Abstract
Hepatitis C virus (HCV) infection is highly prevalent among chronic kidney disease (CKD) subjects under hemodialysis and in kidney transplantation (KT) recipients, being an important cause of morbidity and mortality in these patients. The vast majority of HCV chronic infections in the hemodialysis setting are currently attributable to nosocomial transmission. Acute and chronic hepatitis C exhibits distinct clinical and laboratorial features, which can impact on management and treatment decisions. In hemodialysis subjects, acute infections are usually asymptomatic and anicteric; since spontaneous viral clearance is very uncommon in this context, acute infections should be treated as soon as possible. In KT recipients, the occurrence of acute hepatitis C can have a more severe course, with a rapid progression of liver fibrosis. In these patients, it is recommended to use pegylated interferon (PEG-IFN) in combination with ribavirin, with doses adjusted according to estimated glomerular filtration rate. There is no evidence suggesting that chronic hepatitis C exhibits a more aggressive course in CKD subjects under conservative management. In these subjects, indication of treatment with PEG-IFN plus ribavirin relies on the CKD stage, rate of progression of renal dysfunction and the possibility of a preemptive transplant. HCV infection has been associated with both liver disease-related deaths and cardiovascular mortality in hemodialysis patients. Among those individuals, low HCV viral loads and the phenomenon of intermittent HCV viremia are often observed, and sequential HCV RNA monitoring is needed. Despite the poor tolerability and suboptimal efficacy of antiviral therapy in CKD patients, many patients can achieve sustained virological response, which improve patient and graft outcomes. Hepatitis C eradication before KT theoretically improves survival and reduces the occurrence of chronic graft nephropathy, de novo glomerulonephritis and post-transplant diabetes mellitus.
Keywords: Hepatitis C virus, Chronic kidney disease, End-stage renal disease, Conservative management, Hemodialysis, Kidney transplantation, Diagnosis, Therapy
Core tip: In this review, we discuss the most recent and relevant literature regarding diagnostic aspects, clinical features, outcomes and therapy of chronic hepatitis C in subjects with chronic kidney disease, in the context of conservative management, hemodialysis, and kidney transplantation. In addition, antiviral regimens are summarized and treatment algorithms are proposed.
INTRODUCTION
Over the last two decades, there has been a large body of evidence that supports an intimate relationship between liver and kidney diseases. In the same way that several causes of renal injury can occur in patients with acute liver failure or chronic liver disease, a variety of hepatic lesions can develop in subjects with chronic kidney disease (CKD). Although drug-induced liver injuries, non-alcoholic fatty liver disease and hepatic iron overload are relatively frequent in CKD patients, hepatitis C virus (HCV) infection remains the most common and severe cause of liver disease in this population[1].
HCV infection is a major public health issue, which affects approximately 2.8% of the world’s population[2,3]. HCV infection is highly prevalent among CKD subjects and, consequently, in kidney transplant (KT) recipients[4,5]. In spite of the reduction in HCV seroconversion rates in hemodialysis units, prevalence is still substantially higher than in general population, ranging from 10% to as high as 59%, according to the geographic area[6,7]. A recent meta-analysis performed by Su et al[8] on the incidence of HCV infection in hemodialysis patients confirmed this high variability of incidence rates across regions, with most of this heterogeneity probably related to the level of country development and differences in the primary prevalence of HCV infection in hemodialysis units. By evaluating 22 studies, these authors found a pooled incidence rate of HCV infection of 0.97 (95%CI: 0.66-1.29) in developed countries, and of 4.44 (95%CI: 2.65-6.23) per 100 patients in developing countries[8]. Patients under renal replacement therapy, particularly hemodialysis, are exposed to blood borne pathogens, given the need for intravenous access, and frequent catheter manipulation[9]. These patients are frequently treated in close proximity to one another and share supplies or equipment that can become contaminated. Furthermore, breaches in infection control practices can result in episodes of patient-to-patient HCV transmission[10]. Time in hemodialysis, previous renal transplant and presence of anti-HBc antibodies are associated with HCV infection while use of erythropoietin (EPO) and adherence to universal precaution measures seem to protect against HCV infection[11]. While transfusion of blood products still plays a significant epidemiological role in developing countries, the vast majority of HCV chronic infections in the hemodialysis setting are currently attributable to nosocomial transmission through hand-borne transmission or by the use of contaminated medication vials, such as saline, anesthetic drugs and unfractionated heparin (UFH)[6,9,12]. Although single dose low molecular weight heparin (LMWH) has been increasingly used (particularly in Western Europe), UFH provided in multi-dose vials is the anticoagulant of choice for most maintenance hemodialysis units all over the world, which possibly contributes to HCV transmission when standard precautions are not strictly adopted[13,14].
Since there is a paucity of data for individuals on peritoneal dialysis, this review will focus on diagnostic aspects, clinical outcomes and therapeutic options for hepatitis C in CKD patients receiving conservative management, undergoing hemodialysis, and after kidney transplantation.
ACUTE HEPATITIS C
With the introduction of EPO and the consequent reduction of blood transfusions in hemodialysis patients, the main route of HCV infection is related to environmental transmission of the virus[15,16].
In hemodialysis patients, acute infections are usually asymptomatic and anicteric. Despite the lower levels of alanine aminotransferase (ALT) levels observed in CKD patients[17,18], acute infections are often accompanied of moderate ALT elevations (typically inferior to 10 times the upper limit of normality), followed by anti-HCV seroconversion in 90% of cases, one to seven months after ALT elevation[16,19-21]. Systematic screening of ALT and anti-HCV in hemodialysis patients are strongly recommended (monthly for ALT and 6-monthly for anti-HCV), and even small unexplained increase in serum ALT levels should raise the suspicion of acute HCV infection. The infection is confirmed by the detection of HCV RNA in serum by polymerase chain reaction (PCR) assay, which precedes the appearance of anti-HCV antibodies by several weeks or months[22,23]. In the study of Moreira et al[24], serum samples were collected monthly for 1 year from 281 patients admitted for hemodialysis; six patients seroconverted during the study (incidence = 3.1/1000 person-month). In 1.8% (5/281) of cases, RNA was detected before the appearance of antibodies (up to 5 mo), and in 1.1% (3/281) of cases, RNA was the unique marker of HCV infection.
Viral clearance is very uncommon in hemodialysis patients, occurring in less than 5% of patients[16,19], and therefore acute infections should be treated as soon as the diagnosis is established, whenever possible. Given that documentation of anti-HCV seroconversion is generally feasible in the context of hemodialysis, a pre-treatment liver biopsy is seldom necessary, unless a differential diagnosis is required.
In non-uremic patients, data about treatment of acute hepatitis C are limited and heterogeneous regarding studied populations, regimens and duration of treatment[25]. In hemodialysis patients data are even scarcer, with small sample sizes. In addition, most studies report results with standard interferon[26-31]. Only two studies reported data of pegylated interferon (PEG-IFN) in hemodialysis patients[32,33]. These two and six other studies were evaluated in a meta-analysis about treatment of acute hepatitis C in hemodialysis patients[34] (Table 1). The global rate of sustained virological response (SVR) was 59%, with 9% of dropouts. Although there were no clear differences in efficacy or safety between PEG-IFN and standard IFN, we suggest that PEG-IFN with adjusted doses (Table 2) should be preferentially used, for the sake of better patient compliance and comfort. In non-uremic patients there is no evidence of additional benefit of association with ribavirin[35] but there is no data regarding its use in uremic patients. Nevertheless, the recommendation is to treat HCV acute infection with monotherapy PEG-IFN for six months, regardless of the genotype. It is not recommended to wait 12 wk for spontaneous clearance, since this occurrence is very uncommon in CKD subjects.
Table 1.
Studies on acute hepatitis C treatment in hemodialysis patients
| Ref. | Süleymanlar et al[26] (1998) | Gürsoy et al[27] (2001) | Urbánek et al[28] (2004) | Al-Harbi et al[29] (2005) | Rocha et al[30](2007) | Engel et al[32](2007) | Liu et al[33](2010) | Ferreira et al[31] (2011) |
| n | 3 | 36 | 18 | 9 | 23 | 10 | 35 | 26 |
| Interferon type | IFN | IFN | IFN | IFN | IFN | PEG-IFN α2b | PEG-IFN α2a | IFN |
| PEG-IFN α2a | ||||||||
| Schedule | 4.5 MU tiw | 3 MU or | 10 MU + | 3 MU tiw | 3 MU or | 1 μg/kg qw | 135 μgv qw | 3 MU tiw |
| 6-10 MU tiw | 3 MU tiw | 6 MU tiw | or 135 μg qw | |||||
| Duration | 16 wk | 12 wk | 3 wk + 12 wk | 12 wk | 48 or 24 wk | 24 wk | 24 wk | 48 wk |
| SVR | 100% | 39% | 72% | 67% | 43% | 40% | 89% | 54% |
IFN: Interferon; PEG-IFN: Pegylated interferon; MU: Million units; tiw: Three times a week; qw: Once a week; SVR: Sustained virological response rate by intention-to-treat analysis.
Table 2.
Treatment regimens for hepatitis C virus infection in chronic kidney patients
| Stage of CKD | Estimated GFR | Target dosage of ribavirin | Dosage of Interferon |
| 1 | ≥ 90 | 800 to 1200 mg qd1 | PEG-IFN α2a 180 μg qw or PEG-IFN α2b 1.5 μg/kg qw |
| 2 | 60 to 89 | 600 to 800 mg qd1 | |
| 3 | 30 to 59 | 400 to 600 mg qd1 | |
| 4 | 15 to 29 | 200 mg qd | PEG-IFN α2a 135 μg qwor PEG-IFN α2b 1.0 μg/kg qw |
| 5 | < 15 or HD | Titrated according to patient tolerability2 |
Divided in two doses;
See text for details. GFR: Glomerular filtration rate expressed in mL/min per 1.73 m2; qd: Once a day; PEG-IFN: Pegylated interferon; qw: Once a week; HD: Hemodyalisis.
In KT recipients, the occurrence of acute hepatitis C can be associated with a more severe course, with a rapid progression of fibrosis towards cirrhosis, including the development of fibrosing cholestatic hepatitis or vanishing bile duct syndrome[36-39]. For this reason, antiviral therapy should be rapidly introduced, even if poor tolerability and efficacy are expected. Although there are no comparative studies with IFN monotherapy, the better option would be the treatment with PEG-IFN in combination with ribavirin for 24 to 48 wk, with doses adjusted according to estimated glomerular filtration rate (eGFR) (Table 2).
CHRONIC HEPATITIS C BEFORE KIDNEY TRANSPLANTATION
CKD patients under conservative management
The prevalence of HCV infection is higher in conservative management CKD patients than in general population, being mainly related to parenteral exposure[40-44]. Clinical and laboratory features of chronic HCV infection in CKD individuals under conservative management are not well known, and additional studies are needed to better understand the natural history and clinical impact of chronic HCV infection in this population. Nevertheless, the accuracy of ALT in detecting HCV infection is high[43,44], suggesting that ALT is a good marker of this infection among pre-dialysis patients, in contrast to individuals under hemodialysis[17]. In one study, 39 pre-dialysis patients with chronic HCV infection were compared to HCV-infected hemodialysis subjects[43]. Pre-dialysis patients were older, showed a higher proportion of elevated aminotransferases levels, higher inflammatory activity and more advanced fibrosis on liver histology. However, since comparable fibrosis progression rates were observed, there is no evidence suggesting that chronic hepatitis C exhibits a more aggressive course in CKD subjects under conservative management. Interestingly, high HCV viral loads seems to be common in these patients[43], in contrast to what is observed in hemodialysis subjects, who typically present low levels of serum HCV RNA, a finding possibly related to the clearance of HCV particles during dialysis[45-47].
