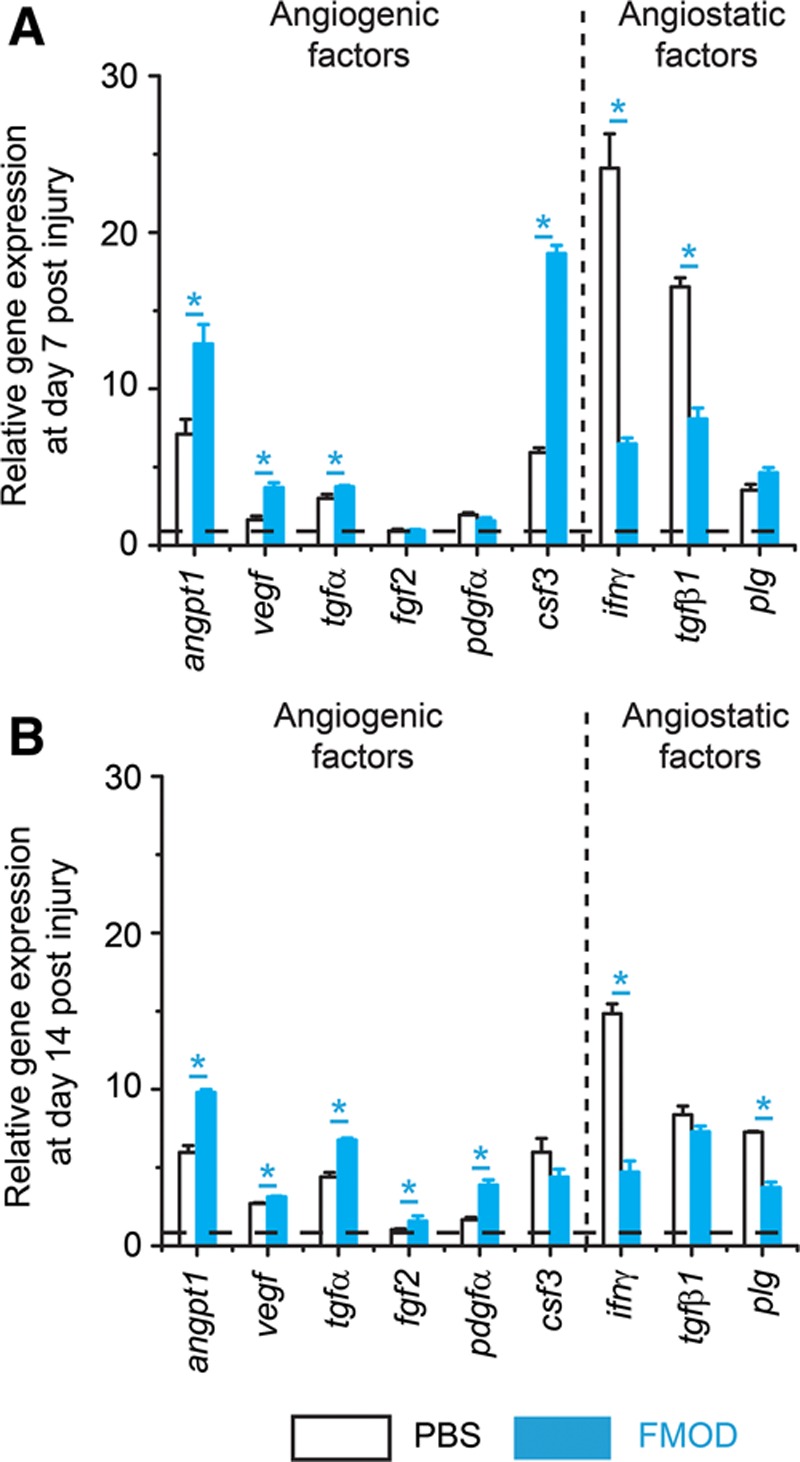
RT2 PCR Array for angiogenic and angiostatic gene expression during adult rat cutaneous wound healing. Gene expressions at day 7 (A) and 14 (B) post injury are shown. Angiogenic genes include angpt1, vegf, tgfα, fgf2, pdgfα, and csf3, whereas angiostatic genes include ifnγ, tgfβ1, and plg. FMOD: 0.4 mg/ml × 50 μl/wounds. Data are presented as mean ± SD (n = 3 different cDNA templates, each template underwent reverse transcription from an RNA pool of 3 wounds harvested from 3 different animals, a total of 9 wounds from 9 animals per treatment were used) and normalized to uninjured rat skin tissue (dashed lines). Significant differences compared by 2-sample t test (P < 0.05) are marked with asterisks.
