Abstract
AIM: To evaluate the biopathologic features and clinical significance of nodal micrometastasis (MI) in early gastric cancer (EGC).
METHODS: Among 1022 EGC patients who underwent gastrectomy with lymphadenectomy of D1 + β or more from March 2001 to December 2005 at the Korean National Cancer Center, available nodal metastasis was found in 90 pT1N1 patients. Nodal metastasis was confirmed by immunohistochemistry (IHC) with cytokeratin and patients were classified into MI and macrometastasis (MA) groups based on the main tumor burden according to the 6th International Union Against Cancer/American Joint Committee on Cancer staging system; the main tumor burden with a diameter of greater than 0.2 mm but no greater than 2 mm as MI, and greater than 2 mm as MA of the representative metastatic node. Proliferative and apoptotic activities of the primary tumor and the nodal metastasis were measured by IHC with Ki-67 and terminal deoxynucleotidyl transferase dUTP nick end labeling, respectively. Biopathologic and clinical features of the patients were analyzed and compared between MI and MA groups. Patients with recurrence were compared with those without recurrence to identify risk factors for recurrence.
RESULTS: Thirty-seven patients showed MI and the other 53 patients revealed MA in the lymph node; the incidence of patients with MI and MA was 41.1% and 58.9%. The main tumor burden was 0.9 and 4.6 mm in the representative metastatic node, respectively. Japanese N2 stations were more frequently involved in MA group (20.9%) than in MI group (10.3%) but the difference was not statistically different (P = 0.338). Proliferative and apoptotic activities of MI were decreased than those of MA (26.7% vs 40.5%, P = 0.004 and 1.0% vs 3.0%, P < 0.001, respectively). However, nodal MI in the current study showed a relatively high proliferative activity and an equivalent apoptotic activity compared to other cancers in the previously published studies. Recurrence was observed in 6 patients during the mean follow up period of 87.6 ± 26.2 mo. The recurrence was significantly associated with the presence of MA (P = 0.041) and lymphovascular invasion of the primary tumor (P = 0.032).
CONCLUSION: Lymphadenectomy of D1 + β or more might be necessary in patients with MI in sentinel node to prevent recurrence by clearing MI involving Japanese N2 station.
Keywords: Early gastric cancer, Sentinel node biopsy, Lymphadenectomy, Micrometastasis, Macrometastasis
Core tip: Nodal micrometastasis in early gastric cancer (EGC) has a relatively high proliferative and an equivalent apoptotic activities compared to other cancers. The incidence of Japanese N2 station micrometastasis involvement is about 10%. Lymphadenectomy of D1+β or more might be necessary if micrometastasis is identified during sentinel node biopsy in EGC.
INTRODUCTION
Nodal metastasis is the one of the important prognostic factors as along with the depth of invasion of the primary tumor and distant metastasis in solid cancers. Nodal metastasis is classified into isolated tumor cell (ITC), micrometastsis (MI) and macrometastasis (MA) depending on the size of metastatic deposit in the lymph node according to the 6th edition of International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) staging system[1]. This classification system was developed through histological examinations, such as immunohistochemisty (IHC), of melanoma and breast cancer during sentinel node biopsy (SNB). However, there are still controversies regarding the clinical significance of MI in a variety of tumors including gastric cancer even though it was favored as a significant prognostic factor[2].
MI was considered as a state of dormancy, showing a balance between proliferation and apoptosis without vascular formation, but causing the recurrence after a prolonged period[3,4]. This hypothesis was evident in animal models and human melanoma and breast cancer[5-7]. However, such biologic information on gastric cancer is very limited with data on proliferative activity only[8,9].
Even though SNB is now performed as a practice for limited lymphadenectomy in melanoma and breast cancer, it has not yet been applied to gastric cancer due to unsatisfactory and heterogeneous sensitivity between practicing surgeons with currently available techniques[10]. However, a recently presented prospective multicenter feasibility trial of SNB in gastric cancer showed optimistic results[11]. A single center’s observational study after applying SNB in early gastric cancer (EGC) also showed promising results in regard to short and long term results[12]. Based on these results, multicenter phase III trial is now planning and quality control studies for it is now underway[13,14]. One of the controversies of SNB application in EGC is the decision of whether radical gastrectomy with lymphadenectomy should be done after detection of MI in the SN[15]. In breast cancer, this issue was confirmed by clinical trials that no further surgical treatment is needed in the case of MI in SNs[16,17]. Applying this approach to gastric cancer is controversial and thus investigation on the clinical significance of MI in EGC should be performed before commencing clinical practice.