Treatment decision relies on the CKD stage (based on eGFR), rate of progression of renal dysfunction and the possibility of a preemptive transplant. A treatment decision algorithm is proposed in Figure 1. Although antiviral therapy is feasible for subjects in all CKD stages, for most patients with CKD stage 4 (eGFR of 15 to 29 mL/min per 1.73 m2) it is preferable to wait until there is indication for initiation of dialysis. This waiting attitude for CKD stage 4 patients is proposed only for those without significant liver fibrosis, considering the particularly low fibrosis progression rate and the poor tolerability of these subjects, as well as the high risk of further deterioration of kidney function and early indication for renal replacement therapy[48,49].
Figure 1.
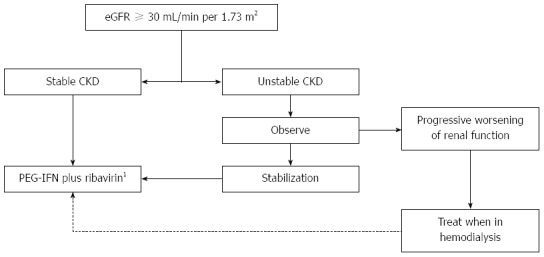
Treatment decision algorithm for hepatitis C virus-infected chronic kidney disease patients under conservative management. 1Adjusted doses according to eGFR. eGFR: Estimated glomerular filtration rate; CKD: Chronic kidney disease; PEG-IFN: Pegylated interferon.
Treatment schedule consists of PEG-IFN α2a and ribavirin, with doses tailored to eGFR (Table 2), for 24 to 48 wk. Dose adjustment according to renal function is particularly needed for ribavirin[50], which concentrates in circulating red blood cells (RBCs)[51,52], causes a relative adenosine triphosphate deficiency and increased susceptibility to oxidative damage, leading to accelerated RBC turnover and hemolytic anemia[53]. Therefore, in CKD subjects, renal function and hemoglobin levels should be carefully monitored during antiviral therapy, due to the increased risk of ribavirin-induced anemia, which can be severe in patients who frequently have multifactorial anemia and other comorbidities (like coronary artery disease). The use of EPO (up to 40000 IU/wk) improves tolerability and promotes the stability of hematological parameters during treatment.
CKD patients under hemodyalisis
HCV infection is an important cause of morbidity and mortality in dialysis patients and has been associated with both liver disease-related deaths (due to complications of cirrhosis and hepatocellular carcinoma) and cardiovascular mortality[54-56]. It is possible that HCV contributes to atherogenesis through aggravation of metabolic syndrome factors and/or by leading to a chronic inflammatory state[57].
In CKD patients undergoing hemodialysis, HCV infection has distinct clinical and laboratory features as compared to the non-uremic population and KT recipients, which can affect the management of those subjects. The prevalence of advanced liver fibrosis is lower (4% to 10%)[58,59], and progression to cirrhosis during hemodialysis seems to be uncommon[60]. In addition, as mentioned above, for yet unknown reasons, ALT levels are lower than those observed in non-uremic patients, even in the presence of significant histological damage, which hampers its utility as a marker of HCV infection[18,61].
Anti-HCV has proven to be a reliable screening test for HCV chronic infection in CKD patients[62]. Although false-negative tests have been observed with first and second generation kits, this became rather unusual with third generation enzyme immunoassays and chemiluminescence assays[62,63].
It should be noted that, although these patients are immunocompromised due to the underlying disease, low HCV viral loads are typically observed[45-47]. The mechanisms involved in this phenomenon are poorly understood and are probably multifactorial. Filtration of viral particles into the dialysate, adherence of the virus to the surface of the dialysis membrane, and destruction of viral particles during the dialysis procedure have been proposed as potential mechanisms[46,47]. It is not clear whether the type of dialysis would significantly affect the clearance of HCV particles. However, it has been suggested that HCV viral load is lower in CKD patients under chronic hemofiltration[64]. Moreover, the phenomenon of intermittent HCV viremia, characterized by low levels of serum HCV viral load intercalated with episodes of undetectable HCV RNA, has been commonly reported in CKD patients under hemodialysis[46,65-68]. This event is responsible for false-negative results in HCV RNA assays in 33% to 67% of anti-HCV-reactive patients[46,65-68], which not only can result in delayed treatment (or no treatment at all), but also contributes to environmental transmission of HCV in dialysis units. Several physiopathogenetic mechanisms have been proposed to explain the intermittent HCV viremia, like heparin interference with the PCR assay used for the detection of HCV RNA[69], mechanical extraction of viral particles adhering to dialyzer membrane[47,70], and induction of interferon production, hepatocyte growth factor, or other cytokines with antiviral properties by the hemodialysis procedure[71-73].
Therefore, isolated undetectable results of HCV RNA should not be interpreted as absence of replication. To better clarify HCV viral kinetics in this population, it is recommended for all anti-HCV-positive CKD patients on hemodialysis to perform sequential HCV RNA monitoring by using a highly sensitive detection method like reverse transcriptase-polymerase chain reaction (RT-PCR) or transcription-mediated amplification[74-77].
Occult HCV infection could conceivably also represent a risk for nosocomial transmission of HCV within hemodialysis units, as well as an additional risk of reactivation and progressive liver disease after KT. However, a study evaluating 417 hemodialysis subjects found only a single case of HCV RNA detectable in peripheral blood mononuclear cells in the absence of HCV RNA in serum, suggesting that occult HCV infection is very rare in CKD patients in hemodialysis[63].
Although widely performed and accepted as the gold-standard method to evaluate hepatic fibrosis, liver biopsy is an invasive technique with associated morbidity[78]. CKD individuals frequently exhibit major hemostatic disorders and hemorrhagic complications, posing additional risks for patients undergoing invasive procedures[79]. Transjugular liver biopsy is an alternative procedure for obtaining liver specimens that has already been evaluated in the CKD population[80,81]. Although safe, this procedure is not widely available and frequently provides small samples, which might underestimate fibrosis staging. Hence, there is a need for the development of accurate noninvasive tests to estimate liver fibrosis, especially among dialysis patients, in whom a higher risk for liver biopsy complications has been observed in most, but not all, studies[80,82-84]. Noninvasive tests such as APRI (AST-to-platelet ratio index), FibroTest® and transient hepatic elastography have shown good diagnostic performance to predict the severity of liver fibrosis in hemodialysis patients with chronic hepatitis C, and can be used as alternative methods to liver biopsy for subjects with contraindications to the procedure or for those who refuse to be biopsied[58,85-87].
Hepatitis C eradication before KT theoretically improves survival and reduces the occurrence of chronic graft nephropathy[88], de novo glomerulonephritis[89] and post-transplant diabetes mellitus[90]. After transplantation, viremia increases significantly[9] and progression of liver fibrosis occurs[91,92], with an evident negative impact on survival after 10 years of transplantation[93]. Moreover, given the risk of treatment-induced graft dysfunction and poor tolerance of interferon-based therapy, antiviral therapy has limited indications in HCV-infected KT subjects[94]. Thus, in KT candidates, treatment should be offered regardless of the degree of histological injury, with the goal of viral eradication. It is highly recommended that common clinical comorbidities such as anemia, retinopathy and cardiovascular disease should be identified and controlled before treatment.
There have been several trials of hepatitis C treatment in hemodialysis patients, mostly uncontrolled and with different therapeutic regimens. These trials have been included in many meta-analysis[95-103], which are listed in Table 3.
Table 3.
Meta-analyses on the treatment of chronic hepatitis C in hemodialysis patients
| Ref. | Year | Trials (n) | Therapy | n | SVR1 |
| Russo et al[95] | 2003 | 11 | IFN | 213 | 33% (21%-51%) |
| Fabrizi et al[96] | 2003 | 14 | IFN | 269 | 37% (28%-48%) |
| Gordon et al[97] | 2008 | 20 | IFN | 459 | 41% (33%-49%) |
| 5 | PEG-IFN | 87 | 37% (9%-77%) | ||
| Fabrizi et al[99] | 2008 | 24 | IFN | 529 | 39% (32%-46%) |
| 4 | PEG-IFN | 116 | 31% (7%-55%) | ||
| Gordon et al[98] | 2009 | 20 | IFN | 428 | 45% |
| Alavian et al[100] | 2010 | 21 | IFN | 491 | 39% (32%-46%) |
| 12 | PEG-IFN | 279 | 39% (27%-52%) | ||
| Fabrizi et al[101] | 2010 | 16 | PEG-IFN | 254 | 33% (24%-43%) |
| Fabrizi et al[102] | 2011 | 10 | PEG-IFN + RBV | 151 | 56% (28%-84%) |
| Fabrizi et al[103] | 2014 | 11 | PEG-IFN + RBV | 287 | 60% (28%-97%) |
Mean overall estimate for sustained virological response (range). IFN: Interferon; PEG-IFN: Pegylated interferon; RBV: Ribavirin.
Overall SVR rates derived from meta-analysis appear not to be very different for the use of standard IFN or PEG-IFN. However, in a randomized, controlled trial, viral load and use of PEG-IFN (vs standard IFN) were predictive of SVR[104]. The addition of ribavirin seems to provide a significant increase in SVR, but demands greater care in pretreatment evaluation and in monitoring and managing of anemia (including EPO supplementation). Studies evaluating combined therapy with interferon and ribavirin used ribavirin doses from 200 mg 3 times a week up to 300 mg/d[105-115]. Dropout rates were highly heterogeneous, ranging from 0% to 71%.
Given its easier dosing schedule and possible higher efficacy, it is recommended to use PEG-IFN (preferably PEG-IFN α2a 135 μg) once a week, after dialysis session, in combination with ribavirin. The ribavirin dose should be titrated according to patient tolerability, as follows: an initial dose of 200 mg once a week is given, followed by increments of 200 mg every two weeks until the maximum dose tolerated (stable levels of hemoglobin above 10 g/dL are often required). After stabilization of ribavirin dosage (usually between 400 to 1200 mg/wk), PEG-IFN is initiated and used for 24 to 48 wk (Figure 2).
Figure 2.
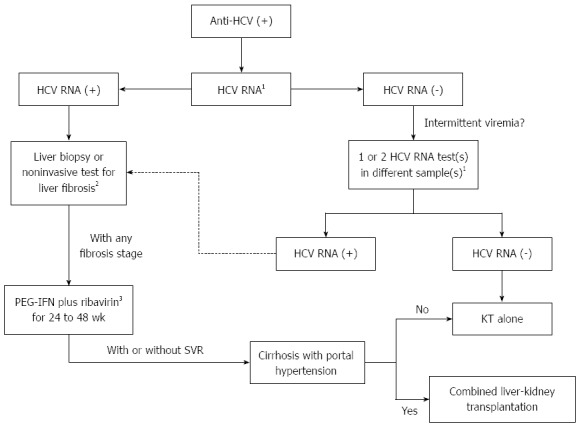
Treatment decision algorithm for hepatitis C virus-infected chronic kidney disease patients under hemodialysis who are candidates for kidney transplantation. 1By real-time PCR or transcription-mediated amplification; 2APRI, FibroTest or transient hepatic elastography; 3Adjusted doses (see text for details). PEG-IFN: Pegylated interferon; KT: Kidney transplantation; SVR: Sustained virological response; HCV: Hepatitis C virus; KT: Kidney transplantation.
HCV viral kinetics can be used to support clinical decisions during treatment. Early virological response has a positive predictive value of 67% to predict SVR and a negative predictive value (NPV) of 75% in patients receiving interferon monotherapy[98]. More recently, it has been observed a NPV of 100% for SVR if HCV RNA is detectable on week 12 of treatment[116].
