Abstract
AIM: To investigate the role of pre-core and basal core promoter (BCP) mutations before and after hepatitis B e antigen (HBeAg) seroconversion.
METHODS: The proportion of pre-core (G1896A) and basal core promoter (A1762T and G1764A) mutant viruses and serum levels of hepatitis B virus (HBV) DNA, hepatitis B surface antigen (HBsAg), and HB core-related antigen were analyzed in chronic hepatitis B patients before and after HBeAg seroconversion (n = 25), in those who were persistently HBeAg positive (n = 18), and in those who were persistently anti-HBe positive (n = 43). All patients were infected with HBV genotype C and were followed for a median of 9 years.
RESULTS: Although the pre-core mutant became predominant (24% to 65%, P = 0.022) in the HBeAg seroconversion group during follow-up, the proportion of the basal core promoter mutation did not change. Median HBV viral markers were significantly higher in patients without the mutations in an HBeAg positive status (HBV DNA: P = 0.003; HBsAg: P < 0.001; HB core-related antigen: P = 0.001). In contrast, HBV DNA (P = 0.012) and HBsAg (P = 0.041) levels were significantly higher in patients with the pre-core mutation in an anti-HBe positive status.
CONCLUSION: There is an opposite association of the pre-core mutation with viral load before and after HBeAg seroconversion in patients with HBV infection.
Keywords: Seroconversion, Hepatitis B core-related antigen, Pre-core, Basal core promoter, Mutation, Hepatitis B surface antigen, Hepatitis B virus DNA
Core tip: The exact roles of pre-core (pre-C) and basal core promoter (BCP) mutations remain unclear before and after hepatitis B e antigen (HBeAg) seroconversion. Here, although the pre-C mutant became predominant in the HBeAg seroconversion group during follow-up, the proportion of the BCP mutation did not change. Hepatitis B virus (HBV) viral markers were significantly higher in patients without the mutations in an HBeAg positive status. HBV DNA and hepatitis B surface antigen levels were higher in patients with the pre-C mutation in an anti-HBe positive status. Taken together, the association of the pre-C mutation on viral load appears to be opposite before and after HBeAg seroconversion in patients with HBV infection.
INTRODUCTION
Hepatitis B virus (HBV) infection is a major health concern that has an estimated 350 to 400 million carriers worldwide. Chronic infection with HBV can cause chronic hepatitis, which may eventually develop into liver cirrhosis and hepatocellular carcinoma[1-4].
In the natural history of chronic HBV infection, seroconversion from hepatitis B e antigen (HBeAg) to its antibody (anti-HBe) is usually accompanied by a decrease in HBV replication and the remission of hepatitis[5-7]. Thus, HBeAg seroconversion is a favorable sign for patients with chronic hepatitis B. However, there are some patients who persistently exhibit elevated HBV DNA levels in the serum and active liver disease, even after seroconversion[8,9].
Several mutations in the HBV genome have been reported to associate with HBeAg seroconversion. When the pre-core (pre-C) and core genes in the HBV genome are transcribed and translated in tandem, HBeAg is produced and secreted into the circulation[10,11]. The G to A mutation at nucleotide (nt) 1896 in the pre-C region (G1896A), which converts codon 28 for tryptophan to a stop codon, is associated with the loss of detectable HBeAg[12,13]. The double mutations of A1762T and G1764A in the basal core promoter (BCP) of the HBV genome have also been shown to reduce HBeAg synthesis by suppressing the transcription of pre-C mRNA[14-16]. However, the detailed mechanisms of HBeAg seroconversion, including the involvement of mutations that decrease the production of HBeAg, have not been fully clarified. Orito et al[17] reported that a predominance of the pre-C mutation was correlated with anti-HBe, while BCP mutations were not associated with either anti-HBe or HBeAg. We previously uncovered that the pre-C and BCP mutations were frequently seen in patients with active replication after HBeAg seroconversion, but not in those with inactive replication[18], which suggested that HBeAg seroconversion was not associated with either mutation in such patients. Since the follow-up duration of these previous reports was limited, this study analyzed the changes in pre-C and BCP mutations among patients who were followed over a longer time course. Furthermore, we assessed the mutations not only in patients who seroconverted from HBeAg to anti-HBe, but also in those whose HBeAg or anti-HBe positive status did not change during follow-up.