FMOD Prompts EC TLS Formation In Vitro
To explore the direct effects of FMOD on EC spouting, the initial step of angiogenesis,20,53 primary HUVECs were seeded in Geltrex matrix (Life Technologies), which contains laminin, collagen IV, entactin, and heparin sulfate proteoglycans to model a wound-healing angiogenic situation. HUVECs spontaneously acquired elongated morphology and formed a capillary network in the gel, clearly visible by 3 hours post seeding (Fig. 6A). A broad range of FMOD (10–250 μg/ml) markedly enhanced HUVEC TLS formation and subsequently established polygon structures referred to as complex meshes (Fig. 6A). Quantitative analyses demonstrated that FMOD significantly increased both dimensional (total length of cellular TLS network per area) and topological parameters (number of junctions, branches, and meshes per area) (Fig. 6B) of HUVEC TLSs. In agreement with previous studies that revealed the positive relationship between EC migration and polygon structure formation,54 we found that FMOD significantly stimulated HUVEC invasion through the Geltrex matrix in vitro (Fig. 7). Therefore, FMOD exhibits its angiogenic function, at least partially, via promotion of EC migration/invasion.
Fig. 6.
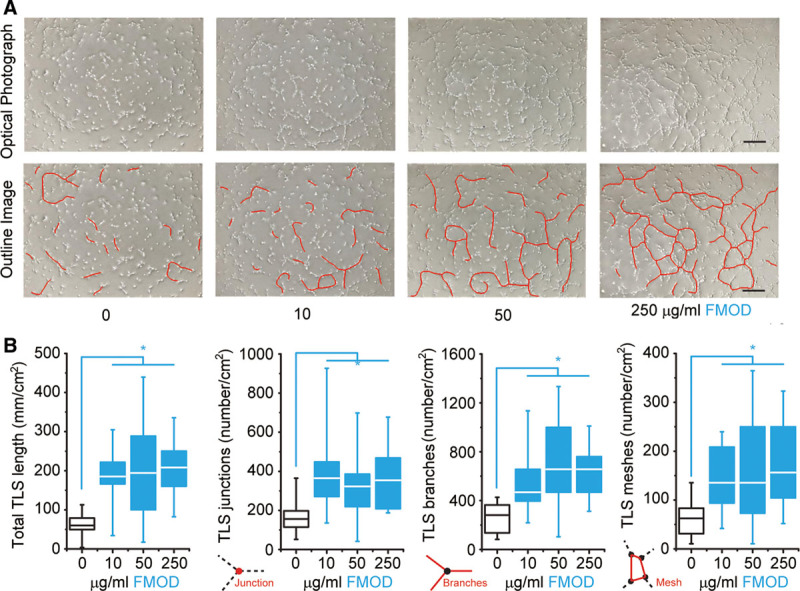
TLS formation by HUVECs on Geltrex matrix in vitro. A, Light microscopy of HUVECs spontaneously formed TLSs (outlined). B, Dimensional and topological parameters of the HUVEC TLS network were quantified. Significant differences compared by Mann-Whitney analysis (P < 0.05) are marked with asterisks (n = 16). Bar = 200 μm.
Fig. 7.