Preliminary reports have suggested that first wave HCV NS3/4A protease inhibitors telaprevir and boceprevir could be used in CKD patients, with good efficacy and safety profile[117-120]. There is no need for dose adjustments for telaprevir or boceprevir since dialysis does not exert a substantial influence on the pharmacokinetics of the drugs[121,122]. It is possible that the next generations of anti-HCV direct-acting antiviral agents (DAAs), such as second and third waves NS3/4A protease inhibitors, NS5A polymerase inhibitors, NS5B polymerase inhibitors and cyclophillin inhibitors will overcome the therapeutic barrier in this population, especially when interferon-free and ribavirin-free regimens become available.
Patients with cirrhosis, particularly those with portal hypertension, may have a decreased survival and increased morbidity after renal transplantation[123]. In these cases, a renal transplantation alone is contraindicated and combined liver-kidney transplantation should be considered. For patients with compensated cirrhosis and without significant portal hypertension, isolated renal transplant appears to be safe[124,125]. In these subjects with advanced fibrosis or cirrhosis, imaging monitoring and upper endoscopy are recommended for the screening of hepatocellular carcinoma and esophageal varices, respectively.
CHRONIC HEPATITIS C AFTER KIDNEY TRANSPLANTATION
Although some studies have failed to find a negative impact on clinical outcomes after KT[126,127], the majority of studies so far reported indicate that HCV infection is associated with increased liver-related mortality and fibrosis progression among HCV-infected KT patients, with a significant reduction in patient and graft survival, possibly related to accelerated fibrogenesis and increased liver damage induced by the use of immunosuppressive regimens[88,91-93,123,128-133]. Recent evidence also suggests that KT recipients with chronic HCV infection have an increased risk of post-transplant de novo glomerulonephritis[89,90,134], diabetes mellitus[90,135], and azathioprine hepatotoxicity[136].
In contrast to what is observed in CKD patients under dialysis, HCV-positive KT recipients more often present with false-negative anti-HCV results, even with newer immunoassays[63,137]. In a recent study, 19 out of 417 KT recipients were HCV RNA-positive and 3 of those patients (16%) were anti-HCV-negative by using chemiluminescence immunoassays[63]. This inability to mount an antibody response against HCV is probably related to the immunosuppressive therapy. Another consequence of immunosuppression is the significant increase in HCV viral load, with no reports of intermittent viremia so far[9,138]. Interestingly, similarly to hemodialysis subjects, there is a very low prevalence of occult HCV infection in KT recipients[63].
There is still no consensus on the best immunosuppressive strategy in HCV-positive KT recipients. Antiviral activity of cyclosporine A (CsA), probably acting by antagonizing the effect of cyclophilin B on HCV replication[139], has been demonstrated both in vitro and in vivo[139-143], and it is possible that CsA may exert a beneficial effect on necroinflammatory activity in HCV-related liver disease among KT recipients[144-146].
Additional differences from hemodialysis patients are the higher prevalence of advanced liver disease in KT recipients[59,91,92], and the better accuracy of aminotransferases for the prediction of significant histological lesions[147-149]. Although liver biopsy is still recommended to evaluate the severity of hepatic lesions in patients on hemodialysis patients as well as in transplant recipients[150,151], several noninvasive methods for the assessment of liver fibrosis have been studied in HCV-positive KT patients, including simple blood tests[58], Fibro Test[85,152], and liver elastography[152,153], with fair diagnostic performances. In selected cases, these methods can be used as alternatives to liver biopsy. Screening for hepatocellular carcinoma and esophageal varices is indicated for patients with advanced fibrosis or cirrhosis.
As for antiviral therapy, there are several heterogeneous studies including small series of HCV-infected KT patients (ranging from 3 to 32 subjects), treated with standard or pegylated IFN alone or in combination with low dose ribavirin[154-170] (Table 4). Seventeen studies have been compiled in 2 meta-analyses[94,171], and the mean overall estimates for SVR with IFN monotherapy (10 studies), IFN plus ribavirin (4 studies) and PEG-IFN plus ribavirin (3 studies) were 16%, 36% and 43%, respectively.
Table 4.
Studies on the treatment of chronic hepatitis C in kidney transplant recipients
| Ref. | Year | n | Therapy | Interferon dose | Duration (mo) | SVR |
| Harihara et al[154] | 1994 | 3 | IFN | 3-6 MU biw | NA | NA |
| Therret et al[155] | 1994 | 13 | IFN | 3-5 MU tiw | About 4 | NA |
| Magnone et al[156] | 1995 | 11 | IFN | 1.5-5.0 MU tiw | 6 | NA |
| Rostaing et al[157] | 1995 | 14 | IFN | 3 MU tiw | About 5 | 0% |
| Ozgür et al[158] | 1995 | 5 | IFN | 4.5 MU tiw | 6 | NA |
| Yasumura et al[159] | 1997 | 6 | IFN | 6 MU tiw | About 7 | 33% |
| Durlik et al[160] | 1998 | 11 | IFN | 3 MU tiw | About 6 | 0% |
| Hanafusa et al[161] | 1998 | 10 | IFN | 9 MU tiw | 6 | 10% |
| Tokumoto et al[162] | 1998 | 6 | IFN | 9 MU tiw | 6 | 50% |
| Baid et al[163] | 2003 | 12 | IFN + RBV | 3 MU tiw | Variable | 33% |
| Tang et al[164] | 2003 | 4 | IFN + RBV | 3 MU tiw | 12 | 50% |
| Shu et al[165] | 2004 | 11 | IFN + RBV | 1 MU tiw | 12 | 27% |
| Izopet et al[166] | 1997 | 15 | IFN | 3 MU tiw | About 5 | 0% |
| Sharma et al[167] | 2006 | 6 | IFN + RBV | 3 MU tiw | About 12 | 33% |
| Pageaux et al[168] | 2009 | 8 | PEG-IFNα2a | 180 μg qw | 6-12 | 50% |
| Aljumah et al[169] | 2012 | 19 | PEG-IFN + RBV | 90-180 μg qw | 12 | 42% |
| Sanai et al[170] | 2013 | 32 | PEG-IFN + RBV | 135-180 μg qw | 12 | 38% |
IFN: Interferon; MU: Million units; biw: Two times a week; NA: Not available; tiw: Three times a week; PEG-IFN: Pegylated interferon; RBV: Ribavirin; SVR: Sustained virological response rate by intention-to-treat analysis.
Despite initial concerns about increased risk of graft dysfunction and loss, ranging from 0% to 40% in early studies[159-162,166], more recent series have shown lower graft rejection rates, between 0 and 5%[167-170]. Advances in immunosuppression therapy and the use of the less immunogenic PEG-IFN are possible explanations for this observation. However, dropout rates remain high (mean incidence of 28%) and do not differ greatly between early and recent studies[154-170], probably due to the worse anemia found in those receiving IFN in combination with ribavirin.
AASLD and KDIGO guidelines recommend against antiviral therapy for HCV-infected KT recipients, with the exceptions of fibrosing cholestatic hepatitis or life-threatening vasculitis[150,172]. Nevertheless, treatment with PEG-IFN and ribavirin should be considered for patients with advanced fibrosis, always taking into account time after transplantation, eGFR and renal function stability.
With the aid of EPO supplementation (doses up to 40000 IU/wk to maintain hemoglobin levels ≥ 10 g/dL), an initial ribavirin dose of 200 mg once a day is given, followed by increments of 200 mg every two weeks until the maximum dose tolerated, with target dosage of ribavirin defined according to eGFR (Table 2). After stabilization of ribavirin dosage (usually between 400 to 800 mg/d), interferon is initiated and used for 48 wk, irrespective of HCV genotype. Even in absence of comparative trials, it is suggested to use PEG-IFN (preferably PEG-IFN α2a 135-180 μg) once a week, in combination with ribavirin (see Figure 3). There are no studies supporting the use of DAAs for the treatment of KT HCV-positive recipients, but it is expected that these patients will benefit from interferon-free regimens.
Figure 3.
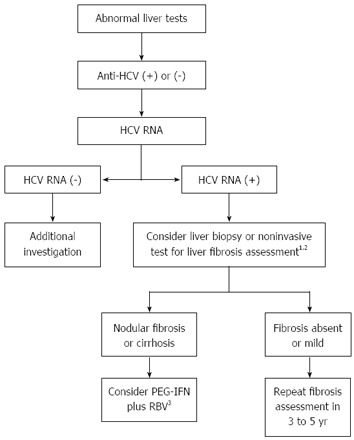
Treatment decision algorithm for hepatitis C virus-infected kidney transplantation recipients. 1Particularly if time after transplantation > 5 years; 2APRI, TX3, FibroTest or transient hepatic elastography; 3Adjusted doses according to eGFR. PEG-IFN: Pegylated interferon; RBV: Ribavirin; eGFR: Estimated glomerular filtration rate.
SIDE EFFECTS OF ANTIVIRAL THERAPY
Treatment with IFN (standard or pegylated) and ribavirin is associated with frequent and sometimes serious side effects[173,174]. Among the latter are autoimmune diseases (worsening or de novo thyroid disorders, diabetes mellitus, psoriasis, etc.), significant hemolytic anemia and severe depression. In a recent meta-analysis of eleven clinical studies published by Fabrizi et al[103], the summary estimate for dropout rate was 0.18 (95%CI: 0.08-0.35), with a large heterogeneity across studies, mainly due to anemia (24%) and infections (13%).
Except from hemolytic anemia, side effects are mainly related to IFN. The majority of the patients receiving IFN presents with a flu-like syndrome, characterized by diffuse myalgia, headache, fatigue and fever. Generally, these symptoms are self-limited and managed by common analgesics. Depression can be induced by IFN in 20% to 30% of the cases, usually after three months of treatment[175]. Being mild to moderate in intensity, IFN-induced depression can generally be handled with conservative measures, by non-psychiatrist professionals. However, if severe depression develops, HCV treatment must be stopped and the patient should be immediately referred to a psychiatrist. IFN-induced cytopenias (thrombocytopenia and leucopenia), are relatively common, typically dose-dependent and rarely associated with clinically significant complications, even in CKD patients. IFN dose reductions and the use of growth factors usually allow the continuation of therapy[173,174].
On the other hand, as previously discussed, the ribavirin-induced hemolytic anemia is very common and troublesome in CKD patients, due to its severity and potential noxious consequences in these subjects with high cardiovascular risk. Initial low doses of ribavirin, early dose reductions, and EPO supplementation are the main strategies for the management of anemia in this context. It should be mentioned that ribavirin can carry an increased risk of birth defects, and proper contraception during and up to six months after therapy must be adopted. Minor side effects like cough and skin rash also seem to be mostly associated with ribavirin[173,174].
With the first-generation HCV protease inhibitors (PIs), boceprevir and telaprevir, complex drug-to-drug interactions and tolerability issues remain a concern[176,177]. Boceprevir is associated with an increased incidence of anemia and dysgeusia and telaprevir is associated with an increased incidence of dermatological disorders, anemia, and anorectal symptoms[178-181]. An approximately 15% to 26% increase in anemia incidence in patients under triple therapy with boceprevir or telaprevir has been observed[178-181]. In these patients, anemia is considered the consequence of the combined effects of ribavirin-induced hemolysis and the bone marrow suppression of IFN and PI. In the same manner of dual therapy, ribavirin dose reductions and EPO are used for the management of anemia, although blood transfusions are also frequently required. Dysgeusia and anorectal symptoms are infrequently severe and often improve under conservative measures and dietetic modifications. A wide spectrum of dermatological disorders has been described in approximately 50% of the patients treated with first-generation PIs, particularly with telaprevir, ranging from simple pruritus with or without rash to severe skin reactions like Stevens-Johnson’s syndrome or DRESS syndrome[182]. Emollients/moisturizers and topical corticosteroids are sufficient for most cases (90% to 95%), but dermatological consultations are frequently needed for more severe cases. Treatment discontinuation is required in about 6% of patients[182].
