Supplemental Digital Content is available in the text.
Abstract
Background:
Conventional suction-assisted lipectomy (SAL) often results in contour irregularity. Selective photothermal heating of adipose tissue by polymer-coated gold nanorods energized by an external near-infrared exposure at 800 nm is introduced in this work to facilitate fat removal.
Methods:
The effects of NanoLipo were examined in food-grade porcine abdominal tissue (skin, fat, and fascia) by histology. The efficacy of NanoLipo was compared with that of conventional SAL in vivo in Yucatan mini pigs by quantification of removed subcutaneous tissue and fatty acids and ultrasound measurement of adipose layer thickness.
Results:
NanoLipo led to the appearance of disruptions in adipose tissue that were not apparent in control groups in ex vivo samples. NanoLipo allowed removal of more subcutaneous tissue (~33% vs ~25% of removed material, P < 0.05) and approximately twice as much free fatty acids (~60% vs ~30% of removed tissue, P < 0.05) in comparison with conventional SAL. Most importantly, NanoLipo led to a greater decrease in adipose layer thickness at 1 month post surgery (P < 0.001).
Conclusions:
NanoLipo facilitates removal of a greater quantity of fat and requires less suction time (4 vs 10 minutes) than conventional SAL. As the safety of poly(ethylene-glycol)-coated gold nanorods is well-established, a clinical trial is currently being organized.
According to the most recent statistics by the American Society for Aesthetic Plastic Surgery, liposuction, including conventional suction-assisted lipectomy (SAL), ultrasound-assisted liposuction, and laser-assisted liposuction, is the most common aesthetic procedure performed by plastic surgeons in the United States.1 However, these procedures are often associated with secondary complications due to irregular fat removal, such as contour deformities, irregular lumpy appearance, and excess skin, leading to patient dissatisfaction.2–4 In this article, a surgical device technology termed “NanoLipo” is introduced. This technology employs a gold nanorod (AuNR) solution energized by external near-infrared laser exposure (Fig. 1) to overcome these challenges by uniformly and selectively heating adipose tissue while sparing surrounding tissue.
Fig. 1.
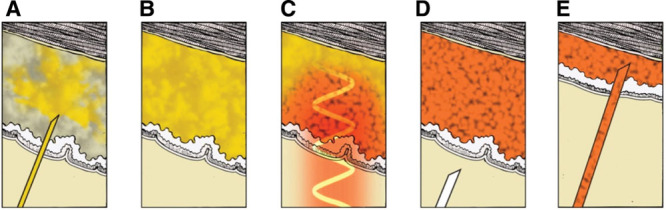
Schematic representation of NanoLipo procedure. A solution of NanoLipo AuNRs is infused into the adipose tissue (A) and permeates it (B). C, An external 800-nm laser is applied to the epidermis; the epidermis temperature is carefully controlled by contact cooling (not shown). D, Adipose tissue loses its mechanical integrity as triglycerides stored in adipocytes are secreted as free fatty acids and glycerol. E, Adipose tissue and the NanoLipo solution are removed using standard liposuction procedures.
NanoLipo heats adipose tissue by surface plasmon resonance (SPR), through which AuNRs absorb laser energy of specific wavelengths in the near-infrared region.5 The wavelength absorbed can be tuned by altering particle shape, size, geometry, and aspect ratio.6 This absorption causes gold electrons to oscillate with the frequency of the electromagnetic field, generating heat with extremely high efficiency.7 Photothermal conversion through SPR takes advantage of the difference in thermal relaxation rates between fat and surrounding tissues to allow very rapid, localized heating of adipose tissue.8,9 Because fat has a lower specific heat capacity (2.3 kJ/g/K for fat vs 4.18 kJ/g/K for water)10,11 and a lower thermal conductivity (0.23 W/m/K for fat vs 0.631 W/m/K for water) than water, it heats faster and dissipates heat slower. Heating by this mechanism selectively softens and loosens adipose tissue, facilitating removal with minimal trauma. Only surgeon-defined regions, where the AuNR solution is infused, absorb laser energy, minimizing the potential for damage to surrounding tissues.
