Abstract
AIM: To ascertain pathologic stage as a prognostic indicator for rectal cancer patients receiving preoperative chemoradiotherapy (PCRT).
METHODS: Patients with mid- and low rectal carcinoma (magnetic resonance imaging - based clinical stage II or III) between 2000 and 2009 and treated with curative radical resection were identified. Patients were divided into two groups: PCRT and No-PCRT. Recurrence-free survival (RFS) was examined according to pathologic stage and addition of adjuvant treatment.
RESULTS: Overall, 894 patients were identified. Of these, 500 patients received PCRT. Adjuvant chemotherapy was delivered to 81.5% of the No-PCRT and 94.8% of the PCRT patients. Adjuvant radiotherapy was given to 29.4% of the patients in the No PCRT group. The 5-year RFS for the No-PCRT group was 92.6% for Stage I, 83.3% for Stage II, and 72.9% for Stage III. The 5-year RFS for the PCRT group was 95.2% for yp Stage 0, 91.7% for yp Stage I, 73.9% for yp Stage II, and 50.7% for yp Stage III.
CONCLUSION: Pathologic stage can predict prognosis in PCRT patients. 5-year RFS is significantly lower among PCRT patients than No-PCRT patients in pathologic stage II and III. These results should be taken into account when considering adjuvant treatment for patients treated with PCRT.
Keywords: Preoperative, Chemoradiotherapy, Rectal cancer, Pathologic stage, Prognosis
Core tip: Strictly speaking, there is no common objective guideline to predict prognosis and give adjuvant treatment according to risk stratification. Patients who show good response were thought to have good prognosis. However, expected value of recurrence-free survival or recurrence rate was not suggested especially in patients who did not show good response to patients receiving preoperative chemoradiotherapy (PCRT). In addition, how to measure the response level was variable. The present study suggests impression of prognosis based on pathologic stage, which is objective, after PCRT and radical resection and show stage-by-stage comparison with those without PCRT to give impression of prognosis by using familiar stage-based prognosis.
INTRODUCTION
Pathologic staging is used to select high-risk patients for adjuvant treatment to reduce disease recurrence and improve survival[1]. Preoperative chemoradiotherapy (PCRT) followed by radical resection is the standard treatment for patients with clinical stage II-III rectal cancer. A tumor down-staging rate of 40%-80% and a pathologic complete response (pCR) rate of 10%-25% can be achieved after PCRT[2-6]. Patients achieving tumor down-staging after preoperative therapy tend to have better local control and increased survival. Conversely, patients with persistent nodal disease after chemoradiation have a very poor prognosis[7-9]. However, there is uncertainty concerning the difference in prognosis according to pathologic stage for patients treated with PCRT. In addition, although several studies have shown that pathologic stage after PCRT (yp stage) followed by radical resection is a significant prognostic indicator, prognostic information is not usually used to inform post-surgical clinical practice for PCRT patients in contrast to patients with rectal cancer who are not treated with PCRT[10-12].
The National Comprehensive Cancer Network guidelines recommend that all patients undergoing PCRT receive postoperative chemotherapy regardless of the pathologic results[13]. This recommendation is based on preoperative clinical staging. Although adjuvant chemotherapy is regarded as the standard treatment[11,14-17] irrespective of the final pathologic stage[11,14-17], evidence supporting the routine use of adjuvant chemotherapy (according to pretreatment clinical stage) for patients with advanced rectal cancer after PCRT is lacking.
In addition, though it has been suggested that patients who do not respond to PCRT have a poor prognosis, the extent of this effect is not clear.
Some studies have indicated that the final pathologic stage is more predictive of long-term outcome (e.g., disease-free survival) than the preoperative clinical stage or degree of down-staging[7,11,18-20]. Thus, it appears plausible to use pathologic stage as a criterion for adjuvant chemotherapy and for formulating a prognosis.