MATERIALS AND METHODS
Patients
Three groups of patients with chronic hepatitis B who were categorized according to HBeAg/anti-HBe positive status were enrolled between 1985 and 2000. The subjects were selected retrospectively from a database of patients who had been followed for at least two years, had not received anti-viral therapy, such as nucleos(t)ide analogues, and whose stored serum samples were available from both the start and end of follow-up. We recruited only patients with HBV genotype C since this genotype is predominant in Japan and because the clinical significance of pre-C and BCP mutations differs among genotypes. The first group consisted of 18 patients whose HBeAg was persistently positive throughout the study period. The second group contained 25 patients in whom HBeAg seroconverted to anti-HBe. The third group was made up of 43 patients whose anti-HBe was persistently positive.
Hepatitis B surface antigen (HBsAg) was confirmed to be positive on at least two occasions a minimum of 6 mo apart in all patients before the start of follow-up. Tests for hepatitis C and human immunodeficiency virus antibodies were negative in all subjects. Patients who demonstrated accompanying hepatocellular carcinoma or signs of hepatic failure at the initial follow-up were excluded from the study.
Stored serum samples were kept frozen at -20 °C or below until assayed. This study was approved by the Ethics Committee of Shinshu University School of Medicine.
Conventional hepatitis B viral markers
Serological markers for HBV, including HBsAg, HBeAg, and anti-HBe, were tested using commercially available enzyme immunoassay kits (Fujirebio Inc., Tokyo, Japan)[19]. HBsAg was quantified[20] using a chemiluminescence enzyme immunoassay (CLEIA)-based HISCL HBsAg assay manufactured by Sysmex Corporation (Kobe, Japan). The assay had a quantitative range of -1.5 to 3.3 log IU/mL. End titer was determined by diluting samples with normal human serum when initial results exceeded the upper limit of the assay range.
Serum HBV DNA was determined using a COBAS TaqMan HBV kit (Roche, Tokyo, Japan)[21] with a quantitative range of 2.1 to 8.9 log copies/mL. According to the manufacturer’s instructions, detection of a positive signal below the quantitative range was described as a positive signal and no signal detection was considered to be a negative signal. Six HBV genotypes (A-F) were evaluated according to the restriction patterns of DNA fragments from the method reported by Mizokami et al[22]. Serum hepatitis B core-related antigen (HBcrAg) levels were measured using a CLEIA HBcrAg assay kit with a fully automated Lumipulse System analyzer (Fujirebio Inc.) as described previously[23,24]. The HBcrAg assay simultaneously measured all antigens (e, core, and p22cr) encoded by the pre-C/core genes of HBV. The immunoreactivity of pro-HBeAg at 10 fg/mL was defined as 1 U/mL. We expressed HBcrAg in terms of log U/mL with a quantitative range of 3.0 to 6.8 log U/mL.
Determination of pre-C and BCP mutations
The pre-C and BCP mutations were determined using nucleic acid samples extracted from 100 μL of serum with a DNA/RNA extraction kit (Smitest EX-R and D; Genome Science Laboratories Co., Ltd., Tokyo, Japan). The stop codon mutation in the pre-C region (A1896) was detected with an enzyme-linked mini-sequence assay kit (Smitest; Genome Science Laboratories). In principle, G1896 in wild type HBV and A1896 in the mutant were determined by mini-sequence reactions using labeled nucleotides that were complementary to either the wild type or mutant[25]. The results were expressed as percent mutation rates according to the definition by Aritomi et al[26] Samples were judged as positive for the pre-C mutation when the mutation rate exceeded 50% in the present study since the mutation rate was found to steadily increase to 100% once surpassing 50%[25].
The double mutation in the BCP was detected using an HBV core promoter detection kit (Smitest; Genome Science Laboratories)[25,26]. This kit detected T1762 and/or A1764 using the polymerase chain reaction (PCR) with primers specific for either wild type or mutant BCP. Results were recorded as wild, mixed, or mutant type. The pre-C and BCP mutations were tested at the start and end of follow-up with kits having manufacturer-established detection limits of 1000 copies/mL.