NanoLipo employs AuNRs that absorb a wavelength within the tissue transparency window (800–900 nm), where competing endogenous chromophores including water and hemoglobin have lower absorption.9 Current Food and Drug Administration (FDA)-approved technologies for laser-assisted liposuction rely predominantly on wavelengths around or beyond 1000 nm, where water absorbs and emits heat.12 Consequently, these methods require the insertion of laser probes into the subcutaneous tissue to liquefy small volumes of fat.13 The point source nature of the heating device makes uniform results difficult to achieve. Surrounding subcutaneous tissue, such as muscle and fibrous connective tissues, will also be heated significantly.14,15 In NanoLipo, an externally applied benign laser source could provide more uniform planar heating than an internally inserted fiber optic laser and increase the safety margin by selective heating of adipose tissue. Finally, an important advantage of the NanoLipo system is the ability to precisely control temperature increases through the concentration of the exogenous solution (Fig. 2), in addition to laser energy, pulsing sequence, and wavelength.7 The amount of heating can thus be finely tuned to achieve the target temperature, while keeping the skin surface temperature lower than 42°C to mechanically weaken adipose tissue without damage to skin.13
Fig. 2.
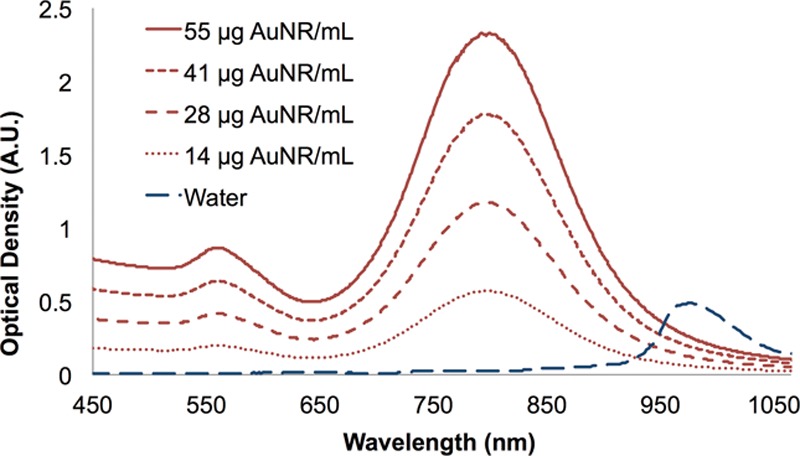
Tunable absorption of AuNR solution used in NanoLipo, which absorbs efficiently in the 800-nm region. Water and other endogenous chromophores do not absorb at this wavelength.
MATERIALS AND METHODS
AuNR Solution
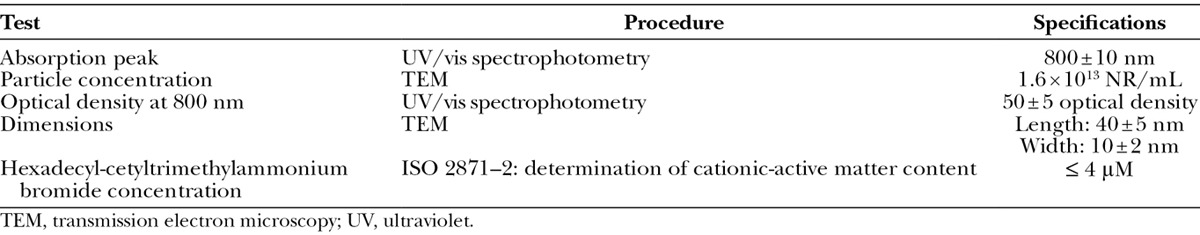
AuNR solution was produced, packaged, and released in accordance with the FDA’s current Good Manufacturing Practice guidelines by NanoSpectra BioSciences Inc. (Houston, Tex.) following the literature.16 AuNRs (10 × 40 nm) were functionalized with poly(ethylene-glycol) (5 kDa) via displacement of hexadecyl-cetyltrimethylammonium bromide, a detergent used in the synthesis of AuNRs. Proposed specifications for AuNRs in the commercially produced NanoLipo system are summarized in Table 1.
Table 1.
Device Specifications for NanoLipo Solution
Laser Source
The Lumenis LightSheer Duet laser system (Lumenis, Yokneam, Israel), a commercially available, FDA-approved device with an 800-nm pulsed diode, was used for all studies. The laser probe, whose application area is 3.5 × 2.2 cm, was set to generate 3 consecutive 30 ms pulses of 6 J/cm2 (46 J/pulse) each pass (ie, 138 J per pass before accounting for any attenuation by the tissue). In animal studies, the skin was cooled using a damp towel to keep skin surface temperatures below 42°C.