In the present study we compared the prognosis (based on pathologic stage) of patients with advanced rectal cancer who received PCRT with that of patients not treated with PCRT, and evaluated the usefulness of yp stage as an outcome predictor and guideline for adjuvant treatment.
MATERIALS AND METHODS
Patient identification
Patients with biopsy-proven mid- and low rectal cancer who were treated with curative surgery at Asan Medical Center between 2000 and 2009 were identified from the institutional colorectal cancer patient database and tumor registry. Cases in which the lower border of the tumor was located ≤ 5 cm from the anal verge (as assessed by proctoscopy or digital rectal examination) were defined as low rectum, and those located > 5 cm, ≤ 10 cm from the anal verge were defined as mid-rectum. Patients with concurrent distant metastasis, concurrent inflammatory bowel disease, hereditary colorectal cancer syndromes, or concurrent malignancy, or those requiring urgent surgery, or with a prior history of immunotherapy or radiotherapy to the pelvis or a prior history of malignancy other than non-skin melanoma or in situ cervical cancer, were excluded. Patients with no identifiable exact clinical stage and pathologic stage were also excluded. The study was approved by the Asan Institutional Review Board.
Clinical staging, pathologic evaluation, and treatment
Preoperative clinical staging was based on magnetic resonance imaging (MRI). MRI diagnosis of T3 lesions was based on the presence of an tumor signal intensity extending through the muscle layers into the perirectal fat, with a broad-based bulging configuration, and continuous with the intramural portion of the tumor. A clinical T4 lesion was defined as direct invasion to an adjacent organ. Positive lymph node (LN) status was ascertained from signal intensity, border characteristics, irregular contour, or heterogeneous texture. In addition, diameter larger than 5 mm was used as a predictor of LN positivity. Upfront resection was recommended for patients with obstructive lesion. For patients with cT3-4 and/or N+, tumor involvement to mesorectal fascia was checked using MRI or CT. When it is possible to get clear mesorectal margin by upfront surgery, the current disease status, possible advantage of PCRT, and expected response rate was explained to patient, and patient involve in selection of treatment plan. If mesorectal fascia involvement was suspected, PCRT was recommended primarily. The PCRT regimen comprised pelvic external beam radiation (45 Gy given in 25 fractions over 5 wk) followed, in most cases, by a boost of 5.4 Gy (in 5 fractions) applied directly to the tumor. This boost was delivered as a second daily fraction during the final week of treatment, taking the cumulative radiation dose to 50.4 Gy. Most of the patients were treated with concurrent chemotherapy comprising 5-fluorouracil and leucovorin (FL) and capecitabine and were included in the PCRT group. FL was delivered via two intravenous bolus injections of 5-fluorouracil (375 mg/m2 per day) and leucovorin (20 mg/m2 per day) for 3 d during the first and fifth weeks of radiotherapy. Capecitabine (825 mg/m2) was given twice daily (orally) during radiotherapy. Surgery was performed 6-8 wk after the completion of radiotherapy according to principle of total mesorectal excision.
Adjuvant chemotherapy is recommended for all No-PCRT patients with pathologic stage III disease and those with stage II with risk factors such as lymphovascular invasion, perineural invasion, preoperative obstruction, and perforation. Adjuvant chemotherapy followed by radical resection is recommended for all medically-fit PCRT patients. The usual adjuvant treatment comprised FL for 4 cycles monthly or capecitabine for 6 cycles. Oxaliplatin regimens were delivered at the discretion of the attending physician. In some cases protocol-based concurrent chemotherapy included the addition of irinotecan or bevacizumab. Postoperative follow-up comprised routine physical examination and carcinoembryonic antigen (CEA) assays every 3-6 mo, and cross-sectional imaging every 6-12 mo over a period of 5 years. Colonoscopy was performed at 6-12 mo after surgery and then every 2-3 years thereafter.