Full HBV genome sequencing
The nucleotide sequences of full-length HBV genomes were determined by a method reported previously[27]. Briefly, two overlapping fragments of an HBV genome were amplified by PCR, and then eight overlapping HBV DNA fragments were amplified by nested PCR. All necessary precautions to prevent cross-contamination were taken and negative controls were included in each assay. The sequencing reaction was performed according to the manufacturer’s instructions (ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kits, Version 3.1; Foster City, CA) with an automated ABI DNA sequencer (Model 3100, Applied Biosystems Carlsbad, CA).
Statistical analyses
The proportions of clinical factors were compared among groups using the χ2 and Fisher’s exact probability tests. Group medians were compared by means of the Mann-Whitney U test and Kruskal-Wallis test. The changes in proportions of the pre-C and BCP mutations between the study start and end points were compared using McNemar’s test. All tests were performed using the IBM SPSS Statistics Desktop for Japan ver. 19.0 (IBM Japan Inc., Tokyo, Japan). P values of less than 0.05 were considered to be statistically significant.
RESULTS
Patients
The clinical and virological backgrounds of the 3 groups are summarized in Table 1. Median age was lowest in patients with seroconversion, intermediate in those with persistent HBeAg, and highest in those with persistent anti-HBe. Gender ratio was similar among the 3 groups. Following our study design, all patients had HBV genotype C.
Table 1.
Clinical and virological backgrounds among 3 groups of patients classified according to changes in hepatitis B e antigen/anti-hepatitis B e
| Characteristic |
HBeAg/anti-HBe status |
P value | ||
| Continuously +/- (n = 18) | From +/- to -/+ (n = 25) | Continuously -/+ (n = 43) | ||
| Age (yr)1 | 44 (24-63) | 37 (18-53) | 51 (25-77) | < 0.001 |
| Gender (M:F) | 11:7 | 14:11 | 24:19 | > 0.2 |
| Follow-up period (yr)1 | 6.3 (2.1-14.6) | 10.8 (2.0-23.7) | 8.5 (2.2-16.6) | 0.006 |
| Genotype C2 | 18 (100) | 25 (100) | 43 (100) | 1 |
| Viral markers at first follow-up | ||||
| HBV DNA (log copies/mL)1 | 8.6 (5.7-> 8.9) | 6.1 (< 2.1-> 8.9) | < 2.1 (< 2.1-8.2) | < 0.001 |
| HBsAg (log IU/mL)1 | 4.6 (1.6-5.5) | 3.6 (-0.9-4.6) | 2.6 (< 0.05-4.3) | < 0.001 |
| HBcrAg (log U/mL)1 | > 6.8 (5.5->6.8) | 6.8 (3.1-> 6.8) | 3.0 (< 3.0-6.8) | < 0.001 |
| Viral markers at final follow-up | ||||
| HBV DNA (log copies/mL)1 | 7.1 (< 2.1-> 8.9) | 3.3 (neg.-6.2) | < 2.1 (neg.-7.0) | < 0.001 |
| HBsAg (log IU/mL)1 | 3.3 (1.0-5.1) | 2.8 (< 0.05-2.8) | 1.3 (< 0.05-4.2) | < 0.001 |
| HBcrAg (log U/mL)1 | 6.7 (4.4-> 6.8) | < 3.0 (< 3.0-6.2) | < 3.0 (< 3.0-5.3) | < 0.001 |
Data are expressed as the median (range);
Data are expressed as a positive number (%). HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HBcrAg: Hepatitis B core-related antigen.
Changes in pre-C and BCP mutations
The presence of the pre-C mutation could be evaluated in 60 (98%) of 61 HBeAg positive samples and 94 (85%) of 111 HBeAg negative samples. We were able to assess the existence of the BCP mutation in 57 (93%) of 61 HBeAg positive samples and 86 (77%) of 111 HBeAg negative samples.