NEW PERSPECTIVES FOR HCV THERAPY IN CKD PATIENTS
In spite of the increment in SVR rates with the use of the first-generation PIs telaprevir and boceprevir in subjects with preserved renal function, the higher incidence of significant side effects (mainly severe anemia and dermatological reactions) and the frequent drug-drug interactions have hampered their widespread use in difficult-to-treat populations, such as CKD patients. Newer DAAs, like sofosbuvir (a nucleotide NS5B polymerase inhibitor), simeprevir (a second generation PI), and daclatasvir (a NS5A replication complex inhibitor), with or without PEG-IFN and/or ribavirin, or used in different combinations with one another, produces SVR rates superior to 90%[183-188]. Besides leading to the highest SVR rates ever seen, these emerging DDAs seems to exhibit a reduced potential for drug-drug interactions and a better safety profile, which will probably facilitate their use for the treatment of HCV infection in CKD subjects. However, the appropriate dose of sofosbuvir has not been identified for subjects with severe renal impairment (eGFR < 30 mL/min per 1.73 m2) or hemodialysis patients and dose adjustments might be necessary[189]. Likewise, although simeprevir is primarily metabolized by the liver and its renal elimination is minimal, the safety of the drug has not been evaluated in patients with CKD stages 4 and 5. Conversely, unpublished data suggested that dose reduction would not be needed for the use of daclatasvir in patients with any stage of renal impairment. Finally, a phase 3 study will evaluate the safety and efficacy of the all-oral and IFN-free combination therapy with ombitasvir (a NS5A replication complex inhibitor), ABT-450 (a second-generation PI) and dasabuvir (a non-nucleoside NS5B polymerase inhibitor), with or without ribavirin, for the treatment of genotype 1-infected CKD patients (ClinicalTrials.gov identifier NCT02207088)[190].
CONCLUSION
HCV infection is highly prevalent among CKD subjects and, consequently, in KT recipients, exerting a significant negative impact on clinical outcomes both before and after KT. Although interferon-based antiviral therapy in CKD patients is associated with poor tolerability and suboptimal efficacy, there has been mounting evidence that many patients can benefit from treatment. In those individuals, accurate characterization of liver disease and adequate assessment of comorbidities are mandatory for optimal management and therapeutic decisions.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 1, 2014
First decision: July 21, 2014
Article in press: December 8, 2014
P- Reviewer: Chuang WL, Lai S, Ohsawa M S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Fabrizi F, Messa P, Basile C, Martin P. Hepatic disorders in chronic kidney disease. Nat Rev Nephrol. 2010;6:395–403. doi: 10.1038/nrneph.2010.37. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.Moyer VA; U. S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 4.Kumagai J, Komiya Y, Tanaka J, Katayama K, Tatsukawa Y, Yorioka N, Miyakawa Y, Yoshizawa H. Hepatitis C virus infection in 2,744 hemodialysis patients followed regularly at nine centers in Hiroshima during November 1999 through February 2003. J Med Virol. 2005;76:498–502. doi: 10.1002/jmv.20389. [DOI] [PubMed] [Google Scholar]
- 5.Hinrichsen H, Leimenstoll G, Stegen G, Schrader H, Fölsch UR, Schmidt WE; PHV Study Group. Prevalence and risk factors of hepatitis C virus infection in haemodialysis patients: a multicentre study in 2796 patients. Gut. 2002;51:429–433. doi: 10.1136/gut.51.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jadoul M, Cornu C, van Ypersele de Strihou C. Incidence and risk factors for hepatitis C seroconversion in hemodialysis: a prospective study. The UCL Collaborative Group. Kidney Int. 1993;44:1322–1326. doi: 10.1038/ki.1993.385. [DOI] [PubMed] [Google Scholar]
- 7.Huang CS, Ho MS, Yang CS, Lee CL, Tan CA. Hepatitis C markers in hemodialysis patients. J Clin Microbiol. 1993;31:1764–1769. doi: 10.1128/jcm.31.7.1764-1769.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Y, Norris JL, Zang C, Peng Z, Wang N. Incidence of hepatitis C virus infection in patients on hemodialysis: a systematic review and meta-analysis. Hemodial Int. 2013;17:532–541. doi: 10.1111/j.1542-4758.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 9.Pereira BJ, Levey AS. Hepatitis C virus infection in dialysis and renal transplantation. Kidney Int. 1997;51:981–999. doi: 10.1038/ki.1997.139. [DOI] [PubMed] [Google Scholar]
- 10.Rao AK, Luckman E, Wise ME, MacCannell T, Blythe D, Lin Y, Xia G, Drobeniuc J, Noble-Wang J, Arduino MJ, et al. Outbreak of hepatitis C virus infections at an outpatient hemodialysis facility: the importance of infection control competencies. Nephrol Nurs J. 2013;40:101–10, 164; quiz 111. [PubMed] [Google Scholar]
- 11.Mbaeyi C, Thompson ND. Hepatitis C virus screening and management of seroconversions in hemodialysis facilities. Semin Dial. 2013;26:439–446. doi: 10.1111/sdi.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arenas MD, Sánchez-Payá J, Muñoz C, Egea JJ, Martín F, Gil MT, Sarró F. [Nosocomial transmission of the hepatitis C virus in hemodialysis: monitors, personnel, or both?] Nefrologia. 2001;21:476–484. [PubMed] [Google Scholar]
- 13.Cronin RE, Reilly RF. Unfractionated heparin for hemodialysis: still the best option. Semin Dial. 2010;23:510–515. doi: 10.1111/j.1525-139X.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanini S, Abbate I, Puro V, Soscia F, Albertoni F, Battisti W, Ruta A, Capobianchi MR, Ippolito G. Molecular epidemiology of a hepatitis C virus epidemic in a haemodialysis unit: outbreak investigation and infection outcome. BMC Infect Dis. 2010;10:257. doi: 10.1186/1471-2334-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izopet J, Sandres-Sauné K, Kamar N, Salama G, Dubois M, Pasquier C, Rostaing L. Incidence of HCV infection in French hemodialysis units: a prospective study. J Med Virol. 2005;77:70–76. doi: 10.1002/jmv.20415. [DOI] [PubMed] [Google Scholar]
- 16.Lemos LB, Perez RM, Matos CA, Silva IS, Silva AE, Ferraz ML. Clinical and laboratory characteristics of acute hepatitis C in patients with end-stage renal disease on hemodialysis. J Clin Gastroenterol. 2008;42:208–211. doi: 10.1097/MCG.0b013e31802dc57f. [DOI] [PubMed] [Google Scholar]
- 17.Guh JY, Lai YH, Yang CY, Chen SC, Chuang WL, Hsu TC, Chen HC, Chang WY, Tsai JH. Impact of decreased serum transaminase levels on the evaluation of viral hepatitis in hemodialysis patients. Nephron. 1995;69:459–465. doi: 10.1159/000188520. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda K, Okuda K, Endo N, Ishiwatari Y, Ikeda R, Hayashi H, Yokozeki K, Kobayashi S, Irie Y. Hypoaminotransferasemia in patients undergoing long-term hemodialysis: clinical and biochemical appraisal. Gastroenterology. 1995;109:1295–1300. doi: 10.1016/0016-5085(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 19.Okuda K, Hayashi H, Yokozeki K, Kobayashi S, Kashima T, Irie Y. Acute hepatitis C among renal failure patients on chronic haemodialysis. J Gastroenterol Hepatol. 1998;13:62–67. doi: 10.1111/j.1440-1746.1998.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 20.Furusyo N, Hayashi J, Kakuda K, Ariyama I, Kanamoto-Tanaka Y, Shimizu C, Etoh Y, Shigematsu M, Kashiwagi S. Acute hepatitis C among Japanese hemodialysis patients: a prospective 9-year study. Am J Gastroenterol. 2001;96:1592–1600. doi: 10.1111/j.1572-0241.2001.03701.x. [DOI] [PubMed] [Google Scholar]
- 21.Espinosa M, Martin-Malo A, Alvarez de Lara MA, Gonzalez R, Rodriguez M, Aljama P. Natural history of acute HCV infection in hemodialysis patients. Clin Nephrol. 2002;58:143–150. doi: 10.5414/cnp58143. [DOI] [PubMed] [Google Scholar]
- 22.Uyttendaele S, Claeys H, Mertens W, Verhaert H, Vermylen C. Evaluation of third-generation screening and confirmatory assays for HCV antibodies. Vox Sang. 1994;66:122–129. doi: 10.1111/j.1423-0410.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 23.Carithers RL, Marquardt A, Gretch DR. Diagnostic testing for hepatitis C. Semin Liver Dis. 2000;20:159–171. doi: 10.1055/s-2000-9939. [DOI] [PubMed] [Google Scholar]
- 24.Moreira R, Pinho JR, Fares J, Oba IT, Cardoso MR, Saraceni CP, Granato C. Prospective study of hepatitis C virus infection in hemodialysis patients by monthly analysis of HCV RNA and antibodies. Can J Microbiol. 2003;49:503–507. doi: 10.1139/w03-065. [DOI] [PubMed] [Google Scholar]
- 25.Maheshwari A, Thuluvath PJ. Management of acute hepatitis C. Clin Liver Dis. 2010;14:169–176, x. doi: 10.1016/j.cld.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Süleymanlar I, Sezer T, Işitan F, Yakupoglu G, Süleymanlar G. Efficacy of interferon alpha in acute hepatitis C in patients on chronic hemodialysis. Nephron. 1998;79:353–354. doi: 10.1159/000045065. [DOI] [PubMed] [Google Scholar]
- 27.Gürsoy M, Gür G, Arslan H, Ozdemir N, Boyacioglu S. Interferon therapy in haemodialysis patients with acute hepatitis C virus infection and factors that predict response to treatment. J Viral Hepat. 2001;8:70–77. doi: 10.1046/j.1365-2893.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 28.Urbánek P, Tesar V, Procházková-Francisci E, Lachmanová J, Marecek Z, Svobodník A. Treatment of early diagnosed HCV infection in hemodialyzed patients with interferon-alpha. Treatment of hepatitis C. Blood Purif. 2004;22:344–350. doi: 10.1159/000079871. [DOI] [PubMed] [Google Scholar]
- 29.Al-Harbi AS, Malik GH, Subaity Y, Mansy H, Abutaleb N. Treatment of acute hepatitis C virus infection with alpha interferon in patients on hemodialysis. Saudi J Kidney Dis Transpl. 2005;16:293–297. [PubMed] [Google Scholar]
- 30.Rocha CM, Perez RM, Narciso JL, Ferreira AP, Lemos LB, Medina-Pestana JO, Silva AE, Ferraz ML. Interferon-alpha therapy within the first year after acute hepatitis C infection in hemodialysis patients: efficacy and tolerance. Eur J Gastroenterol Hepatol. 2007;19:119–123. doi: 10.1097/01.meg.0000252626.73172.f3. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira Ade S, Perez Rde M, Ferraz ML, Lewis-Ximenez LL, Pereira JL, de Almeida PR, de Mattos AA; Acute Hepatitis C Study Group of The Brazilian Society of Hepatology. Acute hepatitis C in Brazil: results of a national survey. J Med Virol. 2011;83:1738–1743. doi: 10.1002/jmv.22175. [DOI] [PubMed] [Google Scholar]