Recurrence-free survival (RFS) was used as the cancer recurrence end point. RFS was defined as the time from surgery to any type of tumor recurrence. Patients who died without evidence of confirmed tumor recurrence were censored at the time of death.
Statistical analysis
Non-parametric data were compared using the Wilcoxon rank sum test. Categorical data were summarized according to frequency within each cohort and compared using the χ2 test. Kaplan-Meier survival analysis was used to determine 5 year RFS, and the log rank test was used to compare RFS with pathologic tumor stage. Cox proportional hazards regression analysis was employed to examine the relationship between various factors and treatment effects. P values < 0.05 were considered statistically significant. All statistical analyses were performed with SPSS (Version 21.0; IBM statistics, New York, NY).
RESULTS
Patient characteristics
A total of 894 patients who underwent curative resection for cT3-4 or N+ (MRI based) mid- and low-rectal cancer during the study period were eligible. Of these, 500 (55.9%) received PCRT. The median patient age was 59 [interquartile range (IQR): 50-66] years, and the majority (63.9%) was male. The median distance of the tumor from the anal verge was 5 (IQR: 3-8) cm, and 49.4% of the patients had low rectal cancer. The median radiation dose was 50.4 (IQR: 45-52.5) Gy. Sphincter-preserving resection was performed for 730 (81.7%) of the patients. The patients in the PCRT group were younger than those in the No-PCRT group, there were more males, and most had a low rectum tumor (Table 1). Sphincter-sparing surgery was performed more frequently in the No-PCRT group. Taking into account only those patients with a low rectal tumor, the sphincter-sparing surgery rates were 62.5% for the PCRT group and 70.9% for the No-PCRT group (P = 0.05; Table 1). Fewer lymph nodes were excised from patients in the PCRT group than from those in the No-PCRT group (median, 13 vs 16, P < 0.001).
Table 1.
Characteristics of patients receiving and not receiving preoperative chemoradiotherapy n (%)
| No-PCRT (n = 394) | PCRT (n = 500) | P value | |
| Age (yr), median (range) | 60 (52-68) | 57 (49-64) | < 0.001 |
| < 50 | 72 (18.3) | 124 (24.8) | |
| 50-65 | 183 (46.4) | 269 (53.8) | |
| > 65 | 139 (35.3) | 107 (21.4) | |
| Gender | 0.028 | ||
| Male | 236 (59.9) | 335 (67.0) | |
| Female | 158 (40.1) | 165 (33.0) | |
| Location | < 0.001 | ||
| Mid-rectum | 277 (70.3) | 175 (35.0) | |
| Low rectum | 117 (29.7) | 325 (65.0) | |
| Sphincter preservation | 358 (90.9) | 372 (74.4) | < 0.001 |
| Among patients with low rectum | 83 (70.9) | 203 (62.5) | 0.053 |
| Clinical stage | 0.86 | ||
| II | 21 (5.3) | 28 (5.6) | |
| III | 373 (94.7) | 472 (94.4) | |
| Pathologic stage1 | |||
| 0 | - | 83 (16.6) | |
| I | 97 (24.6) | 128 (25.6) | |
| II | 145 (36.8) | 135 (27.0) | |
| III | 152 (38.6) | 154 (30.8) | |
| Number of harvested lymph nodes | 16 (11-22) | 13 (9-17) | < 0.001 |
| Length of distal resection margin (cm) | 2.2 (1.4-3.3) | 2.4 (1.4-3.8) | 0.64 |
| Adjuvant chemotherapy1 | 321 (81.5) | 474 (94.8) | < 0.001 |
| Adjuvant radiotherapy | 116 (29.4) | ||
| Follow-up duration (mo) | 60 (39-80) | 56 (43-68) | 0.54 |
The pathologic stage for the PCRT group was based on yp stage. PCRT: Preoperative chemoradiotherapy.