Ex Vivo Studies
Ex vivo studies were performed on food-grade porcine abdominal tissue (pork belly). AuNR or saline solution was injected into the experimental and control areas, respectively. Another region was treated with the laser only. The experimental and laser-only regions were exposed to 20 passes of the Lumenis laser. Frozen sections (10 μm) were stained with hematoxylin and eosin,8 prior to imaging using a NanoZoomer 2.0HT slide scanning microscope (Hamamatsu, Bridgewater, N.J.).
Animal Studies
All animal studies were approved by the University of California, San Diego (UCSD) Institutional Animal Care and Use Committee. Yucatan mini pigs (45–55 kg) were purchased from S&S Farms (Ramona, Calif.), housed at the UCSD pig facility at Elliot Farms, and fed a standard diet (3 meals per day).
NanoLipo Procedure
Abdominal hair was trimmed using clippers and removed using Nair (Church & Dwight, Ewing, N.J.). Following sterilization with surgical betadine, 2 regions were tattooed for conventional SAL or NanoLipo. (See Supplemental Digital Content 1, which displays treatment regions where nonabsorbable sutures were marked, http://links.lww.com/PRSGO/A80.) During all procedures, surface skin temperature was monitored by a FLIR E50 infrared thermal camera (FLIR, Wilsonville, Ore.).
NanoLipo solution was mixed with anesthetic tumescent solution (Ringer’s solution saline with 0.1% lidocaine, 1 ppm epinephrine) to a concentration of 2.5 × 1011 AuNR/mL (14 μg AuNR/mL). One hundred milliliters of the solution was injected into adipose tissue through a small stab incision in a systematic fan pattern to ensure uniform permeation and distribution in the target region (5 × 5 cm). The laser was applied to the marked area over the course of 5 ± 1 minutes to deliver 1000–2000 J of energy over multiple passes, alternating the orientation of the laser application probe to ensure complete coverage of the area. The skin was cooled using a wet towel every 4 passes to maintain a safe skin temperature, as verified by the thermal imaging camera (FLIR E50; FLIR). Subcutaneous tissue was removed by suction-assisted liposuction (Gomco OptiVac G180; Allied Healthcare Products, St. Louis, Mo.), and the incision was closed using an absorbable suture, while the operated areas were marked with a nonabsorbable suture.
Processing of Removed Subcutaneous Tissue
Lipoaspirates were centrifuged at 1000 rpm for 5 minutes to separate the liquid phase, including injection solution, from solid subcutaneous tissue. Both phases were weighed, and subcutaneous tissue was examined under dark-field microscopy at 10× magnification. Cell diameters (along the longest axis, all cells in each field of 3 representative images, totaling ~50 cells) were measured using ImageJ software. To quantify the proportion of removed subcutaneous tissue consisting of free fatty acids and glycerol, 1 g of each subcutaneous tissue sample was digested with 3 mg collagenase/dispase following manufacturer protocol for 1 h at 37°C (Roche) and centrifuged (2000 rpm, 5 minutes) to produce 3 distinct layers (from top to bottom: free fatty acids and glycerol, adipocytes, and fibrous matter). The percentage of removed tissue consisting of free fatty acids and glycerol was determined by measuring the volume of the upper layer containing secreted fatty acids and glycerol, converting it to mass (assuming 1 mL = 1 g), and dividing by the total mass of removed tissue.17
Ultrasound and Skin Appearance Assessment
Ultrasound measurements were taken using a Biosound MyLab30Vet machine (Esaote, Genoa, Italy) with a LA435 Linear Probe 18-10 MHz transducer through a thick layer of ultrasound gel before, immediately after, and at 10 d, 1 month, 2 months, and 3 months post procedure to monitor changes in tissue depth. The effect of user pressure was accounted for by applying maximum pressure and slowly relaxing, acquiring the measurement the moment just before the transducer detached from the surface. Additionally, images were consistently acquired during exhalation to account for any changes in depth due to breathing. Images parallel and perpendicular to the spinal axis of the animal were acquired to obtain complete coverage of the operated area. The machine was set to measure the same depth for each region across all time points.