- 32.Engel M, Malta FM, Gomes MM, Mello IM, Pinho JR, Ono-Nita SK, Carrilho FJ. Acute hepatitis C virus infection assessment among chronic hemodialysis patients in the Southwest Parana State, Brazil. BMC Public Health. 2007;7:50. doi: 10.1186/1471-2458-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu CH, Liang CC, Liu CJ, Lin JW, Chen SI, Hung PH, Tsai HB, Lai MY, Chen PJ, Chen DS, et al. Pegylated interferon alfa-2a monotherapy for hemodialysis patients with acute hepatitis C. Clin Infect Dis. 2010;51:541–549. doi: 10.1086/655682. [DOI] [PubMed] [Google Scholar]
- 34.Fabrizi F, Dixit V, Messa P, Martin P. Interferon therapy of acute hepatitis C in dialysis patients: meta-analysis. J Viral Hepat. 2012;19:784–791. doi: 10.1111/j.1365-2893.2012.01607.x. [DOI] [PubMed] [Google Scholar]
- 35.Santantonio T, Fasano M, Sagnelli E, Tundo P, Babudieri S, Fabris P, Toti M, Di Perri G, Marino N, Pizzigallo E, Angarano G; Acute Hepatitis C Study Group. Acute hepatitis C: a 24-week course of pegylated interferon α-2b versus a 12-week course of pegylated interferon α-2b alone or with ribavirin. Hepatology. 2014;59:2101–2109. doi: 10.1002/hep.26991. [DOI] [PubMed] [Google Scholar]
- 36.Althaf MM, Abdelsalam MS, Rashwan M, Nadri Q. Acute hepatitis C infection in a renal transplant recipient: primacy of the liver or kidney? BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-203643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogachev B, Vorobiov M, Shnaider A, Hausmann M, Zlotnik M, Basok A. Acute viral hepatitis (C - genotype 6a and B) acquired during kidney transplantation by two patients and review of the literature. Clin Nephrol. 2009;72:482–487. [PubMed] [Google Scholar]
- 38.Siddiqui AR, Abbas Z, Luck NH, Hassan SM, Aziz T, Mubarak M, Naqvi SA, Rizvi SA. Experience of fibrosing cholestatic hepatitis with hepatitis C virus in kidney transplant recipients. Transplant Proc. 2012;44:721–724. doi: 10.1016/j.transproceed.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Delladetsima I, Psichogiou M, Sypsa V, Psimenou E, Kostakis A, Hatzakis A, Boletis JN. The course of hepatitis C virus infection in pretransplantation anti-hepatitis C virus-negative renal transplant recipients: a retrospective follow-up study. Am J Kidney Dis. 2006;47:309–316. doi: 10.1053/j.ajkd.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Fabrizi F, Marcelli D, Bacchini G, Guarnori I, Erba G, Locatelli F. Antibodies to hepatitis C virus (HCV) in chronic renal failure (CRF) patients on conservative therapy: prevalence, risk factors and relationship to liver disease. Nephrol Dial Transplant. 1994;9:780–784. [PubMed] [Google Scholar]
- 41.Ilçöl B, Ozener C, Avşar M, Ilçol Y, Lawrence R, Ozer A, Cirakoğlu B, Akoğlu E. Hepatitis C infection in patients with chronic renal failure receiving conservative therapy. Nephrol Dial Transplant. 1997;12:626. doi: 10.1093/ndt/12.3.626a. [DOI] [PubMed] [Google Scholar]
- 42.López-Alcorocho JM, Barril G, Ortiz-Movilla N, Traver JA, Bartolomé J, Sanz P, Selgas R, Carreño V. Prevalence of hepatitis B, hepatitis C, GB virus C/hepatitis G and TT viruses in predialysis and hemodialysis patients. J Med Virol. 2001;63:103–107. [PubMed] [Google Scholar]
- 43.Lemos LB, Perez RM, Lemos MM, Lanzoni VP, Draibe SA, Silva IS, Silva AE, Ferraz ML. Hepatitis C in chronic kidney disease: predialysis patients present more severe histological liver injury than hemodialysis patients? Am J Nephrol. 2007;27:191–196. doi: 10.1159/000100892. [DOI] [PubMed] [Google Scholar]
- 44.Lemos LB, Perez RM, Lemos MM, Draibe SA, Silva IS, Silva AE, Ferraz ML. Hepatitis C among predialysis patients: prevalence and characteristics in a large cohort of patients. Nephron Clin Pract. 2008;108:c135–c140. doi: 10.1159/000114452. [DOI] [PubMed] [Google Scholar]
- 45.Halfon P, Khiri H, Feryn JM, Sayada C, Chanas M, Ouzan D. Prospective virological follow-up of hepatitis C infection in a haemodialysis unit. J Viral Hepat. 1998;5:115–121. doi: 10.1046/j.1365-2893.1998.00089.x. [DOI] [PubMed] [Google Scholar]
- 46.Fabrizi F, Martin P, Dixit V, Brezina M, Cole MJ, Vinson S, Mousa M, Gitnick G. Biological dynamics of viral load in hemodialysis patients with hepatitis C virus. Am J Kidney Dis. 2000;35:122–129. doi: 10.1016/S0272-6386(00)70310-6. [DOI] [PubMed] [Google Scholar]
- 47.Furusyo N, Hayashi J, Ariyama I, Sawayama Y, Etoh Y, Shigematsu M, Kashiwagi S. Maintenance hemodialysis decreases serum hepatitis C virus (HCV) RNA levels in hemodialysis patients with chronic HCV infection. Am J Gastroenterol. 2000;95:490–496. doi: 10.1111/j.1572-0241.2000.01773.x. [DOI] [PubMed] [Google Scholar]
- 48.Willson RA. Nephrotoxicity of interferon alfa-ribavirin therapy for chronic hepatitis C. J Clin Gastroenterol. 2002;35:89–92. doi: 10.1097/00004836-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Gluhovschi C, Gadalean F, Kaycsa A, Curescu M, Sporea I, Gluhovschi G, Petrica L, Velciov S, Bozdog G, Bob F, et al. Does the antiviral therapy of patients with chronic hepatitis exert nephrotoxic effects? Immunopharmacol Immunotoxicol. 2011;33:744–750. doi: 10.3109/08923973.2010.551129. [DOI] [PubMed] [Google Scholar]
- 50.Bruchfeld A, Lindahl K, Schvarcz R, Ståhle L. Dosage of ribavirin in patients with hepatitis C should be based on renal function: a population pharmacokinetic analysis. Ther Drug Monit. 2002;24:701–708. doi: 10.1097/00007691-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Laskin OL, Longstreth JA, Hart CC, Scavuzzo D, Kalman CM, Connor JD, Roberts RB. Ribavirin disposition in high-risk patients for acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1987;41:546–555. doi: 10.1038/clpt.1987.70. [DOI] [PubMed] [Google Scholar]
- 52.Homma M, Matsuzaki Y, Inoue Y, Shibata M, Mitamura K, Tanaka N, Kohda Y. Marked elevation of erythrocyte ribavirin levels in interferon and ribavirin-induced anemia. Clin Gastroenterol Hepatol. 2004;2:337–339. doi: 10.1016/s1542-3565(04)00064-3. [DOI] [PubMed] [Google Scholar]
- 53.De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, Noventa F, Stanzial AM, Solero P, Corrocher R. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997–1004. doi: 10.1053/he.2000.5789. [DOI] [PubMed] [Google Scholar]
- 54.Stehman-Breen CO, Emerson S, Gretch D, Johnson RJ. Risk of death among chronic dialysis patients infected with hepatitis C virus. Am J Kidney Dis. 1998;32:629–634. doi: 10.1016/s0272-6386(98)70027-7. [DOI] [PubMed] [Google Scholar]
- 55.Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697–703. doi: 10.1111/j.1365-2893.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 56.Scott DR, Wong JK, Spicer TS, Dent H, Mensah FK, McDonald S, Levy MT. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010;90:1165–1171. doi: 10.1097/TP.0b013e3181f92548. [DOI] [PubMed] [Google Scholar]
- 57.Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat. 2012;19:601–607. doi: 10.1111/j.1365-2893.2012.01633.x. [DOI] [PubMed] [Google Scholar]
- 58.Schiavon LL, Schiavon JL, Filho RJ, Sampaio JP, Lanzoni VP, Silva AE, Ferraz ML. Simple blood tests as noninvasive markers of liver fibrosis in hemodialysis patients with chronic hepatitis C virus infection. Hepatology. 2007;46:307–314. doi: 10.1002/hep.21681. [DOI] [PubMed] [Google Scholar]
- 59.Vallet-Pichard A, Pol S. Hepatitis C virus infection in hemodialysis patients. Clin Res Hepatol Gastroenterol. 2013;37:340–346. doi: 10.1016/j.clinre.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Okuda K, Yokosuka O. Natural history of chronic hepatitis C in patients on hemodialysis: case control study with 4-23 years of follow-up. World J Gastroenterol. 2004;10:2209–2212. doi: 10.3748/wjg.v10.i15.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fabrizi F, Lunghi G, Finazzi S, Colucci P, Pagano A, Ponticelli C, Locatelli F. Decreased serum aminotransferase activity in patients with chronic renal failure: impact on the detection of viral hepatitis. Am J Kidney Dis. 2001;38:1009–1015. doi: 10.1053/ajkd.2001.28590. [DOI] [PubMed] [Google Scholar]
- 62.Sauné K, Kamar N, Miédougé M, Weclawiak H, Dubois M, Izopet J, Rostaing L. Decreased prevalence and incidence of HCV markers in haemodialysis units: a multicentric French survey. Nephrol Dial Transplant. 2011;26:2309–2316. doi: 10.1093/ndt/gfq696. [DOI] [PubMed] [Google Scholar]
- 63.Baid-Agrawal S, Schindler R, Reinke P, Staedtler A, Rimpler S, Malik B, Frei U, Berg T. Prevalence of occult hepatitis C infection in chronic hemodialysis and kidney transplant patients. J Hepatol. 2014;60:928–933. doi: 10.1016/j.jhep.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 64.Ishida H, Tanabe K, Tokumoto T, Shimizu T, Shimmura H, Yoshioka T, Toma H. Hepatitis C virus decreases in patients with maintenance hemofiltration therapy. Artif Organs. 2004;28:316–318. doi: 10.1111/j.1525-1594.2004.47196.x. [DOI] [PubMed] [Google Scholar]
- 65.Dussol B, de Lamballerie X, Brunet P, Roubicek C, Chicheportiche C, Cantaloube JF, Biagini P, de Micco P, Berland Y. Is hepatitis C virus-RNA detection by nested polymerase chain reaction clinically relevant in hemodialysis patients? Clin Nephrol. 1996;45:257–260. [PubMed] [Google Scholar]
- 66.Galán F, Pérez-Gracia MT, Lozano A, Benavides B, Fernandez-Ruíz E, Rodríguez-Iglesias MA. A 3-year follow-up of HCV-RNA viraemia in haemodialysis patients. Nephrol Dial Transplant. 1998;13:1211–1214. doi: 10.1093/ndt/13.5.1211. [DOI] [PubMed] [Google Scholar]
- 67.Fabrizi F, Bunnapradist S, Lunghi G, Martin P. Kinetics of hepatitis C virus load during hemodialysis: novel perspectives. J Nephrol. 2003;16:467–475. [PubMed] [Google Scholar]
- 68.Dzekova-Vidimliski P, Asani A, Selim G, Gelev S, Polenakovic M, Sikole A. Patterns of viraemia in haemodialysis patients with hepatitis C. Prilozi. 2008;29:201–211. [PubMed] [Google Scholar]
- 69.Satsangi J, Jewell DP, Welsh K, Bunce M, Bell JI. Effect of heparin on polymerase chain reaction. Lancet. 1994;343:1509–1510. doi: 10.1016/s0140-6736(94)92622-0. [DOI] [PubMed] [Google Scholar]