Adjuvant chemotherapy was administered to 81.5% of the patients in the No-PCRT group and to 94.8% of those in the PCRT group. The adjuvant chemotherapy regimen administered to the PCRT group comprised FL (25.7%) or capecitabine (63.4%).
Recurrence and survival
Overall, 5-year RFS was higher in the No-PCRT (80.8%) than the PCRT (74.9%) group (P = 0.01). According to clinical stage, 5-year RFS did not differ between the No-PCRT and PCRT group. In clinical stage II, 5-year RFS was 79.4% with PCRT and 81% with No-PCRT (P = 0.66). In clinical stage III we evaluated 5-year RFS according to cT category. For cT3N+, 5-year RFS was 75.3% with PCRT and 80.7% with No-PCRT (P = 0.1). For cT4N+, it was 61% with PCRT and 63.4% with No-PCRT (P = 0.51).
5-year RFS rates (stratified according to yp stage and p stage) were: 95.2% for yp stage 0; 91.7% for yp stage I; 92.6% for p stage I; 73.8% for yp stage II; 83.3% for p stage II; 50.7% for yp stage III; and 72.9% for p Stage III (Figure 1).
Figure 1.
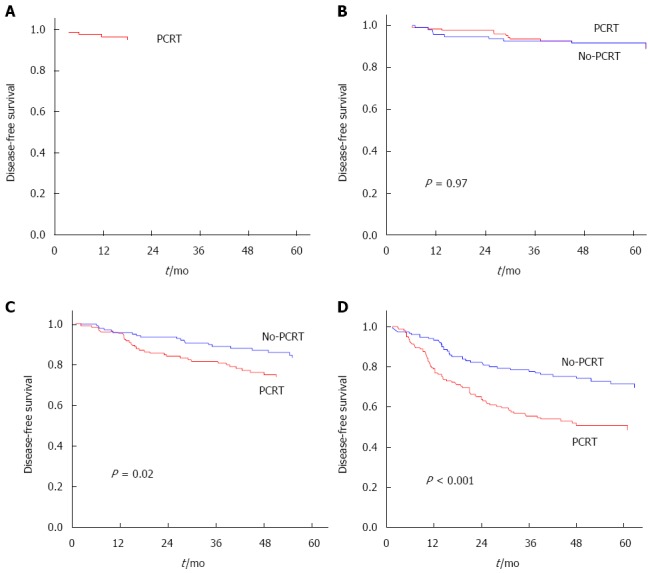
Recurrence-free survival according to pathologic stage. A: p Stage 0; B: p Stage I vs yp Stage I; C: p Stage II vs yp Stage II; D: p Stage III vs yp Stage III. PCRT: Preoperative chemoradiotherapy.
Recurrence-free survival and adjuvant chemotherapy for patients treated with PCRT
Forty patients in the PCRT group received second-line adjuvant chemotherapy: 37 received oxaliplatin-based chemotherapy, 2 received irinotecan-based chemotherapy, and one had target agent. Twenty-three patients with yp Stage III (14.9%) among the patients who received 1st line chemotherapy-based PCRT received a second-line adjuvant chemotherapeutic regimen that was different from the preoperative concurrent chemotherapeutic regimen. 3-year RFS for patients receiving second-line adjuvant chemotherapy was 70.2%, and that for patients receiving the same chemotherapeutic regimen as the preoperative concurrent regimen was 56.7%. Thus, changing the adjuvant chemotherapy regimen did not affect the 3-year RFS of patients with yp Stage III disease (Figure 2). Nevertheless the hazards ratio was more favorable when a 2nd-line regimen was delivered. The risk of recurrence for patients with yp Stage III disease who received second-line chemotherapy was 21% lower than that for patients receiving first-line chemotherapy (HR = 0.79, 95%CI: 0.39-1.63; P = 0.53; Table 2).
Figure 2.