In each image, the distance from the top of the deep fascial membrane to the top of the superficial fascial membrane, which appears white on ultrasound,18 was measured at 4 cross-sections spaced 1 cm apart in each ultrasound image (5 images per treated region) using ImageJ (National Institutes of Health, Bethesda, Md.). Images in which resolution was too low to identify fascial membranes (fewer than 10% of images) were not analyzed. Each measured distance is plotted to illustrate the change in average depth over time. Interpretation of ultrasound was aided by compression testing during image collection, fat layers compress more than fibrous layers.
Statistics
Continuous variables, except for adipose thickness measured by ultrasound, are reported as mean and standard errors. Groups were compared by two-tailed Student’s t test in Excel 2010 (Microsoft, Redmond, Wash.).
RESULTS
Ex Vivo Fat Liquefaction
In the first proof-of-concept experiments, food-grade porcine fatty abdominal tissue was used to determine the minimum concentration of NanoLipo AuNR solution to facilitate removal of the adipose layer at a safe laser power and short duration with minimal heating. Porcine tissue was subcutaneously injected with NanoLipo AuNR solution (0.1 g/L) and irradiated with a laser (800 nm, 2.5 kJ total, 30 ms/pulse), injected with AuNR solution only, irradiated without AuNRs, or left untreated as a control. In the absence of AuNR solution, histology showed no signs of significant lipolysis (Figs. 3A, C). Skin surface temperature was monitored and did not exceed 45°C in NanoLipo-treated samples. NanoLipo-treated regions seemed more translucent than regions treated with laser alone, suggesting liquefaction of fat. (See Supplemental Digital Content 2, which displays details on ex vivo experiments. Top and right, photograph of skin and subcutaneous fat following injection of AuNRs and laser exposure. Below, temperature measurement by thermal camera immediately following laser exposure of AuNR-injected area, http://links.lww.com/PRSGO/A81.) The tissue was also mechanically softer when probed with tweezers. Histology revealed disruption, apparent as voids, in subcutaneous adipose tissue but not in the dermal layer or connective tissue (superficial to the adipose layer) of NanoLipo-treated samples (Fig. 3). Disruption of adipose tissue in these samples made them noticeably more fragile and difficult to section. Although deep connective tissue seems to be disrupted by NanoLipo, insertion of the cannula might also cause such effects.
Fig. 3.
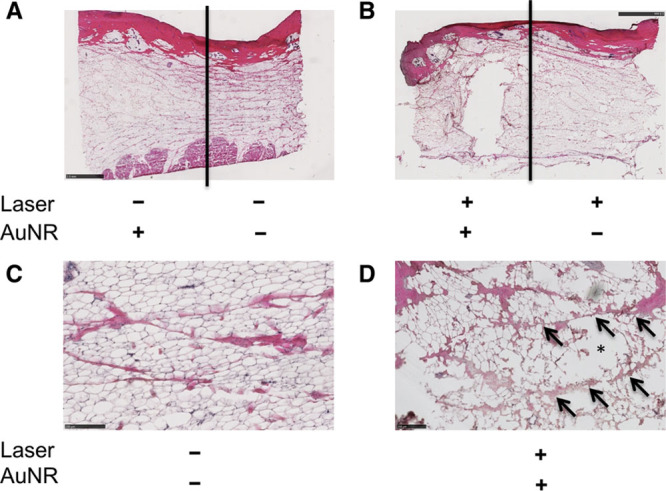
Histological effects of NanoLipo in ex vivo porcine skin and subcutaneous tissue. A, Hematoxylin and eosin (H&E)-stained section of AuNR-injected (left) and untreated (right) samples. B, H&E-stained section of NanoLipo-treated (left) and laser-treated samples. Higher magnification power image of untreated region (C) and NanoLipo-treated region (D); arrows indicate intact connective tissue and asterisk indicates disruptions. Scale bars = 2.5 mm (A and B), 250 μm (C and D).
NanoLipo Efficacy in Yucatan Mini Pigs
We next examined whether NanoLipo enhances fat removal relative to standard liposuction techniques using Yucatan mini pigs. NanoLipo and conventional SAL were performed on 2 abdominal regions on each pig. NanoLipo allowed removal of considerably more subcutaneous tissue (Fig. 4A) and fat than conventional SAL in a comparable amount of time. The NanoLipo method required far less time (4 vs 10 minutes) to remove a similar volume of lipoaspirate and caused less bruising than SAL. Collagenase digestion and centrifugation revealed that the fatty content of tissue removed following NanoLipo was nearly twice that following SAL (Fig. 4B). Dark-field microscopy of adipocytes in the lipoaspirate following NanoLipo showed that the adipocytes from NanoLipo-treated areas were significantly smaller than those from SAL-treated areas (Figs. 4C, D).
