Abstract
Background:
Non–melanoma skin cancer (NMSC) is the most common malignancy in the United States. Recommended treatment for NMSC remains surgical excision following a positive biopsy. Evidence of complete spontaneous regression of residual NMSC exists in the case of small lesions macroscopically removed by shave biopsy, but with a positive microscopic margin. The present study investigates the rate at which residual tumor is present at subsequent excisional biopsy, with the aim to assess if recommendation to forgo surgical excision can be made.
Methods:
A total of 233 shave biopsies of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC) were performed during a 5-year period. All specimens included in the study were less than 2 cm in diameter, were macroscopically removed by shave biopsy, and had a positive initial microscopic margin.
Results:
On subsequent surgical excisional biopsy, 42% of BCC specimens were negative for residual tumor, 38% had residual tumor, but the tumor was completely contained in the excised specimen, and 20% of the specimens had positive margin residual tumor. For SCC specimens, 73% were negative for residual tumor, 21% had residual tumor, but the tumor was completely contained in the excised specimen, and 6% of the specimens had positive margin residual tumor.
Conclusions:
Although reduction of residual tumor at reexcision is noted with both BCC and even more so with SCC, the rate at which this occurs is not sufficient that a general recommendation to forgo surgical excision can be made.
Non–melanoma skin cancer (NMSC) is the most common malignancy in the United States.1,2 The exact prevalence of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) is not known, as reporting of these malignancies to cancer registries is not mandatory. Patients with NMSC are seen and treated by physicians within many different specialties, which further complicates tracking methods. The American Cancer Society reports that of the roughly 3.5 million NMSC diagnosed each year, BCC comprises about 80% of skin cancers, whereas SCC represents nearly all the remaining 20%. These malignancies contribute to more than 3000 NMSC deaths each year, with most due to SCC. The majority of these deaths occurs in older individuals or those with significant comorbidities and weakened immune systems.3
While the incidence of skin cancer continues to increase, so does the cost associated with its treatment. Reports show NMSC as the fifth most costly cancer to treat, representing over 4.5% of all Medicare cancer costs.4 In the last 10 years, approximately $1.45 billion was spent annually on treating NMSC.5 It is important to note that the largest percentage of these treatment expenditures came from surgical excision following biopsy.1 Despite the cost, surgical excision of NMSC lesions is the recommended treatment modality.6
Because treatment of these malignancies comes at such a high cost, it becomes imperative to determine if surgical excision following shave biopsy is in fact necessary. Swetter et al7 reported that 24% of excision specimens in biopsy-proven cases of SCCs and BCCs showed scar with no residual tumor, suggesting tumors regress following shave biopsy. A separate report found the recurrence rate of transected in situ BCC and SCC, either by shave biopsy or surgical resection, to be much lower at 9% and 4.6%, respectively. Similarly, minimally transected invasive NMSCs (defined as tumors narrowly transected at a single peripheral margin or specimen having a small focus of tumor present at one peripheral and/or deep margin) had a very low overall recurrence rate of 5.6%.8
The present study explores, in an elderly population, the frequency at which residual tumor is detected at reexcision in cases of small NMSCs macroscopically resected by shave biopsy, but with microscopically positive margins.
METHODS
A retrospective chart review was conducted at the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah. The review was conducted during a 5-year period and included all patients with BCC and SCC referred to the Plastic Surgery Clinic for a positive deep margin shave biopsy. Only lesions thought to be small enough for complete removal at the time of the initial shave biopsy were included in the study.
The patient’s age, sex, tumor type, location, specimen size, tumor size, and excision results were recorded. No local treatment modality, such as cryotherapy, was used between the time of the shave biopsy and the surgical excision. In addition, none of the patients received other treatments such as chemotherapy or radiation, for their malignancies.
Uncoated double-edged stainless steel Acu-Razor Blades (Acuderm, Fort Lauderdale, Fla.) were used for the initial shave biopsies performed by the dermatology department. The biopsy-site bleeding was controlled using the chemical hemostatic agent Drysol (Person and Covey, Glendale, Calif.). On very rare occasions (approximately 1% of the time), Bovie electrocautery (Bovie Medical Corporation, Clearwater, Fla.) was required to control persistent bleeding. Intraoperative frozen sections were not routinely performed; all pathological results were based on permanent sections. All surgical excisions were performed in the plastic surgery clinic under local anesthesia.