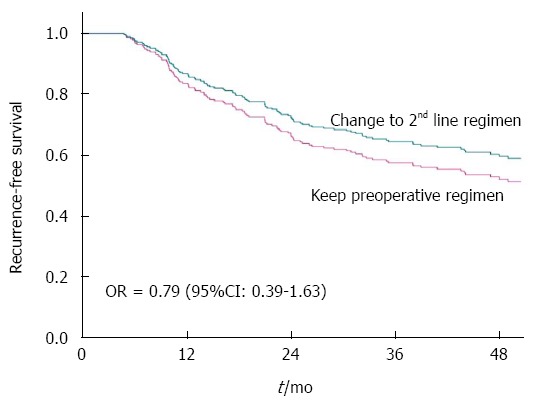
Recurrence-free survival of patients with yp Stage III disease treated with different adjuvant chemotherapy regimens. Patients with yp Stage III that received a different second-line chemotherapy regimen had longer recurrence-free survival than patients receiving a regimen that was the same as the preoperative concurrent regimen.
Table 2.
Univariate and multivariate cox proportional hazards regression models of the clinical factors associated with recurrence-free survival in preoperative chemoradiotherapy patients with pathologically- proven metastatic lymph nodes
| Factor |
Univariate analysis |
Multivariate analysis |
||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex | 0.41 | |||||
| Male | 1.00 | |||||
| Female | 0.85 | 0.57-1.25 | ||||
| Age (yr) | 1.00 | 0.98-1.02 | 0.89 | |||
| Lymphovascular invasion | 0.54 | |||||
| None | 1.00 | |||||
| Present | 1.28 | 0.83-1.98 | ||||
| Perineural invasion | 0.01 | 0.02 | ||||
| None | 1.00 | 1.00 | ||||
| Present | 1.89 | 1.22-2.95 | 1.82 | 1.15-2.87 | ||
| Sphincter preservation | 0.01 | 0.93 | ||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.60 | 0.40-0.89 | 0.96 | 0.35-2.59 | ||
| Tumor grade | 0.34 | |||||
| G1, G2 | 1.00 | |||||
| G3, G4 | 1.31 | 0.75-2.31 | ||||
| Preoperative CEA | 0.45 | |||||
| Normal | 1.00 | |||||
| Increased | 1.22 | 0.73-2.05 | ||||
| Location of tumor | 0.25 | 0.89 | ||||
| Mid-rectum | 1.00 | |||||
| Lower rectum | 1.26 | 0.85-1.87 | 1.03 | 0.66-1.62 | ||
| Number of retrieved lymph node | 0.97 | 0.95-1.01 | 0.14 | |||
| Adjuvant chemotherapy1 | 0.36 | 0.46 | ||||
| Same1 | 1.00 | 1.00 | ||||
| Altered | 0.72 | 0.35-1.47 | 0.76 | 0.36-1.58 | ||
Same: same regimen as that used for preoperative concurrent chemoradiotherapy. Altered: Second-line regimen was different from that used for preoperative chemotherapy; CEA: Carcinoembryonic antigen.
DISCUSSION
In the present study we stratified 5-year RFS according to the final pathologic stage in patients with rectal cancer treated by PCRT followed by radical resection. The AJCC TNM staging system is widely used for prognosis and for predicting the risk of recurrence in rectal cancer patients after surgical resection. However, the TNM staging system was originally based on pathologic findings in patients who did not receive neoadjuvant therapy prior to surgical resection. At present, the applicability and prognostic significance of the TNM staging system for patients that have undergone PCRT is not clear.
Some studies have found that patients showing a good response after PCRT have a more favorable prognosis, even in those patients with initially clinically node-positive disease[7-9,15,18]. On the other hand, patients with persistent nodal disease after chemoradiation have a poorer prognosis[7-9]. Thus, it is important to ascertain whether the use of postoperative chemotherapy should be decided by clinical stage, or by the definitive pathological surgical stage (ypTNM) following chemoradiotherapy.