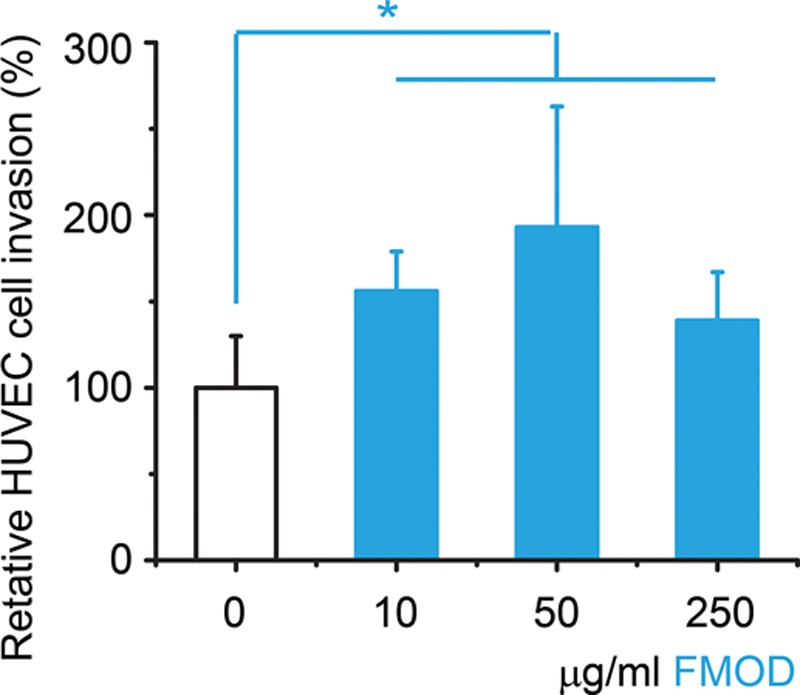
In vitro invasion assay of HUVECs. Data are presented as mean ± SD (n = 6) and normalized to non-FMOD PBS-treated control group. Significant differences compared by 2-sample t test (P < 0.05) are marked with asterisks. One-way analysis of variance revealed that there is no significant difference between 10, 50, and 250 μg/ml FMOD groups.
DISCUSSION
Angiogenesis, a process of neovascular formation from preexisting blood vasculature by sprouting, splitting, and remodeling of the primitive vascular network, results from multiple signals acting on ECs regulated by diverse groups of growth factors and ECM molecules.31,43,53 Until now, most studies on angiogenesis focused on soluble factors, such as Vegf and Fgf2.29–32,41 However, increasing reports reveal that cell-ECM interaction is also critical for EC growth, differentiation, apoptosis, and response to soluble growth factors.10,55,56 For instance, blockage of EC-ECM interactions inhibits neovascularization in vivo and TLS formation in vitro.57–59 These findings indicate that successful angiogenesis requires a dynamic temporally and spatially regulated interaction between ECs, angiogenic factors, and surrounding ECM molecules such as SLRPs.20,21,31
SLRPs are a family of proteins, including decorin, lumican, and FMOD, that are present within the ECM of all tissues.4–10 As recent studies have shown that SLRPs interact with a diversity of cell surface receptors, cytokines, growth factors, and other ECM components resulting in modulation of cell-ECM cross talk and multiple biological processes,10–14,60 the common functionalities of SLRPs are far beyond their structural functions in the ECM.10,60,61 Specifically, intensive studies present a controversial function of decorin in angiogenesis: decorin is angiogenic during development and normal wound healing but is antiangiogenic during tumor angiogenesis due to its ability to interfere with thrombospondin-1, suppress endogenous tumor Vegf production, and evoke stabilization of pericellular fibrillar matrix.10 Additionally, Niewiarowska et al62 revealed that lumican inhibits angiogenesis by reducing proteolytic activity of ECs. However, unlike decorin and lumican, our current study revealed that FMOD is an angiogenic ECM molecule. Although FMOD and lumican present close homology and share the same binding region on type I collagen,63–65 their diverse influences on angiogenesis and epithelial migration3,15,66 further support the hypothesis that FMOD and lumican do not seem to be functionally redundant, especially during cutaneous wound healing.