The aim of this study was to evaluate the biopathologic features and clinical significance of nodal MI in EGC patients and assess the surgical strategy in these patients during application of SNB.
MATERIALS AND METHODS
Patients and eligibility
Gastrectomy with lymphadenectomy of D1 + β or more was performed in 1022 EGC patients except for cases with an absolute indication of endoscopic resection from March 2001 to December 2005 at the Korean National Cancer Center according to the Japanese guidelines[18]. The final pathology was pT1N0 in 896 (87.7%), pT1N1 in 107 (10.5%), pT1N2 in 16 (1.6%), and pT1N3 in 3 (0.3%) according to the 6th UICC/AJCC staging system[1]. For clinical similarity of metastatic SN, patients with pT1N1 were enrolled in the study. However, tissues of nodal metastasis and primary tumor were available only in 90 of 107 pT1N1 EGC patients. The enrolled patients were divided into MI and MA groups by pathologic findings of metastatic nodes according to the 6th UICC/AJCC staging system, and the findings were compared with each other. Adjuvant chemotherapy of 5-Fluorouracil (5-FU) based regimen was performed in node-positive patients with agreement. The mean follow up period of these 90 patients was 87.6 ± 26.2 mo. Patient recruitment and sample collections were performed according to the study protocol approved by the Institutional Review Board, and informed consent was obtained from all patients (NCCNCS-09-231).
Immunohistochemical stain with cytokeratin and Ki-67
The presence of nodal metastasis was confirmed by IHC for cytokeratin and proliferative activity was measured by IHC for Ki-67 according the previous study[19]. Briefly, primary tumor and metastatic lymph nodes were stored in paraffin-embedded block and then tissue sections of 3 μm in thickness were made. The sections were deparaffinized in xylene, rehydrated through a graded series of alcohol, washed in distillated water and heated twice in a microwave oven for 15 min each at 700 W in 10 mmol/L citrate buffer, pH 6.0 or pH 9.0 to retrieve antigen. After this, it was cooled to room temperature (15-30 min). The activity of endogenous peroxidase was blocked by methanol containing 0.3% H2O2 for 10 min and then washed with 0.01 mol/L phosphate buffered saline (PBS). After blocking with 1% normal goat serum for 20 min at room temperature in a humidified chamber, the sections were incubated with primary antibody for 1.5 h at room temperature. The following primary antibodies were used: mouse monoclonal anti-human Ki-67 (clone MIB-1, 1:50) and mouse monoclonal anti-human cytokeratin (clone AE1/AE3, 1:100). After washing in PBS, the specimens were incubated with a biotinylated conjugated-HRP polymer Kit (Super picture, invitrogen, Carlsbad, California) for 30 min at room temperature. As the final step, the slides were developed for 10 min with enzyme substrate 3 and 3-diaminobenzidine (DAB) solution (0.001 mol/L DAB, 0.05 mol/L Tris-HCI buffer, pH 7.6, 0.01 mol/L sodium azide, and 0.006% hydrogen peroxidase). The slides were counterstained with hematoxylin solution for 1 min (DAKO, copenhagen, Denmark). After dehydration, the tissue was sealed with a universal mount (Research Genetics, Huntsville, AL). Controls were prepared in the same manner as detailed for the experimental group, except for the incubation process with primary antibody.
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay
Apoptotic activity was determined in situ from the paraffin embedded tissue sections by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using the DeadEnd™ Colorimetric TUNEL system (Promega, Madison, WI, United States). The specimens were deparaffinized and gradually hydrated, rinsed with cold 1× PBS, and the sections were fixed in 4% paraformaldehyde for 15 min, followed by incubation with proteinase K (20 μg/mL in 10 mmol/L Tris-HCl, pH 8.0) for 20 min. After washing twice, the sections were equilibrated at room temperature for 10 min in equilibration buffer (200 mmol/L potassium cacodylate, 0.2 mmol/L dithiothreitol, 0.25 g/L bovine serum albumin, and 2.5 mmol/L cobalt chloride in 25 mmol/L Tris-HCL, pH 6.6) and then the slides were covered with the terminal deoxynucleotidyl transferase (TdT) enzyme in a TdT labeling reaction mixture (equilibration buffer, biotinylated nucleotide mix, rTdT enzyme = 8:1:1) for 1 h at 37 °C in the dark. The tailing reaction was terminated by 2 × standard saline citrate. The sections were washed three times in PBS and then immersed for 10 min in 0.3% H2O2 to block endogenous peroxidase at room temperature. After washing, the sections were subsequently incubated with peroxidase-labeled streptavidin for 30 min at room temperature. Peroxidase activity was visualized with a DAB color reaction and the slides were counterstained with Mayers’ hematoxylin, dehydrated, and mounted. After mounting, the sections were observed under a microscope. Positive control sections were treated with 1 μg/mL DNase I (Sigma, St. Louis, MO) for 10 min before treatment with TdT buffer. Negative control sections were treated by substituting distilled water for TdT in the reaction mixture.
