Abstract
AIM: To determine whether the expression profiles of EphB receptor and ephrin-B ligand can be used as markers for dysplastic/oncogenic transformation in gastric mucosa.
METHODS: The protein expression and localization of EphB and ephrin-B in normal, ulcerated regenerating, and dysplastic gastric mucosa were examined in a rat experimental model by immunolabeling, and mRNA expression was assessed in four human gastric carcinoma cell lines by reverse transcription-polymerase chain reaction.
RESULTS: Ephrin-B- and EphB-expressing regions were divided along the pit-gland axis in normal gastric units. EphB2 was transiently upregulated in the experimental ulcer, and its expression domain extended to gastric pits and/or the luminal surface where ephrin-B-expressing pit cells reside. EphB2, B3, and B4 and ephrin-B1 were coexpressed in the experimental gastric dysplasia, and more than one ligand-receptor pair was highly expressed in each of the gastric carcinoma cell lines.
CONCLUSION: Robust and stable coexpression of EphB and ephrin-B is a feature common to experimentally induced gastric dysplasia and human gastric carcinoma cell lines as compared to normal gastric and ulcerated regenerating epithelia. Thus, EphB/ephrin-B may be a useful marker combination for dysplastic/oncogenic transformation in gastric cancer.
Keywords: Gastric ulcer, Gastric dysplasia, Gastric carcinoma cell line, Coexpression, EphB, Ephrin-B
Core tip: A constant/high level of EphB and ephrin-B coexpression was identified as a feature common to experimentally induced gastric dysplasia and human gastric carcinoma cell lines, as compared to normal and regenerating gastric epithelia. Based on these, we proposed that the stable/robust EphB and ephrin-B coexpression is a marker of dysplastic/oncogenic transformation. Eph signaling in tumor cells likely has a suppressive role during tumor progression, with Eph and ephrin coexpressed on the same cell engaging in non-productive interactions via lateral inhibition and thereby silencing downstream signaling. These results can be useful for the early and accurate diagnosis of gastric tumors.
INTRODUCTION
The large Eph receptor tyrosine kinase superfamily has 14 members in mammals that are divided into EphA (A1-A8 and A10) and EphB (B1-B4 and B6; EphB5 has only been detected in the chicken) classes on the basis of sequence homology of the extracellular domain[1,2]. Members of these two receptor classes promiscuously bind ligands of the ephrin-A (A1-A5) and -B (B1-B3) classes, respectively. Ephrin-A members are anchored to the plasma membrane through a glycosyl phosphatidylinositol linkage, while ephrin-B is a class of transmembrane proteins. The Eph/ephrin interaction results in bidirectional signal propagation in both receptor- and ligand-expressing cells. Forward signaling by Eph depends mainly on autophosphorylation by the tyrosine kinase domain and association of the receptor with various effector proteins, while reverse signaling by ephrin depends in part on tyrosine phosphorylation of the cytoplasmic region of ephrin-Bs and associated proteins[1,2]. This cell-cell communication is essential to the development and physiology of various tissues and organs, especially in the nervous and vascular systems[1,3]. Accumulating evidence also implicates Eph/ephrin signaling in tumor development and progression: overexpression, reduced expression, and mutations in the receptor and/or ligand affect tumor cell growth, migration, invasion, and metastasis in vitro and in vivo[4,5].
The stomach is lined with a simple columnar epithelium on the luminal surface that forms deep tubular invaginations termed gastric glands. These are connected to gastric pits, which are wide tubular depressions on the luminal surface of the mucosa. The isthmus lies between the pits and glands, with the three comprising a gastric unit. Gastric stem cells located in the isthmus proliferate and differentiate to give rise to pit and gastric gland cells; the former migrate apically towards the gastric lumen, whereas the latter migrate basally towards the neck and then to the base of the glands[6-8]. We previously reported complementary expression patterns for EphB and ephrin-B members in rodent gastric mucosa, with receptors and ligands being preferentially expressed in deeper and superficial regions, respectively, of gastric units[9-11]. This finding indicates that EphB/ephrin-B signaling is mostly restricted to the isthmus, where the overlap between receptor and ligand expression domains is highest. We also showed that EphB signaling in primary gastric epithelial cells promoted cell retraction, and proposed that the EphB-positive progeny of gastric stem cells migrates from the isthmus to the bottom of the gastric glands via contact-mediated repulsion[11].
Up- or downregulation in expression and mutations in genes of Eph receptors or ephrin ligands have been reported in human gastric tumors[12-25], and EphA overexpression in tumors is correlated with cancer progression, metastasis, and/or poor prognosis[16,18,21-23,25]. Less attention has been given to EphB in gastric tumors. A few studies have shown altered or absent expression of certain receptors in tumor samples relative to adjacent normal tissue, with reduction/loss in expression correlated with gastric cancer progression, metastasis, and poor prognosis[20,24]. In colorectal tumors, the expression of EphB receptors is high during early stages of tumor progression, and downregulated at the adenoma-carcinoma transition[26,27].
Detailed comparisons of the expression profiles of Eph receptors and their ephrin ligands between normal, ulcerated regenerating, and dysplastic gastric epithelia and gastric tumors are lacking. In the present study, expression profiles of EphB/ephrin-B in gastric epithelia were assessed in normal and experimentally induced ulcerated and dysplastic tissues, as well as human gastric carcinoma cell lines, to determine whether EphB/ephrin-B expression can serve as a marker for dysplastic/oncogenic transformation in gastric mucosa.
MATERIALS AND METHODS
Animals
F344 male rats (Japan SLC, Inc., Hamamatsu, Japan) were maintained under standard housing and feeding conditions. Tissue samples from normal and gastric ulcer model rats (8-10 wk old) were used for reverse transcription-polymerase chain reaction (RT-PCR) and immuno- and lectin fluorescence labeling experiments, while immunoperoxidase staining was carried out using samples from gastric dysplasia model rats (8-9 mo old). Rats were anesthetized with pentobarbital and the stomach tissue was transcardially perfused with Ca2+/Mg2+-free Hanks’ balanced salt solution and dissected. Animal protocols were approved by the Animal Research Committee of Osaka Prefecture University.