The changes in the proportion of the pre-C mutation between the start and end of follow-up are shown in Figure 1A. Wild type pre-C accounted for 94% of patients whose HBeAg was continuously positive at study onset and remained constant. Wild type pre-C was also predominant at the start of follow-up (76%, 19/25) in patients who experienced HBeAg seroconversion, but the mutant type had become predominant (P = 0.022) by the end of follow-up (65%, 15/23); 11 of 19 wild type pre-C patients converted to mutant type, while 2 of 6 patients with mutant type pre-C reverted to wild type. Mutant type pre-C accounted for 62% of the patients who were continuously positive for anti-HBe at study onset. Such patients with wild type pre-C at the start of follow-up tended to maintain this status (78%), although 22% of initially mutant type pre-C subjects had changed to wild type by the study end point (P = 0.687).
Figure 1.
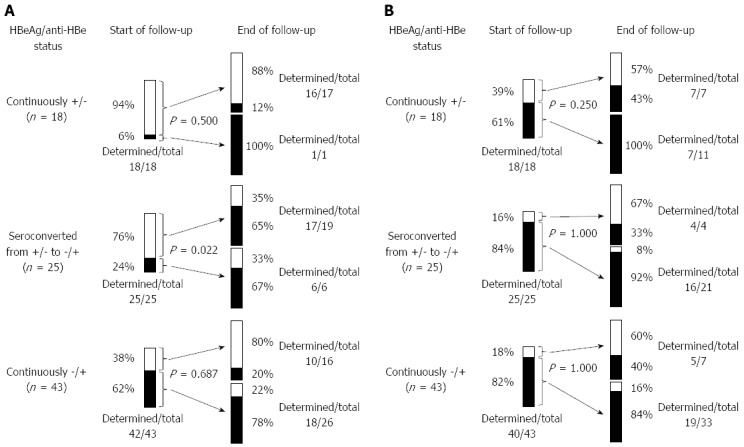
Comparison of changes in pre-core (A) and basal core promoter (B) mutation type among 3 groups of patients classified according to hepatitis B e antigen /anti-hepatitis B e positive status. A: A significant difference was seen in patients with hepatitis B e antigen (HBeAg) seroconversion (P = 0.022). One patient whose pre-core (pre-C) mutation was undetermined at the start of follow-up was wild type at the end point; B: Of the 3 patients whose basal core promoter (BCP) mutation was undetermined at the start of follow-up, 2 were wild type and 1 was undetermined at the end point. HBeAg: Hepatitis B e antigen.
Of the 143 samples with determined BCP mutations, 34 (24%) were wild, 11 (8%) were mixed, and 98 (69%) were mutant types. Because few patients with mixed type BCP reverted to wild type in the present and past studies[18], samples were considered to be positive for the BCP mutation when they were either mixed or mutant type.
The changes in the proportion of the BCP mutation between the start and end of follow-up are shown in Figure 1B. Mutant type BCP accounted for 61% of patients whose HBeAg was continuously positive at study onset and remained constant. In patients who experienced HBeAg seroconversion, mutant type BCP was predominant at the start of follow-up (84%, 21/25) and remained so (80%, 16/20) until final follow-up; 3 of 4 patients with wild type BCP and 15 of 16 patients with mutant type BCP maintained their status throughout the study period. Mutant type BCP initially accounted for 82% of patients who were continuously positive for anti-HBe. Both wild (60%) and mutant (84%) types tended to remain constant until the study end point. When all points of measurement were counted for which both pre-C and BCP mutations were evaluated, the prevalence of the pre-C mutation (18%, 9/57) was significantly lower than that of the BCP mutation (82%, 42/57) in patients with persistent HBeAg (P < 0.001), as well as in subjects with persistent anti-HBe [62% (53/86) vs 78% (67/86), P = 0.030], albeit to a lesser degree.
Comparison of viral loads according to pre-C/BCP mutation and HBeAg/anti-HBe positive status
We next compared the serum levels of HBV DNA, HBsAg, and HBcrAg according to pre-C and BCP mutation and HBeAg and anti-HBe positive status (Figure 2). Both pre-C and BCP mutations could be evaluated in 57 (93%) of 61 HBeAg positive samples and 86 (77%) of 111 HBeAg negative samples. HBV DNA levels were significantly higher in an HBeAg positive status than in an anti-HBe positive status (P < 0.001) and significantly higher in patients without the mutations than in those with at least one mutation in an HBeAg positive status (P < 0.01). On the other hand, HBV DNA levels were significantly lower in patients without the pre-C mutation than in those with it in an anti-HBe positive status (P = 0.012).