The risk of recurrence is high for patients with clinical stage II or III rectal cancer; however, theoretically at least, the risk is not influenced by PCRT because the latter is a local treatment. However, data from this and other studies suggest that the risk of distant and local failure is, in fact, closely associated with the final pathologic stage. Typically a full course of adjuvant chemotherapy is recommended, regardless of the final pathologic stage. There are several reasons for this: preoperative chemotherapy uses a radiosensitizing agent rather than a definitive chemotherapy drug; the seminal randomized trials conducted for PCRT therapy included the use of routine adjuvant chemotherapy; and there may be a presumption that pathological stage is an unreliable prognostic indicator in patients treated with chemoradiotherapy. However, several studies show that pathological stage is in fact a reliable prognostic indicator, and that it may be more accurate than the preoperative clinical stage[7,18-20].
We found that, based on the pathologic stage, 5-year RFS in pathologic stage II and III was lower for the PCRT patients than for the No-PCRT patients. This suggests that the adjuvant chemotherapy for patients with pathologically-proven metastatic lymph nodes after PCRT should be different from that for No-PCRT patients. The authors of the EORTC 22921 study reported benefits of adjuvant chemotherapy for the subgroup showing down-staging after PCRT[21]. They proposed that only those patients achieving a pCR, or those that were down-staged to ypT1-2 after preoperative radiation, would benefit from adjuvant chemotherapy; those with residual ypT3-4 disease would not[21]. The study suggested that adjuvant chemotherapy had a beneficial effect when its administration was based on pathologic stage; however, the results for the ypT and ypN stages were analyzed separately. Adjuvant chemotherapy appeared to benefit patients that were down-staged (in terms of ypT stage) but had no effect according to ypN status[21]. Other studies did not confirm these results, particularly regarding the effect of adjuvant chemotherapy on patients that achieved pCR[15,18]. In view of the favorable outcomes for patients showing complete remission, it may be difficult to improve survival with adjuvant chemotherapy over and above that achieved without adjuvant chemotherapy.
In the present study we were not able to assess the benefits of adjuvant chemotherapy for patients showing complete remission because the number of such patients not receiving adjuvant chemotherapy was too small. However, we did examine the influence of adjuvant chemotherapeutic regimens on the RFS of the patients in the PCRT group with ypN+ disease (who had much poorer oncologic outcomes than those in the non-PCRT group). Those patients in the PRCT group that received changed adjuvant chemotherapy had a more favorable outcome than those who did not, although the difference was not statistically significant.
We also examined the effect of changing the adjuvant chemotherapy regimen used to treat patients with ypT3-4 stage disease. We found that 3-year RFS was higher when second-line chemotherapy was provided, although the difference was smaller (76.5% for patients with an altered second-line regimen vs 69.6% for those with the same regimen as mentioned used preoperatively) than that observed for the ypN+ patients (70.2% for patients with an altered second-line regimen vs 56.7% for those with a same regimen). A previous study reported higher rates of relapse despite adjuvant chemotherapy in patients who did not respond to preoperative treatment, and suggested that FOLFOX (oxaliplatin plus FL) be used for high-risk patients[4].
The 5-year RFS for patients showing complete remission after PCRT was comparable to that for patients with tumors confined within the rectal mucosa, which can be successfully treated by endoscopic resection or local excision. Therefore, organ-preserving treatments may be useful for patients showing complete remission after PCRT. In the present study, however, 90.2% of the latter received adjuvant chemotherapy, and all underwent radical resection. Great care should be taken when adopting an organ-preserving strategy in clinical practice.
The present study has several limitations. First, it was retrospective in nature, which may cause a bias towards the identification of metastasis/recurrence. However, we chose RFS as the outcome measure as it is less likely to be subject to selection bias or to be confounded by other parameters. We also used multivariate regression to adjust for other potential confounders. In addition, we included patients diagnosed as cT3-4 or N+ based only on MRI in order to compensate for selection bias because of the variable accuracy of imaging modalities in the local staging of rectal cancer.