Experimental induction of gastric ulcers
Gastric ulcers were induced with acidified ethanol solution using an established method[28] with minor modifications. Rats weighing 190-210 g were fasted for 24 h but allowed free access to drinking water; 1 mL acidified ethanol solution (80% ethanol in 0.15 mol/L HCl) was orally administrated to each animal using a disposable feeding needle (Fuchigami Ltd., Muko, Japan) on day 0. Stomach tissue samples were processed for histological examination by staining with hematoxylin and eosin (H-E) on days 1, 3, 7, 10, and 14. A 100% survival rate was observed among rats that developed gastric ulcers, and epithelial regeneration was observed by day 3 in stained specimens. Ulcers and regenerating regions were randomly distributed on the mucosal surface of the stomach, which were easily identifiable by naked eye until at least day 7 as small, pale, or petechial/ecchymotic hemorrhagic puncta. Regenerating regions could not be clearly distinguished after day 10 by naked eye; thus, day 7 was selected for histochemical and RT-PCR analyses of regenerating gastric epithelium.
Experimental induction of gastric dysplasia
Dysplasia of gastric glandular epithelia was induced by a previously described method[29] with minor modifications. Briefly, rats weighing 190-210 g were fasted for 24 h but allowed free access to drinking water. A single dose (250 mg/kg) of N-methyl-N’-nitro-N-nitrosoguanidine (MNNG; Kanto Chemical Co., Tokyo, Japan) prepared at 50 mg/mL in 75% dimethylsulfoxide (Sigma-Aldrich, St. Louis, MO, United States) was orally administrated to rats using a disposable feeding needle; 6-7 mo later, rats were sacrificed and their stomachs dissected and fixed. Concave/convex regions on the mucosal surface were cut with a razor blade and processed for histochemistry.
Gastric cancer cell lines and cell culture
The human gastric carcinoma cell lines AZ-521, Kato-III, MKN-7, and SH-10-TC were obtained from the Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer (Tohoku University, Sendai, Japan). AZ-521 cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) containing 10% fetal bovine serum (FBS; Cell Culture Bioscience, Nichirei Biosciences Inc., Tokyo, Japan) and 100 IU/mL penicillin and 100 μg/mL streptomycin (pen/strep; Sigma-Aldrich). Kato-III, MKN-7, and SH-10-TC cells were cultured in Roswell Park Memorial Institute-1640 medium (Sigma-Aldrich) containing 10% FBS and pen/strep. Cells were maintained in a humidified 5% CO2/95% air incubator at 37 °C and used in RT-PCR experiments.
Antibodies
Goat polyclonal antibodies against the extracellular domains of mouse EphB2 (AF467), EphB3 (AF432), EphB4 (AF446), and ephrin-B1 (AF473) were obtained from R&D Systems, Inc. (Minneapolis, MN, United States). A rabbit polyclonal antibody against the ephrin-B carboxy terminus (anti-ephrin-B1/B2/B3, C-18) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). This antibody was used to detect the combined expression of ephrin-B ligands. The rabbit anti-human Ki67 monoclonal antibody (SP6) was from NeoMarkers, Inc. (Fremont, CA, United States). Biotinylated rabbit anti-goat and goat anti-rabbit IgG were from Vector Laboratories, Inc. (Burlingame, CA, United States), and Alexa Fluor 488-conjugated donkey anti-goat IgG was from Molecular Probes, Inc. (Eugene, OR, United States).
RT-PCR
Total RNA was isolated from normal gastric corpus mucosa and regenerating mucosa on day 7 after ulcer induction, as well as human gastric carcinoma cell lines, using Trizol reagent (Invitrogen Japan K.K., Tokyo, Japan). RT-PCR was performed as previously described[30]. Briefly, 1 μg total RNA was transcribed into first-strand cDNA by using M-MLV reverse transcriptase, RNase H- (Promega, Madison, WI, United States) and oligo(dT)18 primer, according to the manufacturer’s instructions. For the detection of endogenous EphB receptors and ephrin-B ligands, 1 μL of the reaction mix (from a total of 25 μL) was amplified over 36 cycles with cDNA as the template. The RT reaction was omitted for the negative control sample. Expression levels of amplified rat EphB1-B4 and B6, and ephrin-B1 and -B2 mRNA were determined from three independent experiments and normalized to the levels of β-actin mRNA as an internal control (amplified over 23 cycles). Expression levels of human GAPDH mRNA (amplified over 23 cycles) were used as an internal control for those of amplified human EphB1-B4, B6, and ephrin-B1 and -B2 mRNA in the samples of human gastric carcinoma cell lines. Primer sequences used to amplify rat EphBs and ephrin-Bs were as previously described[11], and forward and reverse primers for rat β-actin and human EphBs, ephrin-Bs and GAPDH were as follows: β-actin, 5’-GGC ATC CTG ACC CTG AAG TA-3’ and 5’-TCT CAG CTG TGG TGG TGA AG-3’; EphB1, 5’-AAT GGC ATC ATC CTG GAC TA-3’ and 5’-TCA ATC TCC TTG GCA AAC TC-3’; EphB2, 5’-CAA TGC GGA AGA GGT GGA TG-3’ and 5’-GGA TCT CGA AGG TGT ACT GG-3’; EphB3, 5’-GTG AGT GGC TAC GAT GAG G-3’ and 5’-GGA GAT GAG CGA CAT GCA G-3’; EphB4, 5’-GCA GTT CTC TGC CTC AGG A-3’ and 5’-GCT CGA ACT GGC CCA TGA T-3’; EphB6, 5’-CTG AGA GCC GAG TGT TAG TGG-3’ and 5’-AGC TCC CCT TGA GGA AGT GTC-3’; ephrin-B1, 5’-TCA ACC CCA AGT TCC TGA GTG-3’ and 5’-GCG TAG CTT CAG TAG TAG GAC-3’; ephrin-B2, 5’-ACC CAC AGA TAG GAG ACA AA-3’ and 5’-GGT TGA TCC AGC AGA ACT TG-3’; ephrin-B3, 5’-CCT AAC CAG AGG CAT GAA GG-3’ and 5’-TCT CAT AGT GGG GGC AGA AG-3’; and GAPDH, 5’-GTC GGA GTC AAC GGA TTT GG-3’ and 5’-GGA TGA TGT TCT GGA GAG CC-3’.