Figure 2.

Comparison of serum hepatitis B virus DNA, hepatitis B surface antigen, and hepatitis core-related antigen levels among patients with wild (-/-) and mutant types of the pre-core and basal core promoter mutations. Fifty-seven of 61 samples obtained from HBeAg positive cases and 86 of 111 samples obtained from anti-HBeAg positive cases were eligible for analysis. HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; HBcrAg: Hepatitis core-related antigen; pre-C: Pre-core; BCP: Basal core promoter.
A similar tendency to HBV DNA levels was observed for HBsAg levels. HBsAg levels were significantly higher in an HBeAg positive status than in an anti-HBe positive status (P < 0.001) and significantly higher in patients without the mutations than in those with at least one mutation in an HBeAg positive status (P < 0.001). HBsAg levels were significantly higher in patients with the pre-C mutation than in those without it irrespectively of the existence of the BCP mutation (P = 0.041).
HBcrAg levels were significantly lower with presence of pre-C and/or BCP mutations in an HBeAg positive status (P < 0.05, respectively). HBcrAg levels were uniformly low regardless of the presence of mutations in anti-HBe positive status subjects.
Full genome sequences in patients with and without appearance of the pre-C mutation
Full HBV genome sequences were determined after HBeAg seroconversion in 6 patients who seroconverted without the appearance of the pre-C mutation. All patients were positive for BCP mutations: 1 subject had T1753G and C1766T mutations, although the other mutations reported by Okamoto et al[14] were not identified.
DISCUSSION
Although both pre-C and BCP mutations have been associated with HBeAg seroconversion by reducing the production of HBeAg[13-15], their manifestation patterns appear to be different[17]. In the present study, the BCP mutation was already prevalent during the HBeAg positive chronic hepatitis phase and approached 80% around the time of HBeAg seroconversion. On the other hand, the pre-C mutation clearly manifested following the time of seroconversion. These results indicate that the appearance of the pre-C mutation, but not the BCP mutation, is directly associated with seroconversion. It is noteworthy that a considerable number of patients experienced HBeAg seroconversion without evidence of the pre-C G1896A mutation. Furthermore, wild type pre-C remained unchanged in almost all patients whose anti-HBe was continuously positive. Thus, two types of HBeAg seroconversion may exist for chronic HBV in terms of the appearance or absence of the G1896A pre-C mutation. We previously speculated on the possible existence of two seroconversion types in an analysis of HBV patients who experienced seroconversion[18]. Here, we were able to strengthen this notion by including patients who maintained an HBeAg or anti-HBe positive status in a study of longer duration. It should be noted that the absence of the pre-C G1896A mutation does not necessarily indicate the absence of mutations that halt HBeAg production; several patterns of mutations apart from G1896A have been associated with an HBeAg negative phenotype, such as point mutations in the ATG initiation region and deletion/insertion of nucleotides leading to premature termination[13]. Accordingly, we analyzed full genome sequences in 6 patients who seroconverted without the appearance of the pre-C mutation and uncovered T1753G and C1766T mutations in one subject[14] that might be associated with seroconversion. We observed that several patients reverted from mutant pre-C to wild type in the present report. As this important finding has not been confirmed by sequence analysis, we are planning to determine and compare entire genomic sequences using paired samples before and after HBeAg seroconversion in a future study.
We witnessed that serum HBV DNA was significantly lower in patients with the pre-C and/or BCP mutation in an HBeAg positive phase, which indicated that immune processes from the host to eliminate HBV were stronger in individuals with the mutations than in those without. This also supported the generally held belief that pre-C and BCP mutations appear as a result of host immune pressure[14]. Contrary to the HBeAg positive phase, HBV DNA was significantly higher in subjects with the pre-C mutation in an anti-HBe positive phase. Kawabe et al[28] have reported that patients with wild type pre-C demonstrate significantly lower viral loads and ALT levels than those with mutant pre-C among HBeAg negative patients with HBV genotype C infection. Collectively, these results imply that patients with the pre-C mutant have a higher potential to progress to hepatitis after HBeAg seroconversion. This is consistent with the fact that HBeAg negative hepatitis is usually caused by HBeAg non-producing mutant strains of HBV. Indeed, viral replication seems to be considerably suppressed in patients with wild type HBV after achieving HBeAg seroconversion since this strain has the ability to produce HBeAg when actively replicated.
