Abstract
Immunotherapy is a promising treatment option for patients with hepatocellular carcinoma (HCC). Indeed, CD8+ T-cell responses against various tumor antigens occur in these patients. However, these antitumoral T cells show a severely impaired effector function. Several immunosuppressive mechanisms contribute to this T-cell failure, including regulatory T cells and inhibitory receptors.
Keywords: CD8+ T cells, hepatocellular carcinoma, immunotherapy, melanoma, PD-1, regulatory T cells, T-cell failure, tumor-associated antigens
Abbreviations: AFP, α-fetoprotein; HCC, hepatocellular carcinoma; HLA, human leukocyte antigen; IFNγ, interferon-γ; IL, interleukin; MAGE-A1, melanoma-associated gene-A1; NY-ESO-1, New York-esophageal squamous cell carcinoma-1; PD-1, programmed death-1; PD-L1, PD-ligand-1; TAA, tumor-associated antigen; Treg, regulatory T cells
Hepatocellular carcinoma (HCC), one of the most commonly occurring malignancies worldwide, is associated with a persistently high mortality rate and limited therapeutic options.1 Thus, the development of new therapeutic strategies is crucial to improve patient care. In the last decade, immunotherapy has proven to be a promising treatment option in patients with various types of cancer, e.g., melanoma or renal cell carcinoma. Similarly, first immunotherapeutic approaches in patients with HCC resulted in decreased postsurgical recurrence rates, e.g., by adoptive transfer of interleukin-2- (IL-2-) and anti-CD3-stimulated autologous lymphocytes.2
The identification and characterization of naturally occurring antitumoral immune responses is a prerequisite for the development of efficient and safe immunotherapies. Therefore, we studied the breadth, frequency and functionality of tumor-associated antigen- (TAA-) specific CD8+ T cells in patients with HCC. Previously, we analyzed CD8+ T-cell responses against the HCC-specific TAA α-fetoprotein (AFP) in patients with HCC.3 Interestingly, the majority of patients showed AFP-specific CD8+ T-cell responses directed against various, mainly previously undescribed epitopes. These important results confirm the strong immunogenicity of AFP and demonstrate that T cells specific for the self-antigen AFP are present in the natural T-cell repertoire are neither centrally nor peripherally deleted.
Besides AFP many different TAA have been described for HCC. Thus, in our recent study we analyzed CD8+ T cells specific for the TAA glypican-3, melanoma-associated gene-A1 (MAGE-A1) and New York-esophageal squamous cell carcinoma-1 (NY-ESO-1) in addition to AFP in a cohort of 96 HCC patients.4 Interestingly, by using overlapping peptides we detected interferon-γ (IFNγ) producing CD8+ T cells in tumor and non-tumor liver tissue as well as in the peripheral blood for all 4 antigens after nonspecific expansion in vitro. Furthermore, we could identify immunodominant protein regions that contain epitopes restricted by multiple human leukocyte antigen (HLA)-alleles and determine the fine specificity of several novel epitopes. Importantly, the occurrence of TAA-specific CD8+ T-cell responses spread across several TAAs was found to be an independent prognostic factor for progression-free survival in our cohort.
However, in line with our previous study, we failed to detect IFNγ-producing CD8+ T cells for all 4 TAAs after antigen-specific expansion in vitro.3 Thus, we used HLA-A*02- and HLA-A*03-tetramers for direct enumeration of NY-ESO-1 and MAGE-A1 specific CD8+ T cells independent of their effector function. Indeed, we were able to detect TAA-specific CD8+ T cells after specific in vitro expansion among leukocytes in roughly half of the HCC patients analyzed. However, these TAA-specific CD8+ T cells showed a severe functional impairment. This is highly relevant since it has been shown that the induction of IFNγ-producing T cells is one of the major determinants of successful antitumoral vaccination against several malignancies.5 Interestingly, in the same patient cohort antiviral CD8+ T cells showed preserved effector functions after antigen-specific expansion, suggesting that the observed functional impairment is specific for the antitumoral immune response and not due to a general failure of the immune system in HCC patients. Of note, we were able to show the same pattern in patients afflicted with melanoma.
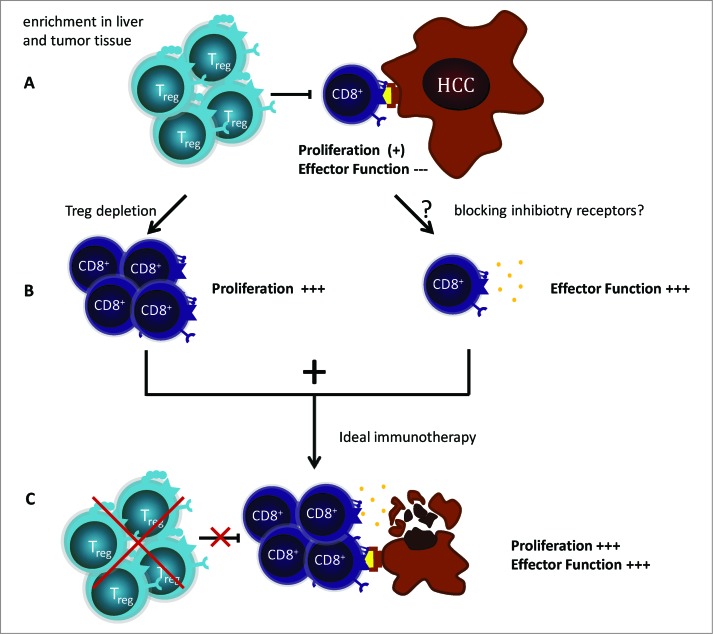
Various mechanisms have been suggested to contribute to the failure of antitumoral immune responses. However, the exact mechanisms of immune suppression in patients with HCC are currently not completely understood. Therefore, we aimed to address several of these inhibitory mechanisms, e.g., the presence of immunosuppressive cells, such as regulatory T cells (Treg), or the expression of inhibitory receptors on the surface of TAA-specific CD8+ T cells (Fig. 1).