In this study, we demonstrated that not only did FMOD markedly enhance vasculogenesis during development, as documented by the in ovo CAM assay, but it also significantly stimulated angiogenesis as evidenced by the Matrigel plug assay and capillary density measurements in adult rodent cutaneous wound models. Additionally, impaired wound angiogenesis in fmod−/− mice could be restored by exogenous FMOD administration. At the cellular level, we confirmed that FMOD boosted HUVEC migration/invasion and TLS formation in vitro. Our previous studies also found that without considerable influence on EC proliferation, FMOD promoted EC cell adhesion, spreading, and actin stress fiber formation for vascularization in vitro.23 Thus, FMOD is an angiogenic ECM molecule that directly modulates EC behaviors. In addition to ECs, mural cells (such as fibroblasts and pericytes) and inflammatory cells (such as monocytes and mast cells) also contribute to wound angiogenesis.53,67 By stimulating expression of various angiogenic factors including angpt1, vegf, tgfα, fgf2, pdgfα, and csf3, FMOD also activated these angiogenesis-related cells in vivo during the wound-healing process. In contrast, Ifnγ and Plg are antiangiogenic, proinflammation molecules involved in wound healing.46–49,51,52,68,69 Additionally, Plg, in particular, also plays an important role in re-epithelialization, as keratinocyte migration over the wound is delayed in Plg-deficient mice.70 In this study, FMOD administration reduced ifnγ and plg levels and increased angiogenesis in adult rodent wounds, which is highly correlated with our previous observation that cutaneous wounds of fmod−/− mice exhibited extended inflammation, elevated epithelial migration, and insufficient angiogenesis.3,15 Moreover, Tgfβ1, a multipotent growth factor that regulates wound healing, promotes EC differentiation in a Vegf-independent manner at early stages of development but inhibits sprouting angiogenesis in differentiated ECs.50 Thus, lower tgfβ1 transcription after wound closure could also contribute to enhanced angiogenesis in FMOD-treated wounds. Consistent with previous studies,23,71 FMOD administration induced a proangiogenic microenvironment for wound healing in vivo by stimulating angiogenic factors and reducing angiostatic molecules.
CONCLUSIONS
In summary, as one of the pioneer groups investigating the influence of SLRPs on wound healing, we elucidated the angiogenic properties of FMOD in wounded scenarios, which function at least partially by promoting EC activation and infiltration in the wound area. Although translation from the preclinical to the clinical setting can be difficult due to an increased number of external factors such as bacterial inhibition, taken together, current studies suggest that FMOD maintains the potential to be an attractive therapeutic candidate for wound management, especially for patients suffering from impaired wound healing due to aberrant cellular infiltration and insufficient angiogenesis, such as in the cases of diabetic wounds.72–74
ACKNOWLEDGMENTS
We thank Andrew Ngo from Department of Chemical Engineering at University of California, Los Angeles, Joyce Wang from Department of Emergency Medicine at State University of New York Downstate/Kings County Hospital Center, and Caroline Chung from University of Pennsylvania for their assistance. We apologize to colleagues whose important work we could not cite due to space restrictions.
Supplementary Material
Footnotes
Presented, in part, at the Plastic Surgery Research Council 58th Annual Meeting, May 2–4, 2013, Santa Monica, Calif., and at the 98th American College of Surgeons Annual Clinical Congress, September 30–October 2, 2012, Chicago, Ill.
Disclosure: Drs. Zheng, Ting, and Soo are inventors on fibromodulin (FMOD)-related patents filed from the University of California, Los Angeles (UCLA). Drs. Zheng, Ting, and Soo are founders of Scarless Laboratories Inc., which sublicenses FMOD-related patents from the UC Regents, who also hold equity in the company. Dr. Soo is also an officer of Scarless Laboratories, Inc. Neither of the other authors has any financial disclosures. This study was supported, in part, by the Plastic Surgery Foundation (2013 National Endowment for Plastic Surgery 269698), National Institutes of Health (NIH)-National Institute of Arthritis and Musculoskeletal and Skin Diseases (R43 AR064126), NIH-National Institute of Dental and Craniofacial Research (R44 DE024692), and National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute Grant UL1TR000124. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
This work was supported by THE PLASTIC SURGERY FOUNDATION.
