Abstract
ECI301 (eMIP), a single amino-acid substituted CCL3 (MIP-1α), enhanced tumor growth inhibition and the abscopal effect (an effect distal to the target) following local antitumor therapy such as radiation, radiofrequency ablation (RFA), or hyperthermia treatment. The recent elucidation of the underlying mechanism may lead to a better antitumor therapy.
Keywords: abscopal effect, alarmin, ECI301, eMIP, HMGB1, HSP70, hyperthermia, MIP-1α, radiation, radiofrequency ablation
Abbreviations: CT, computed tomography; HMGB1, high mobility group box-1 protein; HSP, heat shock protein; MIP-1α, macrophage inflammatory protein 1α; RFA, radiofrequency ablation
Back in 1953, Dr. CP Rhoads, then Director of the Sloan-Kettering Institute, expressed his conviction that chemotherapeutic agents like penicillin would be developed within 10 y.1 However, he was unaware that penicillin cannot kill bacteria by itself due to its isotonic status in the body. In fact, unstable L forms of bacteria can often be isolated from patients with chronic respiratory tract infections who have been treated with cell wall-affecting antibiotics*.2 Before publishing our first paper on a potential new antitumor treatment,3 the authors of this article had studied the function of leukocytes and immune-compromised hosts, especially patients with phagocyte disorders. Whether disorders were caused by congenital defects in their function4,5 or by secondary defects like opsonin deficiency,6 the patients suffered from recurrent life-threatening infections, and infection control of these patients was difficult using antibiotics. This indicates that the simultaneous action of a host-defense mechanism is essential to eradicate infected bacteria.
In contrast to bacteria versus host, there is not much difference between normal and tumor cells. Most traditional chemotherapeutic agents have been developed focusing on the faster growing rate of tumor cells relative to the surrounding normal cells of the same tissue. However, tumor cells with slower dividing rates respond to chemotherapy much more modestly, while many other cells in the body divide at a much faster rate. As a result, various side-effects ensue. The most serious problem is myelo- and immuno-suppression since the host-defense mechanism cannot simultaneously act on the tumor cells that have evaded treatment. Indeed, it has been reported that high dose sequential chemotherapy consistently induces sever leukocyte depletion and that lymphocyte populations never recover.7 Often radiotherapy also induces sever lymphocyte depletion depending on an irradiation position and protocol.
We believe that such treatments that affect the host-defense mechanism must be avoided to achieve the best results. On the other hand, immunotherapy, especially one focusing on unique cells such as NK cells and killer T cells is not powerful enough as stand-alone treatment. Upon bacterial infection, the host reacts using inflammation followed by an adaptive response. Any of this chain of events is essential to eradicate bacteria escaping the drug treatment. Among the immune-therapies examined, Coley's toxin8 injected directly into the tumor is considered to induce acute inflammation as the initial response followed by an adaptive response. However, this may not always be effective because the body does not have high enough numbers of lymphocytes recruited from the peripheral blood.† The bulk of tumor cells must be eliminated or at least injured by some other means, as in the case of antibiotics for bacterial infection.
A model experiment was thus preformed based on a new concept, namely, local antitumor treatment that induces inflammation at the tumor-bearing site followed by enhancement of the host-defense mechanism. We chose irradiation and administration of a derivative of MIP-1α, which was reported to recruit various effector leukocytes. This derivative, named eMIP or ECI301, has a reduced tendency to aggregate compared with MIP-1α. Local ionizing irradiation, not only induces necrosis and apoptosis of tumor cells, but also causes inflammation with concomitant recruitment of leukocytes, which are known to be important for the patient's outcome. However, usually the defense mechanism does not work sufficiently. The results of the model experiments showed striking inhibition of tumor growth with complete tumor eradication from about half of the mice, as well as consistent induction of the abscopal effect.3 The limitations in radiation therapy could thus be overcome, and the remaining cells considered eradicated by immune reaction, since depletion of various leukocytes reversed the suppression effect of eMIP. Administration of docetaxel also spoiled the suppressive effect of radiation and eMIP, suggesting that the immune reaction was inhibited by the chemotherapeutic agent. In addition, we found that eMIP-enhanced tumor growth inhibition could be caused by hyperthermia treatment (42ºC for 30 min) or intra-tumor administration of heat-killed Propionibacterium acnes. Meanwhile, Kaneko et al.9 reported that eMIP augments the RFA-induced antitumor effect against non-RFA-treated hepatocellular carcinoma cells through induction of antitumor immune responses in a CCR1-dependent manner.
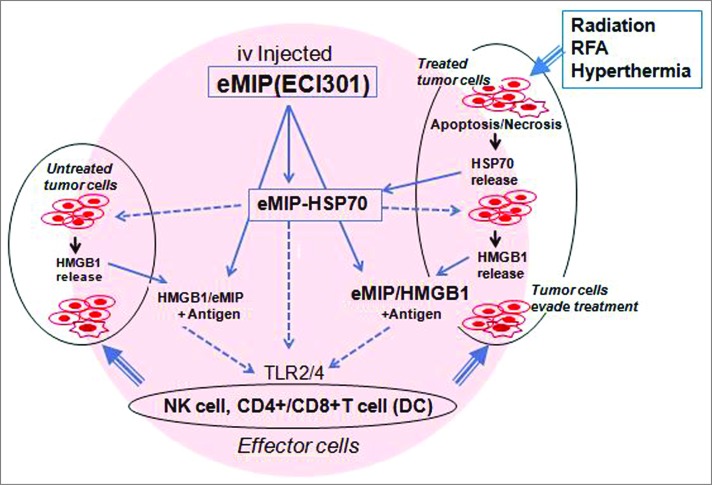
These data suggest the presence of proteins that work in synergy with eMIP to promote inflammation at the local antitumor treated site. In our latest publication in Cancer Research,10 HSP70 and HMGB1, alarmins known to be released early and lately, respectively, from dying tumor cells after irradiation or other local antitumor treatments, were found to play crucial roles in tumor regression by the stimulating host defense mechanism, probably by trapping intravenously-administered eMIP. We also demonstrated that intravenous injection of HSP70 + eMIP inhibited tumor growth. This complex may stimulate HMGB1 release from dying tumor cells and the HMGB1-eMIP complex may act subsequently to HSP70-eMIP (Fig. 1). These complexes are considered to activate effector cells.