Fig. 4.
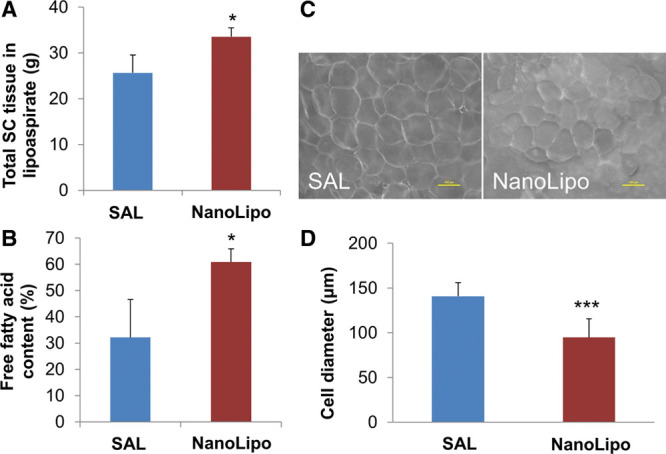
NanoLipo allows removal of more tissue and more fat than standard SAL. A, Subcutaneous (SC) tissue was weighed upon removal. B, Free fatty acids and glycerol were separated from subcutaneous tissue by digestion and centrifugation. Dark-field microscopy (C) and quantification of average diameter of adipocytes in lipoaspirates (D). Scale bar = 100 μM. *P < 0.05; ***P < 0.001. n = 3 procedures in 3 pigs.
Assessment of Tissue Depth Using Ultrasound
The thickness of the adipose layer before and immediately after the procedure and at 1, 2, and 3 months post operation was measured using ultrasound (Fig. 5). Analysis of ultrasound images reveals comparable depth changes between NanoLipo and SAL immediately post operation (Fig. 5C). However, the change in adipose tissue layer thickness at 1 month post surgery is significant in NanoLipo-treated areas (P < 0.001) but not in those treated using SAL. Similarly, reductions in adipose tissue layer thickness at 3 months post surgery were greater in NanoLipo-treated than SAL-treated areas (Figs. 5A, B).
Fig. 5.
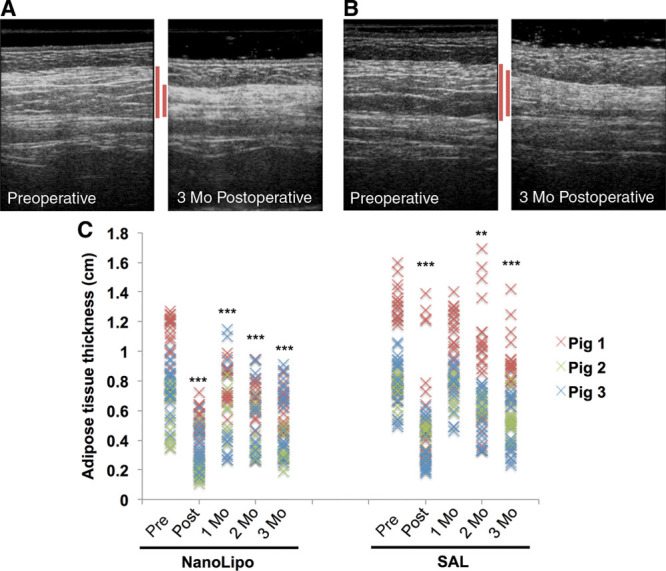
Ultrasound imaging analysis reveals that NanoLipo enhances reductions in tissue thickness. Representative ultrasound images before and 3 months after surgery of NanoLipo-treated region (A) and SAL-treated region (B). Red lines indicate adipose layer targeted by liposuction. C, Quantification of tissue thickness for NanoLipo (left) and SAL (right). **P < 0.01; ***P < 0.001 relative to baseline. n = 3 procedures in 3 pigs; 20 measurements per procedure.