We adopted serum levels of HBsAg, HBcrAg, and HBV DNA in the present study as markers to estimate HBV replication activity. HBsAg and HBcrAg levels have been reported to reflect HBV cccDNA levels in hepatocytes[20,24,29]. HBsAg has also attracted attention as a useful predictor of treatment outcome by interferon and others[30]. Furthermore, the loss of HBsAg is an important indicator in the treatment of HBV carriers. HBcrAg assays simultaneously measure all antigens encoded by the pre-C/core genome, which include the HB core, e, and p22cr antigens, and have been reported to predict the clinical outcome of patients treated with nucleotide or nucleoside analogues[31]. HBsAg patterns according to HBeAg/anti-HBe and pre-C/BCP status were similar to HBV DNA patterns both in HBeAg and anti-HBe positive states; HBsAg was significantly lower in patients with pre-C and/or BCP mutations than in those with wild type pre-C but was significantly higher in patients with the pre-C mutation than in those without it in an anti-HBe positive state. These results confirmed that the pre-C mutation was oppositely associated with viral load in patients before and after HBeAg seroconversion. Since elevated levels of HBV DNA and HBsAg are related to a higher rate of hepatocarcinogenesis, pre-C mutation patterns appear to be clinically important, at least in the context of HBV genotype C patients. We witnessed that the patterns of HBcrAg were similar to those of HBV DNA in the HBeAg positive state but different in the anti-HBe positive state. This difference may reflect the fact that the main antigen measured by the HBcrAg assay is HBeAg.
In conclusion, our findings indicate that the association of the pre-C G1896A mutation on viral load is opposite before and after HBeAg seroconversion in patients with HBV infection in that its presence results in a higher viral load after seroconversion. These observations may shed light on the pathology and treatment of chronic hepatitis B, especially that of an anti-HBe positive status.
ACKNOWLEDGMENTS
We thank Ms Hiroe Banno for her secretarial assistance and Mr. Trevor Ralph for his English editorial assistance.
COMMENTS
Background
Although pre-core (pre-C) and/or basal core promoter (BCP) mutations in the hepatitis B virus (HBV) genome have been reported to associate with hepatitis B e antigen (HBeAg) seroconversion, the detailed mechanisms have not been fully clarified.
Research frontiers
In this study, the authors show that the association of the pre-C mutation on viral load is opposite before and after HBeAg seroconversion in patients with HBV infection in that its presence results in a higher viral load after seroconversion.
Innovations and breakthroughs
Recent reports have highlighted the importance of pre-C and BCP mutations of the HBV genome in association with HBeAg seroconversion. This study analyzed the changes in pre-C and BCP mutations in patients over a long follow-up period. The authors demonstrate that the association of the pre-C mutation on viral load is opposite before and after HBeAg seroconversion in patients with HBV infection.
Applications
This study may shed light on the pathology and treatment of chronic hepatitis B, especially that of an anti-HBe positive status.
Terminology
In the natural history of chronic HBV infection, seroconversion from HBeAg to anti-HBe is usually accompanied by a decrease in HBV replication and the remission of hepatitis. Thus, HBeAg seroconversion is a favorable sign for patients with chronic hepatitis B. However, there are some patients who persistently exhibit elevated HBV DNA levels in the serum and active liver disease, even after seroconversion.
Peer review
The authors investigated the pre-C and/or BCP mutations before and after HBeAg seroconversion. They found that the association of the pre-C mutation on viral load is opposite in patients before and after HBeAg seroconversion. It is an interesting report. However there are several concerns.