Immunolabeling and lectin fluorescence staining
The stomachs of normal rats, and those with experimentally induced ulcers or dysplasia were cut into 3- to 5-mm-thick pieces that were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 4 h at 4 °C. After washing with PBS, the pieces were immersed in 30% sucrose in PBS overnight, embedded in Optimal Cutting Temperature compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan), and sectioned at a thickness of 6-7 μm on a cryostat. Sections were stained by the immunoperoxidase method[30]. Briefly, sections were immersed in 0.3% hydrogen peroxide for 30 min, preincubated with 3% normal rabbit or goat serum in PBS, then incubated with primary antibodies against EphB2, B3, or B4, ephrin-B1 or ephrin-B1/B2/B3 (all at 1 μg/mL) or Ki67 (1:200) overnight at 4 °C. Avidin and biotin (Avidin/Biotin Blocking Kit; Vector Laboratories, Inc., Burlingame, CA, United States) were added to the blocking and primary antibody solutions, respectively, to block endogenous binding sites. Sections were incubated with biotinylated rabbit anti-goat or goat anti-rabbit IgG, followed by treatment with an avidin-biotin peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories, Inc.), and they were developed by immersion in 3,3’-diaminobenzidine substrate (KPL, Gaithersburg, MD, United States). The specificity of the staining was verified by incubation without primary or secondary antibodies. Some immunoperoxidase staining sections were counterstained with haematoxylin. A subset of sections was stained with H-E.
Fluorescein-conjugated Griffonia simplicifolia (GS)-II lectin and rhodamine-conjugated Ulex europaeus agglutinin (UEA)-I lectin (both from Vector Laboratories, Inc.) were used to identify mucous neck and pit cells (i.e., surface mucous cells), respectively[11]. Fluorescence co-labeling of lectins and EphB2 was carried out as previously described[9]. Cryostat sections were preincubated in a humid chamber with 1% bovine serum albumin in PBS, followed by incubation with the primary antibody against EphB2 (2 μg/mL) for 1 h at 32 °C. After washing with PBS, sections were incubated with a mixture of Alexa Fluor 488-conjugated donkey anti-goat IgG and UEA-I (1 μg/mL) for 30 min at 32 °C, and following PBS washes, mounted with Permafluor (Immunotech, Marseille, France). A subset of sections was double-labeled with GS-II (1 μg/mL) and UEA-I for 30 min at 32 °C. Sections were imaged with a fluorescence microscope (IX71; Olympus, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed with StatView software (SAS Institute Inc., Cary, NC, United States). Histograms show mean ± SD. An unpaired t-test was used to compare means and a P value < 0.05 was considered statistically significant.
RESULTS
Expression and localization of EphB and ephrin-B in normal gastric mucosa
Tissue samples of gastric corpus mucosa in normal adult rats were screened by RT-PCR to identify EphB receptors and ephrin-B ligands expressed in the adult stomach. With the exception of ephrin-B3, transcripts for all mammalian EphB and ephrin-B molecules were detected (Figure 1A), with the most prominent expression observed for EphB2-B4, and ephrin-B1 and -B2.
Figure 1.
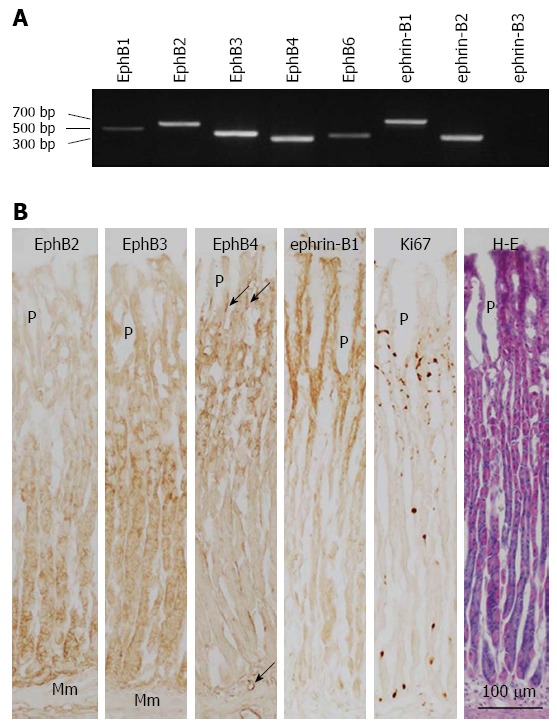
Expression of B-class Eph receptors and ephrin ligands in normal rat gastric corpus mucosa. A: EphB and ephrin-B mRNA expression was determined by reverse transcription-polymerase chain reaction; B: Expression of EphB2-B4, ephrin-B1, and Ki67 in the gastric corpus mucosa was evaluated by immunoperoxidase and hematoxylin and eosin co-staining of frozen sections. Mm: Muscularis mucosae; P: Gastric pit; arrow: Blood vessel.
Immunoperoxidase staining was performed to determine the localization of the most abundant receptors and ligands. The isthmus, where proliferating cells are localized, was identified by Ki67 immunoreactivity. EphB receptors and ephrin B ligands were expressed in the plasma membrane of cells. EphB2 was expressed in gland cells, strongly in the base region where chief cells are located and weakly in the lower portion of the neck (Figure 1B). EphB3 expression was stronger in the neck of gastric glands and weaker in the isthmus and base region. EphB4 was expressed in the isthmus and neck of gastric glands and in blood vessels of the lamina propria mucosae. In contrast, ephrin-B1 immunoreactivity was observed in pit cells lining the lumen, gastric pits, and in cells located in the isthmus (Figure 1B). A similar labeling pattern was seen using the ephrin-B1/B2/B3 antibody, which recognizes all three ephrin-B ligands (data not shown). Thus, in gastric units, pit cells and proliferating isthmus cells express ephrin-B. In summary, cells located in the epithelium overlying the lumen, gastric pits, and isthmus expressed ephrin-B ligands, whereas cells in the neck and based of gastric glands and isthmus expressed EphB receptors; thus, receptors and ligands are differentially expressed within gastric units but are coexpressed in the isthmus, where undifferentiated gastric stem cells and transit-amplifying cells reside.