Second, very few of the patients treated with PCRT followed by radical resection received an altered second-line adjuvant chemotherapy regimen. Because of this (and the retrospective nature of this study) it would be inappropriate to conclude that using the same adjuvant chemotherapy with concurrent preoperative chemotherapeutic regimen based on clinical stage conferred no survival benefit. In addition, oxaliplatin was used as the adjuvant chemotherapeutic regimen since 2007. The number of patients receiving oxaliplatin, along with the shorter follow-up times for these patients, may have affected the final oncologic outcomes.
In conclusion, the final pathologic stage of patients with advanced rectal cancer treated by PCRT can be used to predict oncologic outcome. Thus, we suggest that intensive adjuvant chemotherapy might be considered for patients showing much poorer outcomes than those who are not treated with PCRT. Further large-scale studies should be performed to examine the reliability of pathologic stage as a prognostic indicator and guideline for adjuvant treatment in patients with rectal cancer treated by PCRT based on pathologic stage. It will be important to establish a standard to compare prognoses and to conduct clinical trials with the hope of influencing prognosis.
COMMENTS
Background
Recurrence-risk stratification is necessary to make evidence for adjuvant treatment to reduce disease recurrence and improve survival. Pathologic stage has been used for this purpose. Although preoperative chemoradiotherapy (PCRT) is established as a standard treatment for locally advanced rectal cancer, method for risk-stratification which is useful for post-surgical clinical practice was not settled. In addition the authors did not have overview impression for prognosis for PCRT patients. To give overview impression for prognosis of PCRT, the present study used prognosis based on pathologic stage in patient who did not receive PCRT because it is already well known in setting of clinical practice.
Research frontiers
Risk of recurrence was well stratified based on pathologic stage in PCRT patients. In case of nodal metastasis after PCRT showed much worse prognosis than those with node metastasis without PCRT. In these cases, there was a tendency of improvement of recurrence-free survival when 2nd-line chemotherapy was given. Future investigation is required to decide on clinical suitability of pathologic stage in PCRT patients.
Innovations and breakthroughs
The current study shows not only the difference of prognosis according to pathologic stage in patients treated with PCRT, but also possibility as a standard for adjuvant treatment and measurement of results. The present study also gives impression of prognosis of PCRT patients according to pathologic stage which is familiar to clinicians.
Applications
The final pathologic stage of patients with advanced rectal cancer treated by PCRT can be used to predict oncologic outcome. Adjuvant treatment and surveillance need to be given based on prognostic implication based on pathologic stage. Pathologic stage also would be used as a standard to compare results of treatment in future investigations.
Peer review
The authors presented the data of prognosis based on pathologic stage of locally advanced rectal cancer patients treated with PCRT. The present study showed potential role of pathologic stage as a standard for measurement of treatment outcome peculiarity, although prognostic implication of pathologic stage in PCRT patient were also reported in other similar articles. It would be more useful for clinical practice to evaluate treatment outcome or to give intensive adjuvant treatment in PCRT patients because benefit of each adjuvant treatment in PCRT patients has not been established yet.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 7, 2014
First decision: June 10, 2014
Article in press: August 28, 2014