Footnotes
Supported by Research grant from the Ministry of Health, Labor, and Welfare of Japan.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 8, 2014
First decision: May 29, 2014
Article in press: July 22, 2014
P- Reviewer: Jin DY, Rouet S, Sporea I, Yoshioka K S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 4.Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44 Suppl 19:102–107. doi: 10.1007/s00535-008-2251-0. [DOI] [PubMed] [Google Scholar]
- 5.Hoofnagle JH, Dusheiko GM, Seeff LB, Jones EA, Waggoner JG, Bales ZB. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann Intern Med. 1981;94:744–748. doi: 10.7326/0003-4819-94-6-744. [DOI] [PubMed] [Google Scholar]
- 6.Liaw YF, Chu CM, Su IJ, Huang MJ, Lin DY, Chang-Chien CS. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology. 1983;84:216–219. [PubMed] [Google Scholar]
- 7.Realdi G, Alberti A, Rugge M, Bortolotti F, Rigoli AM, Tremolada F, Ruol A. Seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B virus infection. Gastroenterology. 1980;79:195–199. [PubMed] [Google Scholar]
- 8.Bonino F, Rosina F, Rizzetto M, Rizzi R, Chiaberge E, Tardanico R, Callea F, Verme G. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroenterology. 1986;90:1268–1273. doi: 10.1016/0016-5085(86)90395-1. [DOI] [PubMed] [Google Scholar]
- 9.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 10.Bruss V, Gerlich WH. Formation of transmembraneous hepatitis B e-antigen by cotranslational in vitro processing of the viral precore protein. Virology. 1988;163:268–275. doi: 10.1016/0042-6822(88)90266-8. [DOI] [PubMed] [Google Scholar]
- 11.Garcia PD, Ou JH, Rutter WJ, Walter P. Targeting of the hepatitis B virus precore protein to the endoplasmic reticulum membrane: after signal peptide cleavage translocation can be aborted and the product released into the cytoplasm. J Cell Biol. 1988;106:1093–1104. doi: 10.1083/jcb.106.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, Thomas HC. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989;2:588–591. doi: 10.1016/s0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto H, Yotsumoto S, Akahane Y, Yamanaka T, Miyazaki Y, Sugai Y, Tsuda F, Tanaka T, Miyakawa Y, Mayumi M. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J Virol. 1990;64:1298–1303. doi: 10.1128/jvi.64.3.1298-1303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto H, Tsuda F, Akahane Y, Sugai Y, Yoshiba M, Moriyama K, Tanaka T, Miyakawa Y, Mayumi M. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J Virol. 1994;68:8102–8110. doi: 10.1128/jvi.68.12.8102-8110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckwold VE, Xu Z, Chen M, Yen TS, Ou JH. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J Virol. 1996;70:5845–5851. doi: 10.1128/jvi.70.9.5845-5851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Aoyama K, Ohno N, Iwata K, Akahane Y, Baba K, Yoshizawa H, Mishiro S. The precore/core promoter mutant (T1762A1764) of hepatitis B virus: clinical significance and an easy method for detection. J Gen Virol. 1995;76(Pt 12):3159–3164. doi: 10.1099/0022-1317-76-12-3159. [DOI] [PubMed] [Google Scholar]
- 17.Orito E, Mizokami M, Sakugawa H, Michitaka K, Ishikawa K, Ichida T, Okanoue T, Yotsuyanagi H, Iino S. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Japan HBV Genotype Research Group. Hepatology. 2001;33:218–223. doi: 10.1053/jhep.2001.20532. [DOI] [PubMed] [Google Scholar]
- 18.Misawa N, Matsumoto A, Tanaka E, Rokuhara A, Yoshizawa K, Umemura T, Maki N, Kimura T, Kiyosawa K. Patients with and without loss of hepatitis B virus DNA after hepatitis B e antigen seroconversion have different virological characteristics. J Med Virol. 2006;78:68–73. doi: 10.1002/jmv.20505. [DOI] [PubMed] [Google Scholar]