Expression and localization of EphB and ephrin-B in regenerating gastric mucosa
Expression profiles of EphB and ephrin-B were examined by RT-PCR and immunolabeling in an experimental ulcer model to determine whether complementary receptor/ligand expression patterns are maintained in an actively regenerating gastric epithelium. Gastric corpus mucosa underwent a typical regeneration process 3-14 d after induction of gastric ulcers, as observed in H-E-stained sections. On day 3, epithelial cells covered the luminal surface of the gastric mucosa (Figure 2); on day 7, gastric units composed of incomplete and short gastric glands, with gland cells strongly stained with eosin, were formed in the regenerating mucosa with thick stromal tissues. On day 14, the regeneration of gastric mucosa was almost complete and the regenerated tissue could not be histologically distinguished from normal tissue except for the presence of a relatively abundant stroma in the former. Based on histological assessment of the regeneration process, the gastric mucosa on day 7 was deemed suitable for analysis of EphB and ephrin-B expression because at this time point there were clear signs of active epithelial regeneration.
Figure 2.
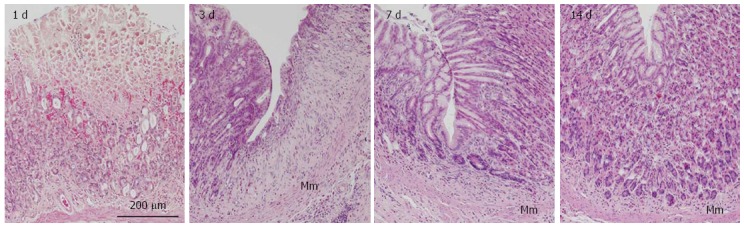
Representative images of rat gastric corpus mucosa showing typical regeneration 1, 3, 7, and 14 d after induction of gastric ulcers by oral administration of acidified ethanol solution (80% ethanol in 0.15 mol/L HCl). Tissue sections were stained with hematoxylin and eosin. Mm: Muscularis mucosae.
The mRNA expression of all EphB receptors and ephrin-B ligands was assessed with the exception of ephrin-B3, for which no expression was observed in either the control or ulcerated gastric corpus mucosa on day 7 by RT-PCR. The expression levels of all molecules were similar to those of the control at this stage of regeneration except for EphB2, which was upregulated by 2.1-fold (P = 0.016; Figure 3).
Figure 3.

Densitometric quantification of EphB1-B4 and B6, and ephrin-B1 and -B2 mRNA expression levels in the gastric corpus mucosa of control (Con) and ulcerated regenerating stomach on day 7 after induction of gastric ulcers (Ulc), as determined by reverse transcription-polymerase chain reaction. EphB2 expression in the regenerating gastric mucosa was higher than in the control (P = 0.016; unpaired t-test).
Immunoperoxidase staining, and immuno- and lectin fluorescence labeling of serial sections were used to determine the localization and changes in expression of the most abundant EphB receptors and ephrin-B ligands in the regenerating gastric corpus mucosa on day 7. Ki67-positive proliferating cells were abundant in the regenerating gastric mucosa, i.e., regenerating gastric units from the base of the gastric pits to the bottom of the gastric glands, as well as in stromal cells (Figures 4 and 5). In regions adjacent to the regenerating gastric mucosa where gastric units elongated to a length similar to the control, Ki67-positive cells were mainly restricted to the isthmus. UAE-I-positive cells formed gastric pits and covered the luminal surface, whereas by lectin fluorescence labeling, GS-II-positive cells were detected throughout the gastric glands in regenerating gastric units (Figure 5). Thus, at this time point, Ki67-positive mucous neck cells had emerged to form the entire length of regenerating gastric glands, which were still extremely thin and lacked a base and part of the neck. EphB2 and B3 were localized in the plasma membrane of gland cells. EphB2 was not detected in control, but was upregulated in gastric units of regenerating areas on day 7 (Figure 4) and detected not only in gastric glands but also in the isthmus and gastric pits occupied by Ki67-positive cells (Figure 5). EphB3 was also upregulated in gastric units of regenerating areas in the isthmus and gastric glands, in contrast to its localization in the control mucosa (Figure 4). The EphB4 expression pattern was similar in regenerating and control mucosa, and was mainly seen in the isthmus and gastric glands of regenerating gastric unit as well as in blood vessels. Ephrin-B1 immunoreactivity was similar in regenerating and control mucosa, and was seen mainly in pit cells (Figure 4). In the regenerated gastric mucosa on day 14, localization of the EphB receptors and ephrin-B1 were the same as that in control rats (data not shown). Thus, the ephrin-B1-expressing region temporarily overlaps with the upper part of the region that highly expresses EphB2 in regenerating gastric units.
Figure 4.
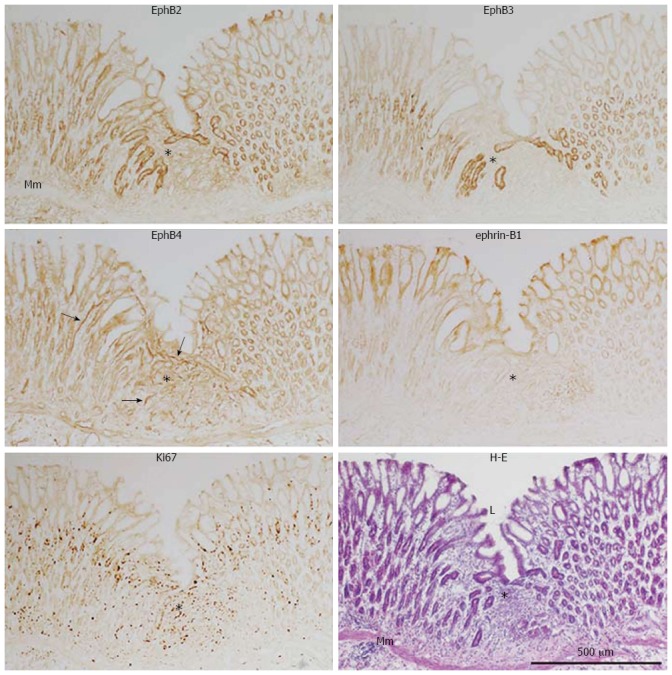
Immunoperoxidase staining of EphB2, B3, and B4, ephrin-B1, and Ki67, with hematoxylin and eosin staining, of frozen sections of the regenerating gastric corpus mucosa 7 d after experimental induction of gastric ulcers (see Figure 2 legend). Mm: Muscularis mucosae; L: Gastric lumen; Arrow: Blood vessel; Asterisk: Regenerating region.