DISCUSSION
SPR in metal nanostructures has been used extensively for photothermal therapy of cancer.19,20 In NanoLipo, we sought to apply SPR-based heating to improve on conventional SAL by selective photothermolysis.9 Selecting an appropriate laser pulse length, among other parameters, allows NanoLipo to precisely increase the amount of energy conferred to the target, that is, adipose tissue. The major advantage of this technology is a consequence of the addition of exogenous energy absorbers (AuNRs) rather than relying on endogenous elements, such as water.
In this work, ultrasound was used to assess whether NanoLipo enhances reductions in adipose tissue thickness and yields more uniform results relative to conventional SAL. A comparison of depth changes between NanoLipo and SAL groups immediately post operation correlated well with the total volume of subcutaneous tissue removed (Fig. 4A). Ultrasound results at 1 month post operation suggest that NanoLipo’s localized thermally aided fat removal resulted in less swelling or that such swelling subsides faster (Fig. 5C). NanoLipo seems to lead to greater reductions in thickness at 3 months post surgery. Tissue depth reduction seems to be more uniform in NanoLipo-treated regions than in those treated with SAL across all time points, as evidenced by a tighter distribution of thicknesses (Fig. 5C).
The results presented herein strongly suggest that NanoLipo may aid in removal of adipose tissue while maintaining the integrity of overlying tissues. Although the NanoLipo system includes one more step than SAL, the amount of time saved during lipoaspiration (4 vs 10 minutes to obtain the same volume) more than makes up for the time spent to apply the laser. This accelerated fat removal may result from release of fatty acids and glycerol from adipocytes, evidenced by reductions in the diameter of cells from lipoaspirate following NanoLipo procedures.
The NanoLipo technique has a high safety margin. Poly(ethylene-glycol)-coated AuNRs have been studied extensively and are essentially innocuous.21,22 The injected dose is also well below the LD50 of AuNRs.23 Furthermore, as AuNRs do not bind to tissue, a large portion of injected AuNRs are immediately removed by aspiration, and as the concentrations delivered (0.01–0.05 g/kg body weight) are well below the expected toxicity limit (3.2 g/kg) for gold,23 long-term AuNR exposure is not expected. A long-term investigation of the distribution of AuNRs in pigs is underway. We anticipate the NanoLipo procedure to be well-tolerated, as the mechanical changes induced by selective photothermolysis are temporary.
NanoLipo would not be significantly more expensive than conventional liposuction. Compatible laser systems, including the Lumenis LightSheer employed in this study, are widely available in cosmetic surgery clinics, and AuNRs at the concentration used here cost less than a dollar per liter of lipoaspirate.
CONCLUSIONS
In this study, we introduce a new system, termed NanoLipo, that facilitates removal of twice as much fat and requires less time (4 vs 10 minutes) than conventional SAL. NanoLipo seems to yield more uniform reductions in adipose layer thickness. A greater proportion of removed tissue consisted of fatty acids, which agrees with our hypothesis that NanoLipo causes fat liquefaction. The presented data suggest that NanoLipo offers advantages over conventional SAL, warranting long-term studies.
ACKNOWLEDGMENTS
We thank the Karen Christman lab (Department of Bioengineering, UC San Diego) for access to cryosectioning equipment, Jennifer Santini and the Light Microscopy Facility (Department of Cellular and Molecular Imaging, UC San Diego) for access to microscopes for histological analysis, Dr. James Day for access to the inductively coupled plasma mass spectrometry facility at the Scripps Isotope Geochemistry Laboratory (Scripps Institute of Oceanography, UC San Diego), and Arnold V. Garcia and the UC San Diego Animal Care team for the contributions to the pig studies.