- 19.Umemura T, Tanaka E, Kiyosawa K, Kumada H. Mortality secondary to fulminant hepatic failure in patients with prior resolution of hepatitis B virus infection in Japan. Clin Infect Dis. 2008;47:e52–e56. doi: 10.1086/590968. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto A, Tanaka E, Morita S, Yoshizawa K, Umemura T, Joshita S. Changes in the serum level of hepatitis B virus (HBV) surface antigen over the natural course of HBV infection. J Gastroenterol. 2012;47:1006–1013. doi: 10.1007/s00535-012-0559-2. [DOI] [PubMed] [Google Scholar]
- 21.Ronsin C, Pillet A, Bali C, Denoyel GA. Evaluation of the COBAS AmpliPrep-total nucleic acid isolation-COBAS TaqMan hepatitis B virus (HBV) quantitative test and comparison to the VERSANT HBV DNA 3.0 assay. J Clin Microbiol. 2006;44:1390–1399. doi: 10.1128/JCM.44.4.1390-1399.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizokami M, Nakano T, Orito E, Tanaka Y, Sakugawa H, Mukaide M, Robertson BH. Hepatitis B virus genotype assignment using restriction fragment length polymorphism patterns. FEBS Lett. 1999;450:66–71. doi: 10.1016/s0014-5793(99)00471-8. [DOI] [PubMed] [Google Scholar]
- 23.Kimura T, Rokuhara A, Sakamoto Y, Yagi S, Tanaka E, Kiyosawa K, Maki N. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J Clin Microbiol. 2002;40:439–445. doi: 10.1128/JCM.40.2.439-445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki F, Miyakoshi H, Kobayashi M, Kumada H. Correlation between serum hepatitis B virus core-related antigen and intrahepatic covalently closed circular DNA in chronic hepatitis B patients. J Med Virol. 2009;81:27–33. doi: 10.1002/jmv.21339. [DOI] [PubMed] [Google Scholar]
- 25.Yamaura T, Tanaka E, Matsumoto A, Rokuhara A, Orii K, Yoshizawa K, Miyakawa Y, Kiyosawa K. A case-control study for early prediction of hepatitis B e antigen seroconversion by hepatitis B virus DNA levels and mutations in the precore region and core promoter. J Med Virol. 2003;70:545–552. doi: 10.1002/jmv.10429. [DOI] [PubMed] [Google Scholar]
- 26.Aritomi T, Yatsuhashi H, Fujino T, Yamasaki K, Inoue O, Koga M, Kato Y, Yano M. Association of mutations in the core promoter and precore region of hepatitis virus with fulminant and severe acute hepatitis in Japan. J Gastroenterol Hepatol. 1998;13:1125–1132. doi: 10.1111/j.1440-1746.1998.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugauchi F, Mizokami M, Orito E, Ohno T, Kato H, Suzuki S, Kimura Y, Ueda R, Butterworth LA, Cooksley WG. A novel variant genotype C of hepatitis B virus identified in isolates from Australian Aborigines: complete genome sequence and phylogenetic relatedness. J Gen Virol. 2001;82:883–892. doi: 10.1099/0022-1317-82-4-883. [DOI] [PubMed] [Google Scholar]
- 28.Kawabe N, Hashimoto S, Harata M, Nitta Y, Murao M, Nakano T, Shimazaki H, Arima Y, Komura N, Kobayashi K, et al. The loss of HBeAg without precore mutation results in lower HBV DNA levels and ALT levels in chronic hepatitis B virus infection. J Gastroenterol. 2009;44:751–756. doi: 10.1007/s00535-009-0061-7. [DOI] [PubMed] [Google Scholar]
- 29.Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, Wong GL, Sung JJ. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5:1462–1468. doi: 10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Li WC, Wang MR, Kong LB, Ren WG, Zhang YG, Nan YM. Peginterferon alpha-based therapy for chronic hepatitis B focusing on HBsAg clearance or seroconversion: a meta-analysis of controlled clinical trials. BMC Infect Dis. 2011;11:165. doi: 10.1186/1471-2334-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka E, Matsumoto A. Guidelines for avoiding risks resulting from discontinuation of nucleoside/nucleotide analogs in patients with chronic hepatitis B. Hepatol Res. 2014;44:1–8. doi: 10.1111/hepr.12108. [DOI] [PubMed] [Google Scholar]