Figure 5.
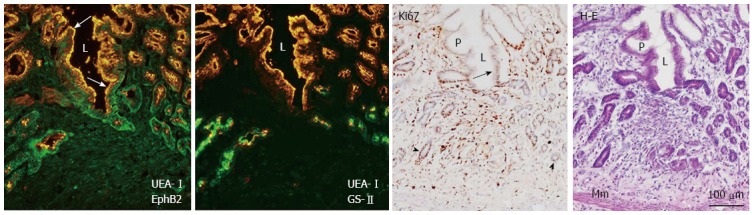
Double immunofluorescence labeling of EphB2 (green) and the pit cell marker Ulex europaeus agglutinin (UEA)-I (orange), and the neck cell marker Griffonia simplicifolia-II (green) and UEA-I, with Ki67 immunoperoxidase and hematoxylin and eosin co-staining in frozen sections of the regenerating gastric corpus mucosa 7 d after induction of gastric ulcers (see Figure 2 legend). L: Gastric lumen; Mm: Muscularis mucosae; P: Gastric pit; Black arrow: Ki67-positive pit cells; Arrowhead: Ki67-positive gland cells; White arrow: UEA-I-positive pit cells.
EphB and ephrin-B localization in the gastric epithelial dysplasia
The expression of EphB and ephrin-B in aberrantly proliferating gastric epithelium was examined by immunoperoxidase staining of experimentally induced gastric dysplasia. A single sublethal dose of MNNG induced dysplastic lesions in the rat gastric mucosa several months later, where hemorrhage was histologically identified and numerous Ki67-positive cells were present, comprising multiple layers in dysplastic epithelia (Figure 6). EphB receptors (EphB2-B4) and ephrin-B1 were highly expressed in the plasma membrane of gastric cells in Ki67-positive and -negative regions (Figure 6); in the former, ephrin-B1 was also detected in the cytoplasm of these cells. EphB4 was also highly expressed in blood vessels. These results demonstrate an overlap of regions expressing high levels of EphB and ephrin-B in gastric dysplasia, suggesting that dysplastic cells coexpress both receptors and ligand. Expression patterns of EphB receptors, ephrin-B1 ligand, and Ki67-positive cells in normal, ulcerated regenerating, and dysplastic gastric epithelia are summarized in Figure 7.
Figure 6.
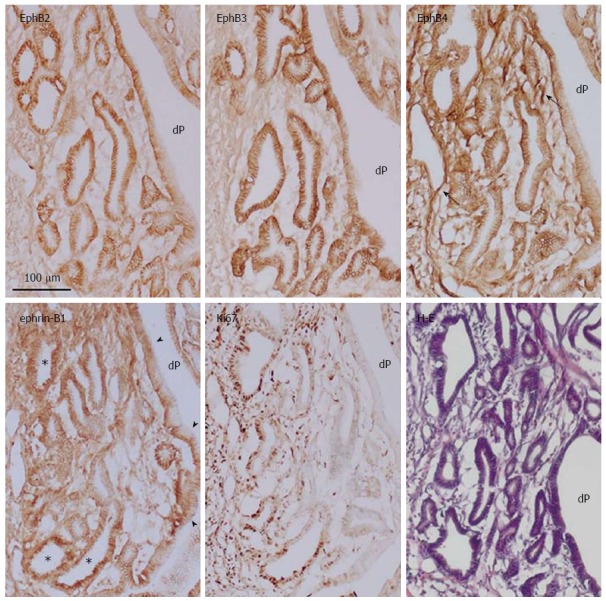
Immunoperoxidase staining of EphB2-B4, ephrin-B1, and Ki67, and hematoxylin and eosin staining of frozen sections of gastric dysplasia in rat induced by oral administration of N-methyl-N′-nitro-N-nitrosoguanidine. dP: Deep and wide aberrant gastric pit; Arrow: Blood vessel; Arrowhead: Membrane localization of ephrin-B1; Asterisk: Cytoplasmic localization of ephrin-B1.
Figure 7.
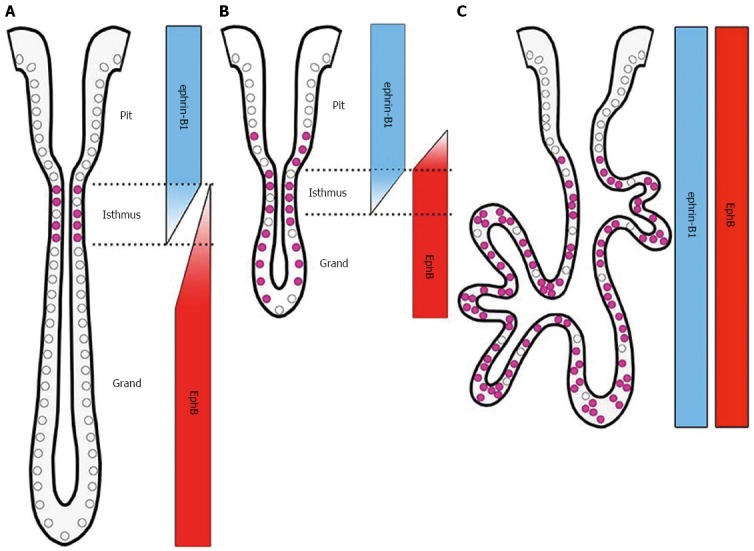
Schematic representation of EphB receptor, ephrin-B1 ligand, and Ki67 expression in normal (A), ulcerated regenerating (B), and dysplastic gastric epithelia (C).