REFERENCES
- 1.Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 2.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Z, Nguyen C, Zhang X, et al. Delayed wound closure in fibromodulin-deficient mice is associated with increased TGF-β3 signaling. J Invest Dermatol. 2011;131:769–778. doi: 10.1038/jid.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khorasani H, Zheng Z, Nguyen C, et al. A quantitative approach to scar analysis. Am J Pathol. 2011;178:621–628. doi: 10.1016/j.ajpath.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezura Y, Chakravarti S, Oldberg A, et al. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Font B, Eichenberger D, Goldschmidt D, et al. Structural requirements for fibromodulin binding to collagen and the control of type I collagen fibrillogenesis–critical roles for disulphide bonding and the C-terminal region. Eur J Biochem. 1998;254:580–587. doi: 10.1046/j.1432-1327.1998.2540580.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg M, Septier D, Oldberg A, et al. Fibromodulin-deficient mice display impaired collagen fibrillogenesis in predentin as well as altered dentin mineralization and enamel formation. J Histochem Cytochem. 2006;54:525–537. doi: 10.1369/jhc.5A6650.2005. [DOI] [PubMed] [Google Scholar]
- 8.Oldberg A, Kalamajski S, Salnikov AV, et al. Collagen-binding proteoglycan fibromodulin can determine stroma matrix structure and fluid balance in experimental carcinoma. Proc Natl Acad Sci U S A. 2007;104:13966–13971. doi: 10.1073/pnas.0702014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svensson L, Aszódi A, Reinholt FP, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 10.Iozzo RV, Goldoni S, Berendsen AD, et al. Small leucine-rich proteoglycans. In: Mecham RP, editor. In: The Extracellular Matrix: an Overview. New York, NY:: Springer Berlin Heidelberg; 2011. pp. 197–231. [Google Scholar]
- 11.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Z, Jian J, Zhang X, et al. Reprogramming of human fibroblasts into multipotent cells with a single ECM proteoglycan, fibromodulin. Biomaterials. 2012;33:5821–5831. doi: 10.1016/j.biomaterials.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 13.Soo C, Beanes S, Dang C, et al. Fibromodulin, a TGF-ß modulator, promotes scarless fetal repair. Surgical Forum. 2001;52:578–581. [Google Scholar]
- 14.Soo C, Hu FY, Zhang X, et al. Differential expression of fibromodulin, a transforming growth factor-beta modulator, in fetal skin development and scarless repair. Am J Pathol. 2000;157:423–433. doi: 10.1016/s0002-9440(10)64555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Z, Lee KS, Zhang X, et al. Fibromodulin-deficiency alters temporospatial expression patterns of transforming growth factor-β ligands and receptors during adult mouse skin wound healing. PLoS One. 2014;9:e90817. doi: 10.1371/journal.pone.0090817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoff A, Rivera AA, Mathis JM, et al. Effect of adenoviral mediated overexpression of fibromodulin on human dermal fibroblasts and scar formation in full-thickness incisional wounds. J Mol Med (Berl) 2007;85:481–496. doi: 10.1007/s00109-006-0148-z. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Z, Zara JN, Nguyen VT, et al. Fibromodulin, a TGF-beta modulator, inhibits scar formation and increases tensile strength. J Am Coll Surgeons. 2011;213:S99. [Google Scholar]
- 18.Zheng Z, Yin W, Zara J, et al. The role of fibromodulin in fibroblast migration and enhanced wound closure. J Am Coll Surgeons. 2010;211:S127–S127. [Google Scholar]
- 19.Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther. 1991;52:407–422. doi: 10.1016/0163-7258(91)90034-j. [DOI] [PubMed] [Google Scholar]
- 20.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 21.Kleinman HK, Malinda KM. Role of angiogenesis in wound healing. In: Mousa SA, editor. In: Angiogenesis Inhibitors and Stimulators: Potential Therapeutic Implications. Austin, TX:: Landes Bioscience; 2000. pp. 102–109. [Google Scholar]