- 70.Okuda K, Hayashi H, Yokozeki K, Irie Y. Destruction of hepatitis C virus particles by haemodialysis. Lancet. 1996;347:909–910. doi: 10.1016/s0140-6736(96)91397-5. [DOI] [PubMed] [Google Scholar]
- 71.Rampino T, Libetta C, Mazzone A, Gregorini M, Soccio G, Ranghino A, Maggio M, Guallini P, Girola S, Dal Canton A. Hepatocyte growth factor protects the liver against hepatitis C virus in patients on regular hemodialysis. J Chemother. 1998;10:164–166. doi: 10.1179/joc.1998.10.2.164. [DOI] [PubMed] [Google Scholar]
- 72.Badalamenti S, Catania A, Lunghi G, Covini G, Bredi E, Brancaccio D, Salvadori M, Como G, Ponticelli C, Graziani G. Changes in viremia and circulating interferon-alpha during hemodialysis in hepatitis C virus-positive patients: only coincidental phenomena? Am J Kidney Dis. 2003;42:143–150. doi: 10.1016/s0272-6386(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 73.Fabrizi F, Messa P, Martin P. Impact of hemodialysis therapy on hepatitis C virus infection: a deeper insight. Int J Artif Organs. 2009;32:1–11. doi: 10.1177/039139880903200101. [DOI] [PubMed] [Google Scholar]
- 74.Khan N, Aswad S, Shidban H, Aghajani M, Mendez R, Mendez R, Comanor L. Improved detection of HCV Infection in hemodialysis patients using a new HCV RNA qualitative assay: experience of a transplant center. J Clin Virol. 2004;30:175–182. doi: 10.1016/j.jcv.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Rao V, Fabrizi F, Pennell P, Schiff E, de Medina M, Lane JR, Martin P, Ivor L. Improved detection of hepatitis C virus infection by transcription-mediated amplification technology in dialysis population. Ren Fail. 2010;32:721–726. doi: 10.3109/0886022X.2010.486499. [DOI] [PubMed] [Google Scholar]
- 76.Bastos DO, Perez RM, Silva IS, Lemos LB, Simonetti JP, Medina-Pestana JO, Silva AE, Ferraz ML. Transcription-mediated amplification (TMA) for the assessment of viremia in hemodialysis patients with hepatitis C. J Med Virol. 2012;84:596–600. doi: 10.1002/jmv.23216. [DOI] [PubMed] [Google Scholar]
- 77.Chevaliez S, Rodriguez C, Pawlotsky JM. New virologic tools for management of chronic hepatitis B and C. Gastroenterology. 2012;142:1303–1313.e1. doi: 10.1053/j.gastro.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 79.Sabovic M, Salobir B, Preloznik Zupan I, Bratina P, Bojec V, Buturovic Ponikvar J. The influence of the haemodialysis procedure on platelets, coagulation and fibrinolysis. Pathophysiol Haemost Thromb. 2005;34:274–278. doi: 10.1159/000093107. [DOI] [PubMed] [Google Scholar]
- 80.Cotler SJ, Diaz G, Gundlapalli S, Jakate S, Chawla A, Mital D, Jensik S, Jensen DM. Characteristics of hepatitis C in renal transplant candidates. J Clin Gastroenterol. 2002;35:191–195. doi: 10.1097/00004836-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 81.Ahmad A, Hasan F, Abdeen S, Sheikh M, Kodaj J, Nampoory MR, Johny KV, Asker H, Siddique I, Thalib L, et al. Transjugular liver biopsy in patients with end-stage renal disease. J Vasc Interv Radiol. 2004;15:257–260. doi: 10.1097/01.rvi.0000109403.52762.c4. [DOI] [PubMed] [Google Scholar]
- 82.Ozdoğan M, Ozgür O, Boyacioğlu S, Coşkun M, Kart H, Ozdal S, Telatar H. Percutaneous liver biopsy complications in patients with chronic renal failure. Nephron. 1996;74:442–443. doi: 10.1159/000189358. [DOI] [PubMed] [Google Scholar]
- 83.Terjung B, Lemnitzer I, Dumoulin FL, Effenberger W, Brackmann HH, Sauerbruch T, Spengler U. Bleeding complications after percutaneous liver biopsy. An analysis of risk factors. Digestion. 2003;67:138–145. doi: 10.1159/000071293. [DOI] [PubMed] [Google Scholar]
- 84.Pawa S, Ehrinpreis M, Mutchnick M, Janisse J, Dhar R, Siddiqui FA. Percutaneous liver biopsy is safe in chronic hepatitis C patients with end-stage renal disease. Clin Gastroenterol Hepatol. 2007;5:1316–1320. doi: 10.1016/j.cgh.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 85.Varaut A, Fontaine H, Serpaggi J, Verkarre V, Vallet-Pichard A, Nalpas B, Imbertbismuth F, Lebray P, Pol S. Diagnostic accuracy of the fibrotest in hemodialysis and renal transplant patients with chronic hepatitis C virus. Transplantation. 2005;80:1550–1555. doi: 10.1097/01.tp.0000183399.85804.02. [DOI] [PubMed] [Google Scholar]
- 86.Liu CH, Liang CC, Liu CJ, Hsu SJ, Lin JW, Chen SI, Hung PH, Tsai HB, Lai MY, Chen PJ, et al. The ratio of aminotransferase to platelets is a useful index for predicting hepatic fibrosis in hemodialysis patients with chronic hepatitis C. Kidney Int. 2010;78:103–109. doi: 10.1038/ki.2010.74. [DOI] [PubMed] [Google Scholar]
- 87.Liu CH, Liang CC, Huang KW, Liu CJ, Chen SI, Lin JW, Hung PH, Tsai HB, Lai MY, Chen PJ, et al. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol. 2011;6:1057–1065. doi: 10.2215/CJN.04320510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh N, Neidlinger N, Djamali A, Leverson G, Voss B, Sollinger HW, Pirsch JD. The impact of hepatitis C virus donor and recipient status on long-term kidney transplant outcomes: University of Wisconsin experience. Clin Transplant. 2012;26:684–693. doi: 10.1111/j.1399-0012.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 89.Cruzado JM, Casanovas-Taltavull T, Torras J, Baliellas C, Gil-Vernet S, Grinyó JM. Pretransplant interferon prevents hepatitis C virus-associated glomerulonephritis in renal allografts by HCV-RNA clearance. Am J Transplant. 2003;3:357–360. doi: 10.1034/j.1600-6143.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 90.Guitard J, Rostaing L, Kamar N. New-onset diabetes and nephropathy after renal transplantation. Contrib Nephrol. 2011;170:247–255. doi: 10.1159/000325778. [DOI] [PubMed] [Google Scholar]
- 91.Zylberberg H, Nalpas B, Carnot F, Skhiri H, Fontaine H, Legendre C, Kreis H, Bréchot C, Pol S. Severe evolution of chronic hepatitis C in renal transplantation: a case control study. Nephrol Dial Transplant. 2002;17:129–133. doi: 10.1093/ndt/17.1.129. [DOI] [PubMed] [Google Scholar]
- 92.de Oliveira Uehara SN, Emori CT, da Silva Fucuta Pereira P, Perez RM, Pestana JO, Lanzoni VP, e Silva IS, Silva AE, Ferraz ML. Histological evolution of hepatitis C virus infection after renal transplantation. Clin Transplant. 2012;26:842–848. doi: 10.1111/j.1399-0012.2012.01635.x. [DOI] [PubMed] [Google Scholar]
- 93.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5:1452–1461. doi: 10.1111/j.1600-6143.2005.00864.x. [DOI] [PubMed] [Google Scholar]
- 94.Fabrizi F, Lunghi G, Dixit V, Martin P. Meta-analysis: anti-viral therapy of hepatitis C virus-related liver disease in renal transplant patients. Aliment Pharmacol Ther. 2006;24:1413–1422. doi: 10.1111/j.1365-2036.2006.03151.x. [DOI] [PubMed] [Google Scholar]
- 95.Russo MW, Goldsweig CD, Jacobson IM, Brown RS. Interferon monotherapy for dialysis patients with chronic hepatitis C: an analysis of the literature on efficacy and safety. Am J Gastroenterol. 2003;98:1610–1615. doi: 10.1111/j.1572-0241.2003.07526.x. [DOI] [PubMed] [Google Scholar]
- 96.Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Meta-analysis: interferon for the treatment of chronic hepatitis C in dialysis patients. Aliment Pharmacol Ther. 2003;18:1071–1081. doi: 10.1046/j.1365-2036.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 97.Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51:263–277. doi: 10.1053/j.ajkd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 98.Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon for hepatitis C virus in hemodialysis--an individual patient meta-analysis of factors associated with sustained virological response. Clin J Am Soc Nephrol. 2009;4:1449–1458. doi: 10.2215/CJN.01850309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fabrizi F, Ganeshan SV, Lunghi G, Messa P, Martin P. Antiviral therapy of hepatitis C in chronic kidney diseases: meta-analysis of controlled clinical trials. J Viral Hepat. 2008;15:600–606. doi: 10.1111/j.1365-2893.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 100.Alavian SM, Tabatabaei SV. Meta-analysis of factors associated with sustained viral response in patients on hemodialysis treated with standard or pegylated interferon for hepatitis C infection. Iran J Kidney Dis. 2010;4:181–194. [PubMed] [Google Scholar]
- 101.Fabrizi F, Dixit V, Messa P, Martin P. Pegylated interferon monotherapy of chronic hepatitis C in dialysis patients: Meta-analysis of clinical trials. J Med Virol. 2010;82:768–775. doi: 10.1002/jmv.21542. [DOI] [PubMed] [Google Scholar]
- 102.Fabrizi F, Dixit V, Martin P, Messa P. Combined antiviral therapy of hepatitis C virus in dialysis patients: meta-analysis of clinical trials. J Viral Hepat. 2011;18:e263–e269. doi: 10.1111/j.1365-2893.2010.01405.x. [DOI] [PubMed] [Google Scholar]
- 103.Fabrizi F, Dixit V, Messa P, Martin P. Antiviral therapy (pegylated interferon and ribavirin) of hepatitis C in dialysis patients: meta-analysis of clinical studies. J Viral Hepat. 2014;21:681–689. doi: 10.1111/jvh.12276. [DOI] [PubMed] [Google Scholar]
- 104.Liu CH, Liang CC, Lin JW, Chen SI, Tsai HB, Chang CS, Hung PH, Kao JH, Liu CJ, Lai MY, et al. Pegylated interferon alpha-2a versus standard interferon alpha-2a for treatment-naive dialysis patients with chronic hepatitis C: a randomised study. Gut. 2008;57:525–530. doi: 10.1136/gut.2007.133884. [DOI] [PubMed] [Google Scholar]
- 105.Bruchfeld A, Ståhle L, Andersson J, Schvarcz R. Interferon and ribavirin therapy in dialysis patients with chronic hepatitis C. Nephrol Dial Transplant. 2001;16:1729. doi: 10.1093/ndt/16.8.1729. [DOI] [PubMed] [Google Scholar]
- 106.Mousa DH, Abdalla AH, Al-Shoail G, Al-Sulaiman MH, Al-Hawas FA, Al-Khader AA. Alpha-interferon with ribavirin in the treatment of hemodialysis patients with hepatitis C. Transplant Proc. 2004;36:1831–1834. doi: 10.1016/j.transproceed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 107.Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat. 2006;13:316–321. doi: 10.1111/j.1365-2893.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 108.Rendina M, Schena A, Castellaneta NM, Losito F, Amoruso AC, Stallone G, Schena FP, Di Leo A, Francavilla A. The treatment of chronic hepatitis C with peginterferon alfa-2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplant. J Hepatol. 2007;46:768–774. doi: 10.1016/j.jhep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 109.van Leusen R, Adang RP, de Vries RA, Cnossen TT, Konings CJ, Schalm SW, Tan AC. Pegylated interferon alfa-2a (40 kD) and ribavirin in haemodialysis patients with chronic hepatitis C. Nephrol Dial Transplant. 2008;23:721–725. doi: 10.1093/ndt/gfm724. [DOI] [PubMed] [Google Scholar]