EphB and ephrin-B expression in gastric cancer cell lines
EphB and ephrin-B mRNA expression was examined by RT-PCR in four human gastric carcinoma cell lines. EphB1 and ephrin-B2 were prominently expressed, and EphB2 and B3, and ephrin-B1 were weakly expressed, in AZ-521 cells. In KATO-III cells, EphB2, and ephrin-B1 and -B2 transcript levels were high, while EphB1, B3, and B4 mRNA was detected at low levels. MKN-7 cells highly expressed EphB2 and B3 and ephrin-B2, while weak expression of EphB1 and B6 was also seen. High levels of EphB1, B2, B3, and B6 and ephrin-B2, and relatively low levels of EphB4 and ephrin-B1 were observed in SH-10-TC cells (Figure 8). These results suggest that unlike in normal gastric epithelium, ephrin-B3 is not expressed in gastric carcinomas; and gastric carcinoma cells coexpress more than one EphB receptor and ephrin-B ligand.
Figure 8.
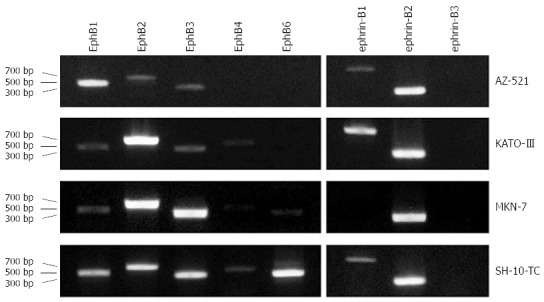
EphB and ephrin-B mRNA expression in human gastric carcinoma cell lines. Transcript levels in AZ-521, KATO-III, MKN-7, and SH-10-TC cells were determined by reverse transcription-polymerase chain reaction. All cell lines coexpressed more than one EphB receptor and/or ephrin-B ligand at a high level.
DISCUSSION
Eph receptor tyrosine kinases and their ephrin ligands function in cell-cell communication, with widespread roles in the development and physiology of various tissues and organs[1,3]. The present study examined whether EphB and ephrin-B expression profiles can serve as an indicator of dysplastic/oncogenic transformation in gastric mucosa. This is the first comprehensive analysis of the expression profiles of B-class Eph receptors and ephrin ligands in normal, ulcerated regenerating, and dysplastic gastric mucosa in a rodent model and human gastric carcinoma cell lines. The ephrin-B- and EphB-expressing regions - in the upper (luminal surface and gastric pits) and lower (gastric gland) compartments, respectively - are divided along the pit-gland axis in normal gastric units, consistent with our previous findings in the mouse[9-11]. We also found that the time-dependent upregulation of EphB2 and B3 expression, as evidenced by immunohistochemical signal intensity, were similar in regenerating and normal, adjacent gastric units. Notably, the region highly expressing EphB2 extended up to the gastric pits and/or the luminal surface where UEA-I-/Ki67-positive pit cells reside. This suggests that proliferating cells in the isthmus and gastric pits temporarily upregulate EphB2 expression during the normal regeneration process. Because there was no alteration in ephrin-B1 signal intensity in these cells, EphB2 and ephrin-B1 are likely coexpressed at constant, high levels in gastric units at this time. Moreover, EphB receptors (EphB2-B4) and ephrin-B1 were upregulated and ubiquitously distributed throughout gastric cells in the dysplastic mucosa, where the Ki67-positive cell region extended down to the bottom of epithelial invaginations and dysplastic cells did not form clear gastric units. This indicates that EphB receptors and ephrin-B1 are coexpressed at high levels in aberrantly proliferating cells in the gastric dysplasia. Based on these findings, it was hypothesized that the emergence of proliferating cells that upregulate expression of both EphB receptors and ephrin-B ligands is an indication of dysplastic/oncogenic transformation. The observed patterns of receptor and ligand mRNA expression in four human gastric carcinoma cell lines provided evidence in support of this hypothesis. Thus, the coexpression of EphB receptors and ephrin-B ligands is also a likely feature of gastric carcinoma. We previously detected the coexpression of certain Eph receptors and ephrin ligands in human tumor samples[31], which suggested that their coexpression in tumors may not be atypical. A comprehensive analysis of receptor-ligand coexpression profiles in human gastric carcinoma samples is required to determine whether this is indeed the case.
Canonical Eph forward signaling has tumor suppressor function, based on studies in which forced Eph activation by soluble and dimerized ephrins inhibited proliferation, survival, migration, and invasion of various types of tumor cell in culture and tumor progression in several mouse models[5]. Certain Eph receptors and ephrin ligands are expressed in vascular endothelial cells in human tumor samples[3,31] and are presumed to promote tumor angiogenesis and thus its progression[5], while dysregulation of Eph/ephrin signaling affects tumor progression and metastasis in vitro and in vivo[4,5,19]. In the present study, the coexpression of more than one receptor and/or ligand was observed in dysplastic gastric cells and gastric carcinoma cell lines. This can lead to ineffectual lateral interactions in tumor cells by mutual inhibition in intercellular interactions[2,5,32]. It is well known that EphB receptors may be overexpressed during early stages of tumor progression and subsequently downregulated at the adenoma-carcinoma transition in colorectal tumors[26]. In mouse colorectal tumor models, the expansion and invasiveness of the initial tumor cells expressing EphB receptors at high levels were inhibited by contact with ephrin-B-expressing normal cells enclosing the tumor compartment: the forward signaling caused cell-cell repulsion between tumorigenic and normal cells, as well as adhesion between tumor cells[26,27].