Referral for surgical excision was placed within 1 month of the initial shave biopsy, and the procedure took place within 2 months of referral. The excisional biopsy procedure included a full-thickness excision of the lesion with a minimum of 5-mm clear margins. If positive margins were seen on microscopic evaluation at the time of excision, a second surgical excision (reexcision) with wider margins was performed.
All shave biopsies and excisional specimens were sent to the pathology service for evaluation. The specimens were segmented at 2–3 mm intervals. These sections were then embedded in paraffin. Four-micrometer-thick slices, at 4 different layers 30 µm apart, were obtained from each section. All specimens were examined after standard hematoxylin-eosin staining. This allowed full-thickness viewing according to the standard formalin-fixed paraffin-embedding procedure.
RESULTS
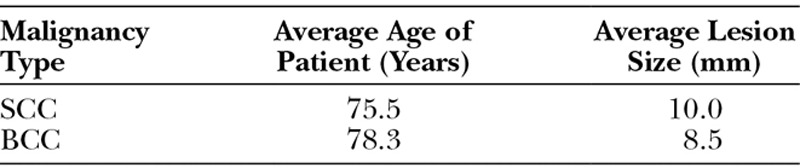
During the 5-year study period, a total of 233 shave biopsies of BCC or SCC with positive deep margins were identified. Of those 233 biopsies, 152 were BCC, and the remaining 81 were SCC. The study subjects included 231 men and 2 women. The average age of patients with BCC and SCC was 78.3 and 75.5, respectively. The average lesion size was 8.5 mm (range, 3–17 mm) for BCC and 10.0 mm (range, 1–12 mm) for SCC (Table 1). The most common location was the head and neck for both BCC (94.7%) and SCC (74.1%) (Table 2 and Fig. 1).
Table 1.
Study Demographics

Table 2.
Lesion Location by Malignancy Type

Fig. 1.
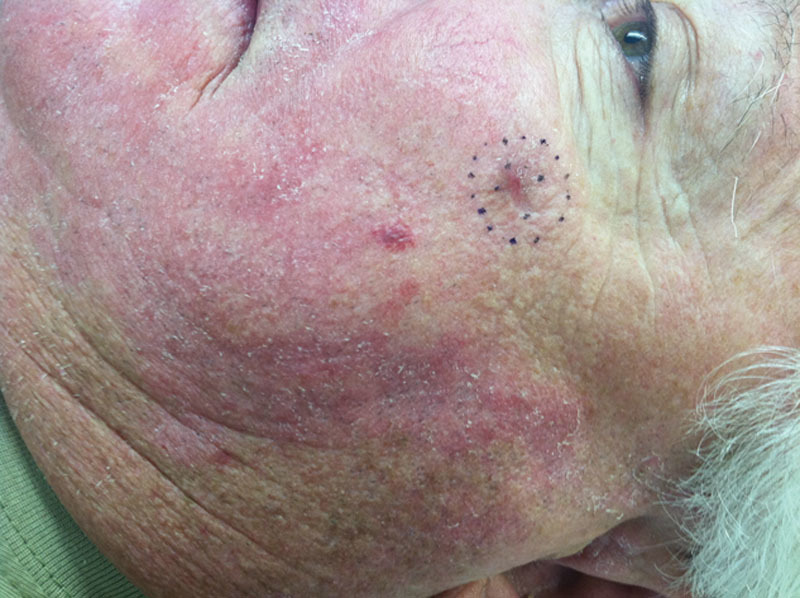
Typical lesion sent for surgical excision following positive margin shave biopsy.
When analyzing the results of surgical specimens obtained at surgical excision of the area of positive BCC shave biopsy, the following results were obtained: 42% of surgical excision specimens were negative for residual tumor, 38% had residual tumor, but the tumor was completely contained within the excised specimen, and the remaining 20% of the specimens had residual tumor that was not completely removed by the surgical excision. The results for surgical excision of SCC showed that 73% of specimens were negative for residual tumor, 21% had residual tumor, but the tumor was completely contained within the excised specimen, and 6% of the specimens had residual tumor that was not completely removed by the surgical excision (Table 3).
Table 3.