- 22.West DC, Thompson WD, Sells PG, et al. Angiogenesis assays using chick chorioallantoic membrane. In: Murray JC, editor. In: Methods in Molecular Medicine. Vol. 46. Tortowa, N.J.: Humana Press Inc.; 2011. Angiogenesis Protocols. [DOI] [PubMed] [Google Scholar]
- 23.Jian J, Zheng Z, Zhang K, et al. Fibromodulin promoted in vitro and in vivo angiogenesis. Biochem Biophys Res Commun. 2013;436:530–535. doi: 10.1016/j.bbrc.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maragoudakis ME, Haralabopoulos GC, Tsopanoglou NE, et al. Validation of collagenous protein synthesis as an index for angiogenesis with the use of morphological methods. Microvasc Res. 1995;50:215–222. doi: 10.1006/mvre.1995.1054. [DOI] [PubMed] [Google Scholar]
- 25.Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 27.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 29.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 30.Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signaling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 31.Katoh M. Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling networks (review). Int J Mol Med. 2013;32:763–767. doi: 10.3892/ijmm.2013.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Pompili VJ, Das H. Neovascularization and hematopoietic stem cells. Cell Biochem Biophys. 2013;67:235–245. doi: 10.1007/s12013-011-9298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Brown LF, Yeo KT, Berse B, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nissen NN, Polverini PJ, Koch AE, et al. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 38.Schreiber AB, Winkler ME, Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986;232:1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- 39.Grotendorst GR, Soma Y, Takehara K, et al. EGF and TGF-alpha are potent chemoattractants for endothelial cells and EGF-like peptides are present at sites of tissue regeneration. J Cell Physiol. 1989;139:617–623. doi: 10.1002/jcp.1041390323. [DOI] [PubMed] [Google Scholar]
- 40.Gille J, Swerlick RA, Caughman SW. Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J. 1997;16:750–759. doi: 10.1093/emboj/16.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Presta M, Dell’Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Cochran BH, Reffel AC, Stiles CD. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983;33:939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- 43.Eming SA, Medalie DA, Tompkins RG, et al. Genetically modified human keratinocytes overexpressing PDGF-A enhance the performance of a composite skin graft. Hum Gene Ther. 1998;9:529–539. doi: 10.1089/hum.1998.9.4-529. [DOI] [PubMed] [Google Scholar]
- 44.Gruber BL, Marchese MJ, Kew R. Angiogenic factors stimulate mast-cell migration. Blood. 1995;86:2488–2493. [PubMed] [Google Scholar]
- 45.Marx M, Perlmutter RA, Madri JA. Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis. J Clin Invest. 1994;93:131–139. doi: 10.1172/JCI116936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friesel R, Komoriya A, Maciag T. Inhibition of endothelial cell proliferation by gamma-interferon. J Cell Biol. 1987;104:689–696. doi: 10.1083/jcb.104.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuruoka N, Sugiyama M, Tawaragi Y, et al. Inhibition of in vitro angiogenesis by lymphotoxin and interferon-gamma. Biochem Biophys Res Commun. 1988;155:429–435. doi: 10.1016/s0006-291x(88)81104-5. [DOI] [PubMed] [Google Scholar]
- 48.Kommineni VK, Nagineni CN, William A, et al. IFN-gamma acts as anti-angiogenic cytokine in the human cornea by regulating the expression of VEGF-A and sVEGF-R1. Biochem Biophys Res Commun. 2008;374:479–484. doi: 10.1016/j.bbrc.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato N, Nariuchi H, Tsuruoka N, et al. Actions of TNF and IFN-gamma on angiogenesis in vitro. J Invest Dermatol. 1990;95(6 Suppl):85S–89S. doi: 10.1111/1523-1747.ep12874809. [DOI] [PubMed] [Google Scholar]
- 50.Mallet C, Vittet D, Feige JJ, et al. TGFbeta1 induces vasculogenesis and inhibits angiogenic sprouting in an embryonic stem cell differentiation model: respective contribution of ALK1 and ALK5. Stem Cells. 2006;24:2420–2427. doi: 10.1634/stemcells.2005-0494. [DOI] [PubMed] [Google Scholar]