Less attention has been given to the EphB receptors in gastric tumors. The overexpression or reduced and/or loss of EphB1 and B2 expression has been reported in human gastric tumor samples relative to adjacent normal tissue, which has been correlated with gastric cancer progression, metastasis, and poor prognosis[20,24]. Thus, as in the case of colorectal carcinoma, early upregulation of EphB receptor expression followed by a downregulation at the adenoma-carcinoma transition likely occurs in gastrointestinal tumors. However, EphB-expressing cells located in the basal region of dysplastic gastric mucosae were not surrounded by cells expressing normal levels of ephrin-B, unlike the localization of receptors and ligands in the early stage of colorectal tumors. Moreover, EphB and ephrin-B coexpression was observed in all gastric carcinoma cell lines examined in the present study, implying that EphB receptors may not be acting as tumor suppressors through lateral inhibition. Nonetheless, the coexpression of EphB/ephrin-B may be a useful marker for gastric tumor progression; further investigation will be required to determine whether, for instance, tyrosine phosphorylation status in these proteins is correlated with expression profiles and tumor progression.
Xenograft studies have shown that ephrin-B1 signaling can promote gastric tumor cell invasion[33,34], and is therefore likely implicated in tumor progression. The present investigation detected cells strongly coexpressing EphB receptors and ephrin-B1 in dysplastic Ki67-positive and adjacent Ki67-negative regions; ephrin-B1 was also frequently localized in the cytoplasm of proliferating cells. Eph-ephrin complexes and surrounding plasma membrane are internalized via endocytosis in cells expressing either the receptor or ligand. This is the terminal event in the Eph-ephrin interaction, after which Eph forward and/or ephrin reverse signaling drives cell-cell repulsion[2,3], thereby blocking intercellular communication via the loss of cell-cell adhesion. Thus, the cytoplasmic localization of ephirn-B1 in dysplastic proliferating cells, but not in normal cells or regenerating gastric cells, could mark the progression of gastric tumors.
In conclusion, a constant and high level of EphB and ephrin-B coexpression was identified as a feature common to experimentally induced gastric dysplasia and human gastric carcinoma cell lines, as compared to normal gastric and ulcerated regenerating epithelia. Based on these findings, it is proposed that the stable and robust upregulation of coexpressed EphB and ephrin-B is a marker of dysplastic/oncogenic transformation. Eph forward signaling in tumor cells likely has a suppressive role during tumor progression, with Eph and ephrin coexpressed on the same cell engages in non-productive interactions via lateral inhibition and thereby silencing downstream signaling. These results can be useful for the early and accurate diagnosis of gastric tumors for the timely initiation of therapeutic interventions.
COMMENTS
Background
Up- or down-regulation in expression and mutations in genes of Eph receptors or ephrin ligands have been reported in human gastric tumors, and EphA overexpression in tumors is correlated with cancer progression, metastasis, and/or poor prognosis. Less attention has been given to EphB in gastric tumors while in colorectal tumors the expression of EphB receptors is high during early stages of tumor progression and downregulated at the adenoma-carcinoma transition.
Research frontiers
Canonical Eph forward signaling has tumor suppressor function, based on studies in which forced Eph activation inhibited proliferation, survival, migration, and invasion of various types of tumor cell in culture and tumor progression in several mouse models. Certain Eph receptors and ephrin ligands are expressed in vascular endothelial cells in human tumor samples and are presumed to promote tumor angiogenesis and thus its progression, while dysregulation of Eph/ephrin signaling affects tumor progression and metastasis in vitro and in vivo.
Innovations and breakthroughs
To determine whether EphB and ephrin-B expression profiles can be used as markers for dysplastic/oncogenic transformation in gastric mucosa, the protein expression and localization of EphB and ephrin-B in normal, ulcerated regenerating, and dysplastic gastric mucosa were examined in a rat experimental model by immunolabeling, and mRNA expression was assessed in four human gastric carcinoma cell lines by reverse transcription-polymerase chain reaction (RT-PCR).
Applications
Robust and stable coexpression of EphB and ephrin-B was a feature common to experimentally induced gastric dysplasia and human gastric carcinoma cell lines as compared to normal gastric and ulcerated regenerating epithelia. Thus, EphB/ephrin-B could be a useful marker combination for dysplastic/oncogenic transformation in gastric cancer.
Terminology
Eph receptors and ephrin ligands: The Eph receptor tyrosine kinases have 14 members, EphA (A1-A8 and A10) and EphB (B1-B4 and B6), in mammals. EphAs and EphBs promiscuously bind ephrin-A (A1-A5) and ephrin-B (B1-B3) ligands, respectively. Ephrin-As are glycosylphosphatidylinositol-anchored membrane proteins while ephrin-Bs are transmembrane proteins. The Eph/ephrin interaction results in bidirectional signal propagation in both receptor- and ligand-expressing cells. Eph forward signaling depends mainly on autophosphorylation and association of the receptor with various effector proteins, while ephrin reverse signaling in part on tyrosine phosphorylation of the cytoplasmic region of ephrin-Bs and associated proteins.
Peer review
This paper examined the expression profiles of EphB and ephrin-B in normal, ulcerated regenerating, and dysplastic gastric mucosa in a rat experimental model as well as human gastric carcinoma cell lines by immunolabeling and/or RT-PCR. The authors found a constant/high level of EphB and ephrin-B coexpression identified as a feature common to experimentally induced gastric dysplasia and human gastric carcinoma cell lines, as compared to normal gastric and ulcerated regenerating epithelia. These results may be useful for the early and accurate diagnosis of gastric tumors.