Results of Surgical Excisional Biopsy
When a second surgical excision with wider margin was performed for the cases with residual tumor after initial excisional biopsy, all specimens were found to have negative margins.
DISCUSSION
NMSC is currently the most common malignancy in the country, and the cost associated with its treatment, already significant, is escalating.1,6 The National Comprehensive Cancer Network recommends surgical excision of non–melanoma skin malignancies as the primary treatment modality.6 The premise of the current study is that initial shave biopsy coupled with close clinical follow-up of small NMSC lesions in a geriatric population could prove to be more cost effective than surgical excision, given a low chance of local recurrence.
The incidence of NMSC in the United States has increased significantly over the past 15 years, with the highest rates seen in the population older than 65 years.1,6 Patients included in this study had a mean age of 76 years, the average US male life expectancy, which allowed us to observe spontaneous regression rates of NMSC following shave biopsy in this high-risk group.9
The findings of our study were similar to those of previous studies which showed that in biopsy-proven cases of SCCs and BCCs, the reexcision specimen was negative for any residual tumor at a fairly high frequency.10 Although the precise mechanism for these findings is unclear, several alternatives are plausible. One alternative hypothesizes that inflammation associated with the acute healing process following shave biopsy leads to clearance of the residual tumor cells.7,8,11 Another possibility is that once most of the tumor burden is mechanically removed, the patient’s immune system is able to clear the minimal residual tumor cell load. Alternatively, the pathological preparation of excisional specimens could cause a false-negative result simply by the nature of which pathologic examination is conducted (ie, not all possible sections of the specimen are examined under the microscope). Finally, another possibility is that on pathologic examination, the initial shave biopsy specimen seems to have a positive margin. In reality, the entire tumor is removed, and the tumor edge is destroyed by the transecting blade, thereby leaving no residual tumor cell at the excision site. To elucidate if one or more of these mechanisms are responsible for the large portion of negative biopsies observed is beyond the scope of the current study and will require further investigation.
A significant portion of surgical excision specimens were negative for residual tumor: 42% of BCC and 73% of SCC. Regardless of these large numbers, a general recommendation to forgo surgical excision of such lesions cannot be made as 58% of patients with BCC and 27% of patients with SCC had residual tumor. These frequencies are too high to be left untreated over a long period of time. Nonetheless, certain subgroups of patients might benefit from a conservative approach involving close clinical follow-up. Such groups include those with significant comorbidities associated with short life expectancies. Obviously, if excision is not performed in these groups, close clinical follow-up would be mandatory, with excision reserved for clinical recurrence. In order for a definite recommendation to be made in this clinical scenario, future studies should assess the time to clinical local recurrence of tumor. Until this is accurately determined, the decision to forgo formal excision and proceed with close observation should be considered on a case-by-case basis.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Joseph AK, Mark TL, Mueller C. The period prevalence and costs of treating nonmelanoma skin cancers in patients over 65 years of age covered by Medicare. Dermatol Surg. 2001;27:955–959. doi: 10.1046/j.1524-4725.2001.01106.x. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Skin Cancer: Basal and Squamous Cell. 2012. Available at: http://www.cancer.org/cancer/skincancer-basalandsquamouscell/. Accessed February 11, 2014.
- 4.Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 5.Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004: A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. 2013. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed February 11, 2014.
- 7.Swetter SM, Boldrick JC, Pierre P, et al. Effects of biopsy-induced wound healing on residual basal cell and squamous cell carcinomas: rate of tumor regression in excisional specimens. J Cutan Pathol. 2003;30:139–146. doi: 10.1034/j.1600-0560.2003.000002.x. [DOI] [PubMed] [Google Scholar]
- 8.Rieger KE, Linos E, Egbert BM, et al. Recurrence rates associated with incompletely excised low-risk nonmelanoma skin cancer. J Cutan Pathol. 2010;37:59–67. doi: 10.1111/j.1600-0560.2009.01340.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:40–42. [PubMed] [Google Scholar]
- 10.Rowe DE, Carroll RJ, Day CL., Jr Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315–328. doi: 10.1111/j.1524-4725.1989.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y, Duff ML, Sammons DL, et al. Retrospective chart review of skin cancer presence in the wide excisions. World J Clin Cases. 2014;2:52–56. doi: 10.12998/wjcc.v2.i3.52. [DOI] [PMC free article] [PubMed] [Google Scholar]