- 110.Carriero D, Fabrizi F, Uriel AJ, Park J, Martin P, Dieterich DT. Treatment of dialysis patients with chronic hepatitis C using pegylated interferon and low-dose ribavirin. Int J Artif Organs. 2008;31:295–302. doi: 10.1177/039139880803100404. [DOI] [PubMed] [Google Scholar]
- 111.Hakim W, Sheikh S, Inayat I, Caldwell C, Smith D, Lorber M, Friedman A, Jain D, Bia M, Formica R, et al. HCV response in patients with end stage renal disease treated with combination pegylated interferon alpha-2a and ribavirin. J Clin Gastroenterol. 2009;43:477–481. doi: 10.1097/MCG.0b013e318180803a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu CH, Liang CC, Liu CJ, Tsai HB, Hung PH, Hsu SJ, Chen SI, Lin JW, Lai MY, Chen JH, et al. Pegylated interferon alpha-2a plus low-dose ribavirin for the retreatment of dialysis chronic hepatitis C patients who relapsed from prior interferon monotherapy. Gut. 2009;58:314–316. doi: 10.1136/gut.2008.165076. [DOI] [PubMed] [Google Scholar]
- 113.Alsaran K, Sabry A, Shaheen N. Pegylated interferon alpha-2a for treatment of chronic HCV infection in hemodialysis patients: a single Saudi center experience. Int Urol Nephrol. 2011;43:865–873. doi: 10.1007/s11255-010-9756-1. [DOI] [PubMed] [Google Scholar]
- 114.Liu CH, Huang CF, Liu CJ, Dai CY, Liang CC, Huang JF, Hung PH, Tsai HB, Tsai MK, Chen SI, et al. Pegylated interferon-α2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: a randomized trial. Ann Intern Med. 2013;159:729–738. doi: 10.7326/0003-4819-159-11-201312030-00005. [DOI] [PubMed] [Google Scholar]
- 115.Liu CH, Liu CJ, Huang CF, Lin JW, Dai CY, Liang CC, Huang JF, Hung PH, Tsai HB, Tsai MK, et al. Peginterferon alfa-2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 2 receiving haemodialysis: a randomised trial. Gut. 2014:Apr 19; Epub ahead of print. doi: 10.1136/gutjnl-2014-307080. [DOI] [PubMed] [Google Scholar]
- 116.Fucuta Pereira Pda S, Uehara SN, de Mello Perez R, Feldner AC, de Melo IC, de Souza e Silva IS, Silva AE, Ferraz ML. Is early virological response as predictive of the hepatitis C treatment response in dialysis patients as in non-uremic patients? Int J Infect Dis. 2013;17:e50–e53. doi: 10.1016/j.ijid.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 117.Dumortier J, Guillaud O, Gagnieu MC, Janbon B, Juillard L, Morelon E, Leroy V. Anti-viral triple therapy with telaprevir in haemodialysed HCV patients: is it feasible? J Clin Virol. 2013;56:146–149. doi: 10.1016/j.jcv.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 118.Knapstein J, Galle PR, Zimmermann T. Antiviral triple therapy with boceprevir in a chronic hepatitis C haemodialysis patient awaiting kidney re-transplantation. Dig Liver Dis. 2014;46:88–89. doi: 10.1016/j.dld.2013.08.133. [DOI] [PubMed] [Google Scholar]
- 119.Slim J, Scangarello N, Samaha P, Dazley J. A case of sustained virologic response of HCV with telaprevir-based therapy in a patient with HIV and end stage kidney disease. Int J STD AIDS. 2014;25:830–832. doi: 10.1177/0956462414521164. [DOI] [PubMed] [Google Scholar]
- 120.Wiegand J, Maasoumy B, Buggisch P, Buslau A, Schiefke I, Berg T, Wedemeyer H, Sarrazin C, Hinrichsen H. Letter: Telaprevir triple therapy in chronic hepatitis C genotype 1 patients receiving haemodialysis. Aliment Pharmacol Ther. 2014;39:1342–1344. doi: 10.1111/apt.12748. [DOI] [PubMed] [Google Scholar]
- 121.Treitel M, Marbury T, Preston RA, Triantafyllou I, Feely W, O’Mara E, Kasserra C, Gupta S, Hughes EA. Single-dose pharmacokinetics of boceprevir in subjects with impaired hepatic or renal function. Clin Pharmacokinet. 2012;51:619–628. doi: 10.1007/BF03261935. [DOI] [PubMed] [Google Scholar]
- 122.de Kanter CT, den Hollander JG, Verweij-van Wissen CP, Burger DM. Telaprevir pharmacokinetics in a hepatitis C virus infected patient on haemodialysis. J Clin Virol. 2014;60:431–432. doi: 10.1016/j.jcv.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 123.Mathurin P, Mouquet C, Poynard T, Sylla C, Benalia H, Fretz C, Thibault V, Cadranel JF, Bernard B, Opolon P, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257–263. doi: 10.1002/hep.510290123. [DOI] [PubMed] [Google Scholar]
- 124.Paramesh AS, Davis JY, Mallikarjun C, Zhang R, Cannon R, Shores N, Killackey MT, McGee J, Saggi BH, Slakey DP, et al. Kidney transplantation alone in ESRD patients with hepatitis C cirrhosis. Transplantation. 2012;94:250–254. doi: 10.1097/TP.0b013e318255f890. [DOI] [PubMed] [Google Scholar]
- 125.Campos S, Parsikia A, Zaki RF, Ortiz JA. Kidney transplantation alone in ESRD patients with hepatitis C cirrhosis. Transplantation. 2012;94:e65–e66. doi: 10.1097/TP.0b013e318274abc1. [DOI] [PubMed] [Google Scholar]
- 126.Alric L, Di-Martino V, Selves J, Cacoub P, Charlotte F, Reynaud D, Piette JC, Péron JM, Vinel JP, Durand D, et al. Long-term impact of renal transplantation on liver fibrosis during hepatitis C virus infection. Gastroenterology. 2002;123:1494–1499. doi: 10.1053/gast.2002.36610. [DOI] [PubMed] [Google Scholar]
- 127.Kamar N, Rostaing L, Selves J, Sandres-Saune K, Alric L, Durand D, Izopet J. Natural history of hepatitis C virus-related liver fibrosis after renal transplantation. Am J Transplant. 2005;5:1704–1712. doi: 10.1111/j.1600-6143.2005.00918.x. [DOI] [PubMed] [Google Scholar]
- 128.Legendre C, Garrigue V, Le Bihan C, Mamzer-Bruneel MF, Chaix ML, Landais P, Kreis H, Pol S. Harmful long-term impact of hepatitis C virus infection in kidney transplant recipients. Transplantation. 1998;65:667–670. doi: 10.1097/00007890-199803150-00011. [DOI] [PubMed] [Google Scholar]
- 129.Breitenfeldt MK, Rasenack J, Berthold H, Olschewski M, Schroff J, Strey C, Grotz WH. Impact of hepatitis B and C on graft loss and mortality of patients after kidney transplantation. Clin Transplant. 2002;16:130–136. doi: 10.1034/j.1399-0012.2002.1o034.x. [DOI] [PubMed] [Google Scholar]
- 130.Ridruejo E, Díaz C, Michel MD, Soler Pujol G, Martínez A, Marciano S, Mandó OG, Vilches A. Short and long term outcome of kidney transplanted patients with chronic viral hepatitis B and C. Ann Hepatol. 2010;9:271–277. [PubMed] [Google Scholar]
- 131.Rostami Z, Nourbala MH, Alavian SM, Bieraghdar F, Jahani Y, Einollahi B. The impact of Hepatitis C virus infection on kidney transplantation outcomes: A systematic review of 18 observational studies: The impact of HCV on renal transplantation. Hepat Mon. 2011;11:247–254. [PMC free article] [PubMed] [Google Scholar]
- 132.Fabrizi F, Martin P, Dixit V, Messa P. Meta-analysis of observational studies: hepatitis C and survival after renal transplant. J Viral Hepat. 2014;21:314–324. doi: 10.1111/jvh.12148. [DOI] [PubMed] [Google Scholar]
- 133.Periera BJ, Wright TL, Schmid CH, Levey AS. The impact of pretransplantation hepatitis C infection on the outcome of renal transplantation. Transplantation. 1995;60:799–805. [PubMed] [Google Scholar]
- 134.Ozdemir BH, Ozdemir FN, Sezer S, Colak T, Haberal M. De novo glomerulonephritis in renal allografts with hepatitis C virus infection. Transplant Proc. 2006;38:492–495. doi: 10.1016/j.transproceed.2005.12.109. [DOI] [PubMed] [Google Scholar]
- 135.Fabrizi F, Messa P, Martin P, Takkouche B. Hepatitis C virus infection and post-transplant diabetes mellitus among renal transplant patients: a meta-analysis. Int J Artif Organs. 2008;31:675–682. doi: 10.1177/039139880803100801. [DOI] [PubMed] [Google Scholar]
- 136.Pol S, Cavalcanti R, Carnot F, Legendre C, Driss F, Chaix ML, Thervet E, Chkoff N, Brechot C, Berthelot P, et al. Azathioprine hepatitis in kidney transplant recipients. A predisposing role of chronic viral hepatitis. Transplantation. 1996;61:1774–1776. doi: 10.1097/00007890-199606270-00019. [DOI] [PubMed] [Google Scholar]
- 137.Preiksaitis JK, Cockfield SM, Fenton JM, Burton NI, Chui LW. Serologic responses to hepatitis C virus in solid organ transplant recipients. Transplantation. 1997;64:1775–1780. doi: 10.1097/00007890-199712270-00026. [DOI] [PubMed] [Google Scholar]
- 138.Roth D, Zucker K, Cirocco R, Burke G, Ciancio G, Esquenazi V, Swanson SJ, Miller J. A prospective study of hepatitis C virus infection in renal allograft recipients. Transplantation. 1996;61:886–889. doi: 10.1097/00007890-199603270-00007. [DOI] [PubMed] [Google Scholar]
- 139.Nakagawa M, Sakamoto N, Tanabe Y, Koyama T, Itsui Y, Takeda Y, Chen CH, Kakinuma S, Oooka S, Maekawa S, et al. Suppression of hepatitis C virus replication by cyclosporin a is mediated by blockade of cyclophilins. Gastroenterology. 2005;129:1031–1041. doi: 10.1053/j.gastro.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 140.Fernandes F, Poole DS, Hoover S, Middleton R, Andrei AC, Gerstner J, Striker R. Sensitivity of hepatitis C virus to cyclosporine A depends on nonstructural proteins NS5A and NS5B. Hepatology. 2007;46:1026–1033. doi: 10.1002/hep.21809. [DOI] [PubMed] [Google Scholar]
- 141.Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282–1288. doi: 10.1053/jhep.2003.50449. [DOI] [PubMed] [Google Scholar]
- 142.Villamil F, Levy G, Grazi GL, Mies S, Samuel D, Sanjuan F, Rossi M, Lake J, Munn S, Mühlbacher F, et al. Long-term outcomes in liver transplant patients with hepatic C infection receiving tacrolimus or cyclosporine. Transplant Proc. 2006;38:2964–2967. doi: 10.1016/j.transproceed.2006.08.131. [DOI] [PubMed] [Google Scholar]