Figure 1.
Tumor-associated antigen specific T cells are a promising immunotherapeutic target in hepatocellular carcinoma. (A) Tumor-associated antigen (TAA)-specific CD8+ T cells are present in the natural T-cell repertoire. However, they show a severe functional impairment. Several immunosuppressive mechanisms contribute to this T-cell failure, such as regulatory T cells (Tregs) and inhibitory receptors. Indeed, Tregs are enriched in liver and tumor tissue. Furthermore, the inhibitory receptor programmed cell death 1 (PD-1) is expressed heterogeneously on TAA-specifically expanded CD8+ T cells . (B) Depletion of Tregs prior to antigen-specific expansion in vitro induces an increased proliferation of TAA-specific CD8+ T cells but does not restore effector function. The mechanisms contributing to the impaired effector function are not fully understood. However, blockage of the PD-1/PD-L1 pathway results in a restored IFNγ production in single cases. (C) The aim of an efficient immunotherapeutic approach is to induce proliferation and effector function in TAA-specific CD8+ T cells. Thus, it is crucial to address several immune suppressive mechanisms in future trials in order to unlock the immune system's full antitumoral potential.
Indeed, we could show an enrichment of CD4+ Tregs in HCC lesions. The depletion of Tregs prior to antigen-specific expansion in vitro increased proliferation of TAA-specific CD8+ T cells in the majority of cell lines; however, cytokine production could not be restored. Thus, different or additional mechanisms are responsible for the observed dysfunctionality of TAA-specific CD8+ T cells in HCC patients.
Since recent Phase I clinical trials blocking the inhibitory receptor programmed cell death-1 (PDCD1, better known as PD-1) or its ligand PD-ligand-1 (PD-L1) in cancer patients with various malignancies have shown promising results, we also analyzed if PD-1 plays a role in TAA-specific CD8+ T-cell failure in HCC.6,7 Interestingly, we detected a heterogeneous expression of PD-1 on TAA-specifically expanded CD8+ T cells from patients with HCC. In line with this finding, blocking the PD-1/PD-L1 signaling pathway prior to cultivation resulted in heterogeneous effects, including the restoration of IFNγ production in one cell line and a fulminant proliferation of TAA-specific CD8+ T cells in another. Thus, blocking inhibitory receptors might be a promising treatment approach for patients with HCC but requires further refinement, and possibly, the combination of different immunomodulatory protocols.
In conclusion, we could show that TAA-specific CD8+ T cells are present in the natural T-cell repertoire despite their specificity for self-antigens. Thus, tumor vaccines with the aim to boost or induce antitumoral T-cell responses seem to be promising treatment options for patients with HCC. Most recent vaccine trials have focused on the HCC-specific TAA AFP 8; however, by detecting broad immune responses not only against AFP but also against MAGE-A1, glypican-3 and NY-ESO-1, our study suggests that future vaccine trials should include various tumor antigens to achieve better clinical results. Indeed, a broad immune response was associated with improved patient survival. These findings are reinforced by promising clinical response rates in HCC patients after administration of dendritic cells pulsed with tumor-lysate, thus containing multiple TAA.9 However, previous vaccination studies in HCC patients have failed to achieve sufficient long lasting clinical results. In our study, we could show that multiple inhibitory mechanisms, e.g., the presence of inhibitory Tregs, contribute to CD8+ T-cell failure in patients with HCC. Thus, it is crucial to address these immunosuppressive mechanisms, and their interaction in patients, in future trials in order to unlock the immune system's full antitumoral potential and promote patient survival.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. El-Serag HB. Hepatocellular carcinoma. New Engl J Med 2011; 365:1118-27; PMID:; http://dx.doi.org/ 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 2. Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356:802-7; PMID:; http://dx.doi.org/ 10.1016/S0140-6736(00)02654-4 [DOI] [PubMed] [Google Scholar]
- 3. Thimme R, Neagu M, Boettler T, Neumann-Haefelin C, Kersting N, Geissler M, Makowiec F, Obermaier R, Hopt UT, Blum HE, et al. Comprehensive analysis of the alpha-fetoprotein-specific CD8+ T cell responses in patients with hepatocellular carcinoma. Hepatology (Baltimore, Md) 2008; 48:1821-33; PMID:; http://dx.doi.org/ 10.1002/hep.22535 [DOI] [PubMed] [Google Scholar]
- 4. Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology (Baltimore, Md) 2014; 59:1415-26; PMID:; http://dx.doi.org/ 10.1002/hep.26731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:; http://dx.doi.org/ 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med 2012; 366:2455-65; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med 2012; 366:2443-54; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res 2006; 12:2817-25; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-2856 [DOI] [PubMed] [Google Scholar]
- 9. Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology (Baltimore, Md) 2009; 49:124-32; PMID:; http://dx.doi.org/ 10.1002/hep.22626 [DOI] [PubMed] [Google Scholar]