P- Reviewer: Cheung HYS, Kato J, Yoshida N S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 2.Chan AK, Wong AO, Langevin J, Jenken D, Heine J, Buie D, Johnson DR. Preoperative chemotherapy and pelvic radiation for tethered or fixed rectal cancer: a phase II dose escalation study. Int J Radiat Oncol Biol Phys. 2000;48:843–856. doi: 10.1016/s0360-3016(00)00692-1. [DOI] [PubMed] [Google Scholar]
- 3.Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, Khanduja KS. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895–903. doi: 10.1007/s10350-004-6325-7. [DOI] [PubMed] [Google Scholar]
- 4.Janjan NA, Crane C, Feig BW, Cleary K, Dubrow R, Curley S, Vauthey JN, Lynch P, Ellis LM, Wolff R, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24:107–112. doi: 10.1097/00000421-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, Cellini F, Barbaro B, Cogliandolo S, Nuzzo G, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664–674. doi: 10.1016/s0360-3016(02)02764-5. [DOI] [PubMed] [Google Scholar]
- 6.Mohiuddin M, Hayne M, Regine WF, Hanna N, Hagihara PF, McGrath P, Marks GM. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int J Radiat Oncol Biol Phys. 2000;48:1075–1080. doi: 10.1016/s0360-3016(00)00732-x. [DOI] [PubMed] [Google Scholar]
- 7.Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Saltz LB, Goodman KA, Minsky BD, Wong WD, Weiser MR. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. 2008;113:57–64. doi: 10.1002/cncr.23516. [DOI] [PubMed] [Google Scholar]
- 8.Suzue S, Irikura T. Studies on hepatic agents. I. Synthesis of aminoacyl (and hydroxyacyl) aminoacetonitriles. Chem Pharm Bull (Tokyo) 1968;16:1417–1432. doi: 10.1248/cpb.16.1417. [DOI] [PubMed] [Google Scholar]
- 9.Bujko K, Michalski W, Kepka L, Nowacki MP, Nasierowska-Guttmejer A, Tokar P, Dymecki D, Pawlak M, Lesniak T, Richter P, et al. Association between pathologic response in metastatic lymph nodes after preoperative chemoradiotherapy and risk of distant metastases in rectal cancer: An analysis of outcomes in a randomized trial. Int J Radiat Oncol Biol Phys. 2007;67:369–377. doi: 10.1016/j.ijrobp.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 10.Chapet O, Romestaing P, Mornex F, Souquet JC, Favrel V, Ardiet JM, d’Hombres A, Gerard JP. Preoperative radiotherapy for rectal adenocarcinoma: Which are strong prognostic factors? Int J Radiat Oncol Biol Phys. 2005;61:1371–1377. doi: 10.1016/j.ijrobp.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Hoff PM, Eng C, Wolff RA, Janjan NA, Delclos ME, et al. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol. 2006;29:219–224. doi: 10.1097/01.coc.0000214930.78200.4a. [DOI] [PubMed] [Google Scholar]
- 12.Kim TH, Chang HJ, Kim DY, Jung KH, Hong YS, Kim SY, Park JW, Oh JH, Lim SB, Choi HS, et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys. 2010;77:1158–1165. doi: 10.1016/j.ijrobp.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Rectal Cancer. NCCN; 2013. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 14.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 15.Fietkau R, Barten M, Klautke G, Klar E, Ludwig K, Thomas H, Brinckmann W, Friedrich A, Prall F, Hartung G, et al. Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum. 2006;49:1284–1292. doi: 10.1007/s10350-006-0570-x. [DOI] [PubMed] [Google Scholar]
- 16.Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 17.Ghadimi BM, Grade M, Difilippantonio MJ, Varma S, Simon R, Montagna C, Füzesi L, Langer C, Becker H, Liersch T, et al. Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol. 2005;23:1826–1838. doi: 10.1200/JCO.2005.00.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99–107. doi: 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Chang GJ, Rodriguez-Bigas MA, Eng C, Skibber JM. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer. 2009;115:5432–5440. doi: 10.1002/cncr.24622. [DOI] [PubMed] [Google Scholar]
- 20.Kuo LJ, Liu MC, Jian JJ, Horng CF, Cheng TI, Chen CM, Fang WT, Chung YL. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol. 2007;14:2766–2772. doi: 10.1245/s10434-007-9471-z. [DOI] [PubMed] [Google Scholar]
- 21.Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Piérart M, Calais G. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379–4386. doi: 10.1200/JCO.2007.11.9685. [DOI] [PubMed] [Google Scholar]