- 143.Rayhill SC, Barbeito R, Katz D, Voigt M, Labrecque D, Kirby P, Miller R, Stolpen A, Wu Y, Schmidt W. A cyclosporine-based immunosuppressive regimen may be better than tacrolimus for long-term liver allograft survival in recipients transplanted for hepatitis C. Transplant Proc. 2006;38:3625–3628. doi: 10.1016/j.transproceed.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 144.Kamar N, Selves J, Sandres-Saune K, Durand D, Izopet J, Rostaing L. Does cyclosporine have a beneficial effect on the course of chronic hepatitis C infection after renal transplantation? Transplant Proc. 2006;38:1329–1332. doi: 10.1016/j.transproceed.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 145.Schiavon LL, Carvalho-Filho RJ, Narciso-Schiavon JL, Barbosa DV, Lanzoni VP, Ferraz ML, Silva AE. Impact of cyclosporine-based immunosuppressive therapy on liver histology of hepatitis C virus-infected renal transplant patients. Hepatology. 2008;48:348–349. doi: 10.1002/hep.22331. [DOI] [PubMed] [Google Scholar]
- 146.Manuel O, Baid-Agrawal S, Moradpour D, Pascual M. Immunosuppression in hepatitis C virus-infected patients after kidney transplantation. Contrib Nephrol. 2012;176:97–107. doi: 10.1159/000332387. [DOI] [PubMed] [Google Scholar]
- 147.Fabrizi F, Martin P, Ponticelli C. Hepatitis C virus infection and renal transplantation. Am J Kidney Dis. 2001;38:919–934. doi: 10.1053/ajkd.2001.28576. [DOI] [PubMed] [Google Scholar]
- 148.Giordano HM, França AV, Meirelles L, Escanhoela CA, Nishimura NF, Santos RL, Quadros KR, Mazzali M, Alves-Filho G, Soares EC. Chronic liver disease in kidney recipients with hepatitis C virus infection. Clin Transplant. 2003;17:195–199. doi: 10.1034/j.1399-0012.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 149.Perez RM, Ferreira AS, Medina-Pestana JO, Lanzoni VP, Silva AE, Ferraz ML. Is alanine aminotransferase a good marker of histologic hepatic damage in renal transplant patients with hepatitis C virus infection? Clin Transplant. 2005;19:622–625. doi: 10.1111/j.1399-0012.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 150.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;(109):S1–99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 151.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 152.Alric L, Kamar N, Bonnet D, Danjoux M, Abravanel F, Lauwers-Cances V, Rostaing L. Comparison of liver stiffness, fibrotest and liver biopsy for assessment of liver fibrosis in kidney-transplant patients with chronic viral hepatitis. Transpl Int. 2009;22:568–573. doi: 10.1111/j.1432-2277.2009.00834.x. [DOI] [PubMed] [Google Scholar]
- 153.Muñoz R, Ramírez E, Fernandez I, Martin A, Romero M, Romero E, Dominguez-Gil B, Hernandez A, Morales E, Andres A, et al. Correlation between fibroscan, liver biopsy, and clinical liver function in patients with hepatitis C virus infection after renal transplantation. Transplant Proc. 2009;41:2425–2426. doi: 10.1016/j.transproceed.2009.06.103. [DOI] [PubMed] [Google Scholar]
- 154.Harihara Y, Kurooka Y, Yanagisawa T, Kuzuhara K, Otsubo O, Kumada H. Interferon therapy in renal allograft recipients with chronic hepatitis C. Transplant Proc. 1994;26:2075. [PubMed] [Google Scholar]
- 155.Therret E, Pol S, Legendre C, Gagnadoux MF, Cavalcanti R, Kreis H. Low-dose recombinant leukocyte interferon-alpha treatment of hepatitis C viral infection in renal transplant recipients. A pilot study. Transplantation. 1994;58:625–628. doi: 10.1097/00007890-199409150-00018. [DOI] [PubMed] [Google Scholar]
- 156.Magnone M, Holley JL, Shapiro R, Scantlebury V, McCauley J, Jordan M, Vivas C, Starzl T, Johnson JP. Interferon-alpha-induced acute renal allograft rejection. Transplantation. 1995;59:1068–1070. doi: 10.1097/00007890-199504150-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D. Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation. 1995;59:1426–1431. doi: 10.1097/00007890-199505270-00012. [DOI] [PubMed] [Google Scholar]
- 158.Ozgür O, Boyacioğlu S, Telatar H, Haberal M. Recombinant alpha-interferon in renal allograft recipients with chronic hepatitis C. Nephrol Dial Transplant. 1995;10:2104–2106. [PubMed] [Google Scholar]
- 159.Yasumura T, Nakajima H, Hamashima T, Nakai I, Yoshimura N, Ohmori Y, Oka T. Long-term outcome of recombinant INF-alpha treatment of chronic hepatitis C in kidney transplant recipients. Transplant Proc. 1997;29:784–786. doi: 10.1016/s0041-1345(96)00101-7. [DOI] [PubMed] [Google Scholar]
- 160.Durlik M, Gaciong Z, Rowińska D, Rancewicz Z, Lewandowska D, Kozłowska B, Wyzgał J, Soluch L, Walewska-Zielecka B, Rowiński W, et al. Long-term results of treatment of chronic hepatitis B, C and D with interferon-alpha in renal allograft recipients. Transpl Int. 1998;11 Suppl 1:S135–S139. doi: 10.1007/s001470050445. [DOI] [PubMed] [Google Scholar]
- 161.Hanafusa T, Ichikawa Y, Kishikawa H, Kyo M, Fukunishi T, Kokado Y, Okuyama A, Shinji Y, Nagano S. Retrospective study on the impact of hepatitis C virus infection on kidney transplant patients over 20 years. Transplantation. 1998;66:471–476. doi: 10.1097/00007890-199808270-00010. [DOI] [PubMed] [Google Scholar]
- 162.Tokumoto T, Tanabe K, Ishikawa N, Simizu T, Oshima T, Noguchi S, Gouya N, Nakazawa H, Hashimoto E, Fuchinoue S, et al. Effect of interferon-alfa treatment in renal transplant recipients with chronic hepatitis C. Transplant Proc. 1998;30:3270–3272. doi: 10.1016/s0041-1345(98)01024-0. [DOI] [PubMed] [Google Scholar]
- 163.Baid S, Tolkoff-Rubin N, Saidman S, Chung R, Williams WW, Auchincloss H, Colvin RB, Delmonico FL, Cosimi AB, Pascual M. Acute humoral rejection in hepatitis C-infected renal transplant recipients receiving antiviral therapy. Am J Transplant. 2003;3:74–78. doi: 10.1034/j.1600-6143.2003.30113.x. [DOI] [PubMed] [Google Scholar]
- 164.Tang S, Cheng IK, Leung VK, Kuok UI, Tang AW, Wing Ho Y, Neng Lai K, Mao Chan T. Successful treatment of hepatitis C after kidney transplantation with combined interferon alpha-2b and ribavirin. J Hepatol. 2003;39:875–878. doi: 10.1016/s0168-8278(03)00358-1. [DOI] [PubMed] [Google Scholar]
- 165.Shu KH, Lan JL, Wu MJ, Cheng CH, Chen CH, Lee WC, Chang HR, Lian JD. Ultralow-dose alpha-interferon plus ribavirin for the treatment of active hepatitis C in renal transplant recipients. Transplantation. 2004;77:1894–1896. doi: 10.1097/01.tp.0000131151.07818.d7. [DOI] [PubMed] [Google Scholar]
- 166.Izopet J, Rostaing L, Ton-That H, Dubois M, Cazabat M, Charlet JP, Sayada C, Duffaut M, Durand D, Puel J. Kinetics of HCV viremia in kidney transplant recipients during and after alpha-interferon therapy. Am J Nephrol. 1997;17:417–420. doi: 10.1159/000169133. [DOI] [PubMed] [Google Scholar]
- 167.Sharma RK, Bansal SB, Gupta A, Gulati S, Kumar A, Prasad N. Chronic hepatitis C virus infection in renal transplant: treatment and outcome. Clin Transplant. 2006;20:677–683. doi: 10.1111/j.1399-0012.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 168.Pageaux GP, Hilleret MN, Garrigues V, Bismuth M, Audin-Mamlouk H, Zarski JP, Mourad G. Pegylated interferon-alpha-based treatment for chronic hepatitis C in renal transplant recipients: an open pilot study. Transpl Int. 2009;22:562–567. doi: 10.1111/j.1432-2277.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- 169.Aljumah AA, Saeed MA, Al Flaiw AI, Al Traif IH, Al Alwan AM, Al Qurashi SH, Al Ghamdi GA, Al Hejaili FF, Al Balwi MA, Al Sayyari AA. Efficacy and safety of treatment of hepatitis C virus infection in renal transplant recipients. World J Gastroenterol. 2012;18:55–63. doi: 10.3748/wjg.v18.i1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sanai FM, Mousa D, Al-Mdani A, Al-Shoail G, Al-Ashgar H, Al Meshari K, Al-Qahtani A, Saadeh M, Bzeizi KI, Aleid H. Safety and efficacy of peginterferon-α2a plus ribavirin treatment in renal transplant recipients with chronic hepatitis C. J Hepatol. 2013;58:1096–1103. doi: 10.1016/j.jhep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 171.Wei F, Liu J, Liu F, Hu H, Ren H, Hu P. Interferon-based anti-viral therapy for hepatitis C virus infection after renal transplantation: an updated meta-analysis. PLoS One. 2014;9:e90611. doi: 10.1371/journal.pone.0090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Carvalho-Filho RJ, Dalgard O. Individualized treatment of chronic hepatitis C with pegylated interferon and ribavirin. Pharmgenomics Pers Med. 2010;3:1–13. doi: 10.2147/pgpm.s4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sulkowski MS, Cooper C, Hunyady B, Jia J, Ogurtsov P, Peck-Radosavljevic M, Shiffman ML, Yurdaydin C, Dalgard O. Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol. 2011;8:212–223. doi: 10.1038/nrgastro.2011.21. [DOI] [PubMed] [Google Scholar]
- 175.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: A review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 176.Kiser JJ, Burton JR, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10:596–606. doi: 10.1038/nrgastro.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chopra A, Klein PL, Drinnan T, Lee SS. How to optimize HCV therapy in genotype 1 patients: management of side-effects. Liver Int. 2013;33 Suppl 1:30–34. doi: 10.1111/liv.12080. [DOI] [PubMed] [Google Scholar]
- 178.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 181.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 182.Cacoub P, Bourlière M, Lübbe J, Dupin N, Buggisch P, Dusheiko G, Hézode C, Picard O, Pujol R, Segaert S, et al. Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol. 2012;56:455–463. doi: 10.1016/j.jhep.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 183.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 184.Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 185.Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 186.Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, Horban A, Brown A, Peeters M, Lenz O, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669–79.e3. doi: 10.1053/j.gastro.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 187.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 188.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 189.Cornpropst MT, Denning JM, Clemons D, Marbury TC, Alcorn H, Smith WB, Sale M, Fang L, Berrey MM, Symonds WT. The effect of renal impairment and end stage renal disease on the single-dose pharmacokinetics of PSI-7977. J Hepatol. 2012;56(Suppl.2):S443. [Google Scholar]
- 190.Cornpropst MT . AbbVie. Ombitasvir/ABT-450/Ritonavir and Dasabuvir With or Without Ribavirin in Treatment- Naïve HCV Genotype 1-Infected Adults With Chronic Kidney Disease. In: ClinicalTrials , editor. gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. p. [cited 2014 Aug 20], NLM Identifier: NCT02207088. [Google Scholar]