Figure 1.
A possible action mechanism of the combination therapy. Local antitumor treatments induce apoptosis and necrosis of rapidly dividing tumor cells. HSP70 released from dying tumor cells traps intravenous-administrated eMIP, and eMIP-HSP70 complex induces HMGB1 release from dying tumor cells. HMGB1 also binds eMIP. eMIP-HSP70 and eMIP-HMGB1 promote eradication of tumor cells that may evade the treatments by stimulating NK cells and CD4+ and CD8+ cells, directly or indirectly.
At least two points must be taken into account upon clinical application of the concept: (i) a relatively huge volume of tumor per body weight was treated in our animal model. Enough HSP70 and/or HMGB1 needs to be released during local antitumor treatment in patients. Administration of HSP70 + eMIP might be needed after local tumor treatment; and (ii) conventional radiation therapy targets (unavoidably) nearby healthy tissue. Our irradiation in animal models was more tumor-direct.‡ Even using precisely targeted radiotherapy, a larger area than the tumor area detected by CT is usually necessary to treat. Under such conditions, whether or not eMIP can be delivered to the correct site needs to be examined.
Notes
*In this article, the word antibiotics is synonymous with anti-bacterial chemotherapeutic agents.
†One gram of tumor mass contains 109 cells whereas the total number of lymphocytes in peripheral blood (which can recruit) is of the order of 109∼1010.
‡Mice were held in the decubitus position, and ionizing radiation was delivered just above the tumor. The reference point for the prescription dose was set to cover the target volume of the tumor homogenously.3
References
- 1.Moss R. in Questioning Chemotherapy. NY: Equinox Press; 1995 [Google Scholar]
- 2.Kasai T, Tomita T, Kanegasaki S, Homma JY. Synergistic effects of a macrolide and a cell wall-affecting antibiotic on Pseudomonas aeruginosa in vitro and in vivo. J Antibiotics 1982; 35:1086-92; PMID:; http://dx.doi.org/ 10.7164/antibiotics.35.1086 [DOI] [PubMed] [Google Scholar]
- 3.Shiraishi K, Ishiwata Y, Nakagawa K, Yokochi S, Taruki C, Akuta T, Ohtomo K, Matsushima K, Tamatani T, Kanegasaki S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of MIP-1α. Clinical Cancer Res 2008; 14:1159-66; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4485 [DOI] [PubMed] [Google Scholar]
- 4.Ishibashi F, Nunoi H, Matsuda I, Kanegasaki S. Statistical and mutational analysis of chronic granulomatous disease in Japan with special reference to gp91- and p22-phox deficiency. Hum. Genet 2000; 106:473-81; PMID:; http://dx.doi.org/ 10.1007/s004390000288 [DOI] [PubMed] [Google Scholar]
- 5.Nunoi H, Yamazaki T, Tsuchiya H, Kato S, Malech HL, Matsuda I, Kanegasaki S. A heterozygous mutation of β-actin associated with neutrophil dysfunction and recurrent infection. Proc Natl Acad Sci U S A 1999; 96:8693-98; PMID:; http://dx.doi.org/ 10.1073/pnas.96.15.8693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ono Y, Kunii O, Kobayashi K, Kanegasaki S. Evaluation of opsono- phagocytic dysfunctions in severely burned patients by luminol-dependent chemiluminescence. Microbiol Immunol 1993; 37:563-71; PMID:; http://dx.doi.org/ 10.1111/j.1348-0421.1993.tb01678.x [DOI] [PubMed] [Google Scholar]
- 7.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, Wexler LH, Adde MA, McClure LL, Gress RE. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994; 84:2221-28; PMID: [PubMed] [Google Scholar]
- 8.McCarthy EF.The toxin of William B Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006; 26:154-58; PMID: [PMC free article] [PubMed] [Google Scholar]
- 9.Iida N, Nakamoto Y, Baba T, Nakagawa H, Mizukoshi E, Naito M, Mukaida M, Kaneko S. Antitumor effect after radiofrequency ablation of murine hepatoma is augmented by an active variant of CC chemokine ligand 3/macrophage inflammatory protein-1α. Cancer Res 2010; 70:6556-66; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0096 [DOI] [PubMed] [Google Scholar]
- 10.Kanegasaki S, Matsushima K, Shiraishi K, Nakagawa K, Tsuchiya T. Macrophage inflammatory protein derivative ECI301 enhances the alarmin-associated abscopal benefits of tumor radiotherapy. Cancer Res 201474:5070-78; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0551 [DOI] [PubMed] [Google Scholar]