- 51.O’Reilly MS. Angiostatin: an endogenous inhibitor of angiogenesis and of tumor growth. EXS. 1997;79:273–294. [PubMed] [Google Scholar]
- 52.Oh CW, Hoover-Plow J, Plow EF. The role of plasminogen in angiogenesis in vivo. J Thromb Haemost. 2003;1:1683–1687. doi: 10.1046/j.1538-7836.2003.00182.x. [DOI] [PubMed] [Google Scholar]
- 53.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 54.Aranda E, Owen GI. A semi-quantitative assay to screen for angiogenic compounds and compounds with angiogenic potential using the EA.hy926 endothelial cell line. Biol Res. 2009;42:377–389. [PubMed] [Google Scholar]
- 55.Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim Biophys Acta. 2004;1654:13–22. doi: 10.1016/j.bbcan.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Krishnan L, Hoying JB, Nguyen H, et al. Interaction of angiogenic microvessels with the extracellular matrix. Am J Physiol Heart Circ Physiol. 2007;293:H3650–H3658. doi: 10.1152/ajpheart.00772.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Britsch S, Christ B, Jacob HJ. The influence of cell-matrix interactions on the development of quail chorioallantoic vascular system. Anat Embryol (Berl) 1989;180:479–484. doi: 10.1007/BF00305123. [DOI] [PubMed] [Google Scholar]
- 58.Belotti D, Foglieni C, Resovi A, et al. Targeting angiogenesis with compounds from the extracellular matrix. Int J Biochem Cell Biol. 2011;43:1674–1685. doi: 10.1016/j.biocel.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Strömblad S, Cheresh DA. Cell adhesion and angiogenesis. Trends Cell Biol. 1996;6:462–468. doi: 10.1016/0962-8924(96)84942-7. [DOI] [PubMed] [Google Scholar]
- 60.Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs). J Cell Commun Signal. 2009;3:323–335. doi: 10.1007/s12079-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niewiarowska J, Brézillon S, Sacewicz-Hofman I, et al. Lumican inhibits angiogenesis by interfering with α2β1 receptor activity and downregulating MMP-14 expression. Thromb Res. 2011;128:452–457. doi: 10.1016/j.thromres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Matheson S, Larjava H, Häkkinen L. Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. J Periodontal Res. 2005;40:312–324. doi: 10.1111/j.1600-0765.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- 64.Kalamajski S, Oldberg A. Homologous sequence in lumican and fibromodulin leucine-rich repeat 5-7 competes for collagen binding. J Biol Chem. 2009;284:534–539. doi: 10.1074/jbc.M805721200. [DOI] [PubMed] [Google Scholar]
- 65.Svensson L, Närlid I, Oldberg A. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett. 2000;470:178–182. doi: 10.1016/s0014-5793(00)01314-4. [DOI] [PubMed] [Google Scholar]
- 66.Yamanaka O, Yuan Y, Coulson-Thomas VJ, et al. Lumican binds ALK5 to promote epithelium wound healing. PLoS One. 2013;8:e82730. doi: 10.1371/journal.pone.0082730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imhof BA, Aurrand-Lions M. Angiogenesis and inflammation face off. Nat Med. 2006;12:171–172. doi: 10.1038/nm0206-171. [DOI] [PubMed] [Google Scholar]
- 68.Maheswaran V, Schmidt Z, Koshova O, et al. Effects of plasmin on endothelial cells. N-S Arch Pharmacol. 2012;385:90. [Google Scholar]
- 69.Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012;92:509–519. doi: 10.1189/jlb.0212056. [DOI] [PubMed] [Google Scholar]
- 70.Romer J, Bugge TH, Pyke C, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2:287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 71.Adini I, Ghosh K, Adini A, et al. Melanocyte-secreted fibromodulin promotes an angiogenic microenvironment. J Clin Invest. 2014;124:425–436. doi: 10.1172/JCI69404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibran NS, Jang YC, Isik FF, et al. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122–128. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 73.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 74.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