Supplementary Material
Footnotes
Disclosure: Dr. A. Almutairi is cofounder and chief technology officer of eLux Medical. Dr. K. M. Almutairi is cofounder of eLux Medical Inc. and is interim chief medical officer. Drs. Rubin and B. E. DiBernardo are both scientific and clinical advisors to eLux Medical Inc. eLux Medical advisors have stock options and cofounders have stock. None of the other authors has any financial disclosures. “NanoLipo” is a trademark of eLux Medical Inc. This study was funded, in part, by King Abdulaziz City for Science and Technology and the National Institutes of Health through the KACST-UC San Diego Center of Excellence in Nanomedicine and a New Innovator Award (1 DP2 OD006499). The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Ahmad J, Eaves FF, 3rd, Rohrich RJ, et al. The American Society for Aesthetic Plastic Surgery (ASAPS) survey: current trends in liposuction. Aesthet Surg J. 2011;31:214–224. doi: 10.1177/1090820X10395508. [DOI] [PubMed] [Google Scholar]
- 2.Illouz YG. Complications of liposuction. Clin Plast Surg. 2006;33:129–163, viii. doi: 10.1016/j.cps.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Teimourian B, Adham MN. A national survey of complications associated with suction lipectomy: what we did then and what we do now. Plast Reconstr Surg. 2000;105:1881–1884. doi: 10.1097/00006534-200004050-00043. [DOI] [PubMed] [Google Scholar]
- 4.Teimourian B. Complications associated with suction lipectomy. Clin Plast Surg. 1989;16:385–394. [PubMed] [Google Scholar]
- 5.Maestro LM, Haro-Gonzalez P, Coello JG, et al. Absorption efficiency of gold nanorods determined by quantum dot fluorescence thermometry. Appl Phys Lett. 2012;100 [Google Scholar]
- 6.Jain PK, Lee KS, El-Sayed IH, et al. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 7.Govorov AO, Richardson HH. Generating heat with metal nanoparticles. Nano Today. 2007;2:30–38. [Google Scholar]
- 8.Anderson RR, Farinelli W, Laubach H, et al. Selective photothermolysis of lipid-rich tissues: a free electron laser study. Lasers Surg Med. 2006;38:913–919. doi: 10.1002/lsm.20393. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 10.El-Brawany MA, Nassiri DK, Terhaar G, et al. Measurement of thermal and ultrasonic properties of some biological tissues. J Med Eng Technol. 2009;33:249–256. doi: 10.1080/03091900802451265. [DOI] [PubMed] [Google Scholar]
- 11.Bowman HF, Balasubramaniam TA. A new technique utilizing thermistor probes for the measurement of thermal properties of biomaterials. Cryobiology. 1976;13:572–580. doi: 10.1016/0011-2240(76)90150-4. [DOI] [PubMed] [Google Scholar]
- 12.Kou L, Labrie D, Chylek P. Refractive indices of water and ice in the 0.65- to 2.5-μm spectral range. Appl Opt. 1993;32:3531–3540. doi: 10.1364/AO.32.003531. [DOI] [PubMed] [Google Scholar]
- 13.DiBernardo BE. Randomized, blinded split abdomen study evaluating skin shrinkage and skin tightening in laser-assisted liposuction versus liposuction control. Aesthet Surg J. 2010;30:593–602. doi: 10.1177/1090820X10380707. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg SN, Gazelle GS, Halpern EF, et al. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3:212–218. doi: 10.1016/s1076-6332(96)80443-0. [DOI] [PubMed] [Google Scholar]
- 15.Thomas LW. The chemical composition of adipose tissue of man and mice. Q J Exp Physiol Cogn Med Sci. 1962;47:179–188. doi: 10.1113/expphysiol.1962.sp001589. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Juste J, Pastoriza-Santos I, Liz-Marzan LM, et al. Gold nanorods: synthesis, characterization and applications. Coord Chem Rev. 2005;249:1870–1901. [Google Scholar]
- 17.Erdim M, Tezel E, Numanoglu A, et al. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J Plast Reconstr Aesthet Surg. 2009;62:1210–1214. doi: 10.1016/j.bjps.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Leahy S, Toomey C, McCreesh K, et al. Ultrasound measurement of subcutaneous adipose tissue thickness accurately predicts total and segmental body fat of young adults. Ultrasound Med Biol. 2012;38:28–34. doi: 10.1016/j.ultrasmedbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Arvizo R, Bhattacharya R, Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opin Drug Deliv. 2010;7:753–763. doi: 10.1517/17425241003777010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukianova-Hleb EY, Ren X, Zasadzinski JA, et al. Plasmonic nanobubbles enhance efficacy and selectivity of chemotherapy against drug-resistant cancer cells. Adv Mater. 2012;24:3831–3837. doi: 10.1002/adma.201103550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rayavarapu RG, Petersen W, Hartsuiker L, et al. In vitro toxicity studies of polymer-coated gold nanorods. Nanotechnology. 2010;21:145101. doi: 10.1088/0957-4484/21/14/145101. [DOI] [PubMed] [Google Scholar]
- 22.Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3:34–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Hainfeld JF, Slatkin DN, Focella TM, et al. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2006;79:248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]