Footnotes
Supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science, No. 21580367 (to Ogawa K).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 28, 2014
First decision: May 29, 2014
Article in press: July 22, 2014
P- Reviewer: Gao LB, Krieg A S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 2.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 3.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Merlos-Suárez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008;20:194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 7.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–296. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 8.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 9.Ishii M, Nakajima T, Ogawa K. Complementary expression of EphB receptors and ephrin-B ligand in the pyloric and duodenal epithelium of adult mice. Histochem Cell Biol. 2011;136:345–356. doi: 10.1007/s00418-011-0849-4. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa K, Saeki N, Igura Y, Hayashi Y. Complementary expression and repulsive signaling suggest that EphB2 and ephrin-B1 are possibly involved in epithelial boundary formation at the squamocolumnar junction in the rodent stomach. Histochem Cell Biol. 2013;140:659–675. doi: 10.1007/s00418-013-1129-2. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa K, Takemoto N, Ishii M, Pasquale EB, Nakajima T. Complementary expression and repulsive signaling suggest that EphB receptors and ephrin-B ligands control cell positioning in the gastric epithelium. Histochem Cell Biol. 2011;136:617–636. doi: 10.1007/s00418-011-0867-2. [DOI] [PubMed] [Google Scholar]
- 12.Davalos V, Dopeso H, Velho S, Ferreira AM, Cirnes L, Díaz-Chico N, Bilbao C, Ramírez R, Rodríguez G, Falcón O, et al. High EPHB2 mutation rate in gastric but not endometrial tumors with microsatellite instability. Oncogene. 2007;26:308–311. doi: 10.1038/sj.onc.1209780. [DOI] [PubMed] [Google Scholar]
- 13.Iwase T, Tanaka M, Suzuki M, Naito Y, Sugimura H, Kino I. Identification of protein-tyrosine kinase genes preferentially expressed in embryo stomach and gastric cancer. Biochem Biophys Res Commun. 1993;194:698–705. doi: 10.1006/bbrc.1993.1878. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka H, Tanaka M, Kanamori M, Yoshii S, Ihara M, Wang YJ, Song JP, Li ZY, Arai H, Otsuki Y, et al. Expression profile of EFNB1, EFNB2, two ligands of EPHB2 in human gastric cancer. J Cancer Res Clin Oncol. 2002;128:343–348. doi: 10.1007/s00432-002-0355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiyokawa E, Takai S, Tanaka M, Iwase T, Suzuki M, Xiang YY, Naito Y, Yamada K, Sugimura H, Kino I. Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res. 1994;54:3645–3650. [PubMed] [Google Scholar]
- 16.Miyazaki K, Inokuchi M, Takagi Y, Kato K, Kojima K, Sugihara K. EphA4 is a prognostic factor in gastric cancer. BMC Clin Pathol. 2013;13:19. doi: 10.1186/1472-6890-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T, et al. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42–47. doi: 10.1111/j.1349-7006.2005.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oki M, Yamamoto H, Taniguchi H, Adachi Y, Imai K, Shinomura Y. Overexpression of the receptor tyrosine kinase EphA4 in human gastric cancers. World J Gastroenterol. 2008;14:5650–5656. doi: 10.3748/wjg.14.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimura H, Wang JD, Mori H, Tsuboi M, Nagura K, Igarashi H, Tao H, Nakamura R, Natsume H, Kahyo T, et al. EPH-EPHRIN in human gastrointestinal cancers. World J Gastrointest Oncol. 2010;2:421–428. doi: 10.4251/wjgo.v2.i12.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JD, Dong YC, Sheng Z, Ma HH, Li GL, Wang XL, Lu GM, Sugimura H, Jin J, Zhou XJ. Loss of expression of EphB1 protein in gastric carcinoma associated with invasion and metastasis. Oncology. 2007;73:238–245. doi: 10.1159/000127421. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Dong Y, Wang X, Ma H, Sheng Z, Li G, Lu G, Sugimura H, Zhou X. Expression of EphA1 in gastric carcinomas is associated with metastasis and survival. Oncol Rep. 2010;24:1577–1584. doi: 10.3892/or_00001020. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Li G, Ma H, Bao Y, Wang X, Zhou H, Sheng Z, Sugimura H, Jin J, Zhou X. Differential expression of EphA7 receptor tyrosine kinase in gastric carcinoma. Hum Pathol. 2007;38:1649–1656. doi: 10.1016/j.humpath.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Xi HQ, Wu XS, Wei B, Chen L. Aberrant expression of EphA3 in gastric carcinoma: correlation with tumor angiogenesis and survival. J Gastroenterol. 2012;47:785–794. doi: 10.1007/s00535-012-0549-4. [DOI] [PubMed] [Google Scholar]
- 24.Yu G, Gao Y, Ni C, Chen Y, Pan J, Wang X, Ding Z, Wang J. Reduced expression of EphB2 is significantly associated with nodal metastasis in Chinese patients with gastric cancer. J Cancer Res Clin Oncol. 2011;137:73–80. doi: 10.1007/s00432-010-0861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan W, Chen Z, Wu S, Ge J, Chang S, Wang X, Chen J, Chen Z. Expression of EphA2 and E-cadherin in gastric cancer: correlated with tumor progression and lymphogenous metastasis. Pathol Oncol Res. 2009;15:473–478. doi: 10.1007/s12253-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 26.Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 27.Cortina C, Palomo-Ponce S, Iglesias M, Fernández-Masip JL, Vivancos A, Whissell G, Humà M, Peiró N, Gallego L, Jonkheer S, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 28.Mizui T, Doteuchi M. Effect of polyamines on acidified ethanol-induced gastric lesions in rats. Jpn J Pharmacol. 1983;33:939–945. doi: 10.1254/jjp.33.939. [DOI] [PubMed] [Google Scholar]
- 29.Hirose M, Yamaguchi S, Fukushima S, Hasegawa R, Takahashi S, Ito N. Promotion by dihydroxybenzene derivatives of N-methyl-N’-nitro-N-nitrosoguanidine-induced F344 rat forestomach and glandular stomach carcinogenesis. Cancer Res. 1989;49:5143–5147. [PubMed] [Google Scholar]
- 30.Ogawa K, Wada H, Okada N, Harada I, Nakajima T, Pasquale EB, Tsuyama S. EphB2 and ephrin-B1 expressed in the adult kidney regulate the cytoarchitecture of medullary tubule cells through Rho family GTPases. J Cell Sci. 2006;119:559–570. doi: 10.1242/jcs.02777. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 32.Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- 33.Tanaka M, Kamata R, Takigahira M, Yanagihara K, Sakai R. Phosphorylation of ephrin-B1 regulates dissemination of gastric scirrhous carcinoma. Am J Pathol. 2007;171:68–78. doi: 10.2353/ajpath.2007.070033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka M, Kamata R, Yanagihara K, Sakai R. Suppression of gastric cancer dissemination by ephrin-B1-derived peptide. Cancer Sci. 2010;101:87–93. doi: 10.1111/j.1349-7006.2009.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