Pathologic evaluation
Classification of nodal metastasis was done according to the 6th UICC/AJCC staging system[1]. The main tumor burden with a diameter of no greater than 0.2 mm was defined as ITC, greater than 0.2 mm but no greater than 2 mm as MI, and finally, greater than 2 mm as MA of the representative metastatic node. The location and pattern of nodal metastasis were classified according to previous studies on melanoma and gastric cancer[20,21]. The location of nodal tumor was classified as marginal sinus, intermediate, parenchymal or diffuse type. The pattern of nodal tumor was classified as single cluster, multiple clusters, or diffuse type. Proliferative activity measured by Ki-67 reactivity and apoptotic activity measured by TUNEL assay were defined as the percentage of positive tumor cells per 500 observed tumor cells in the most intensively reacted area. If the number of tumor cells was less than 500, the total tumor cell count itself was used as the denominator.
Statistical analysis
Continuous variables were compared using the Student t test or Mann-Whitney U test according to the sample size of comparing groups. The χ2 test or Fisher’s exact test was used for comparing categorical variables as the above principle. A scattered plot was created with Pearson’s correlation coefficient for proliferative and apoptotic activities of primary and metastatic nodal tumors. P values were two sided and values of < 0.05 were considered statistically significant. All data were analyzed using SAS version 9 (SAS Institute Inc., Cary, NC) and interpreted by a biostatistics specialist.
RESULTS
Clinicopathologic features of enrolled patients
The incidence of patients with MI and MA was 41.1% and 58.9% with the mean main tumor burden of 0.9 and 4.6 mm, respectively (P < 0.001) (Table 1). Japanese N2 station involvement in MI and MA was 10.3% and 20.9%, respectively. The location of nodal tumor in the MA group was mostly at the non marginal sinus and this finding was significantly different with the MI group (P < 0.001). The pathologic features regarding the primary tumor was not different between the two groups. All the clinical features were not different between the two groups except recurrence which occurred in 6 patients of the MA group (P < 0.001).
Table 1.
Clinicopathologic features according to the classification of lymph node metastasis (n = 90)
| Micrometastasis | Macrometastasis | P value | |
| (n = 37) | (n = 53) | ||
| Age (yr) | 56.9 ± 12.9 | 59.2 ± 11.1 | 0.377 |
| Sex | 0.665 | ||
| Male | 22 (59.5) | 34 (64.2) | |
| Female | 15 (40.5) | 19 (35.8) | |
| Depth of invasion | 0.966 | ||
| Mucosa | 5 (13.5) | 7 (13.2) | |
| Submucosa | 32 (86.5) | 46 (86.8) | |
| Tumor size (cm) | 4.7 ± 2.8 | 4.5 ± 2.0 | 0.652 |
| Histology | 0.522 | ||
| Differentiated | 18 (48.6) | 30 (56.6) | |
| Undifferentiated | 19 (51.4) | 23 (43.4) | |
| Lauren classification | 0.162 | ||
| Intestinal | 19 (51.4) | 35 (66.0) | |
| Diffuse, mixed | 18 (48.6) | 18 (34.0) | |
| Lymphovascular invasion | 0.832 | ||
| Absent | 17 (45.9) | 23 (43.4) | |
| Present | 20 (54.1) | 30 (56.6) | |
| Metastatic LNs (n) | 1.7 ± 1.3 | 2.2 ± 1.3 | 0.085 |
| Japanese N2 station involvement1 | 0.338 | ||
| No | 26 (89.7) | 34 (79.1) | |
| Yes | 3 (10.3) | 9 (20.9) | |
| Main tumor burden in LN (mm) | 0.9 ± 0.5 | 4.6 ± 4.6 | < 0.001 |
| Pattern of metastasis in LNs | 0.694 | ||
| Single cluster | 14 (37.8) | 18 (34.0) | |
| Multiple cluster | 22 (59.5) | 33 (62.3) | |
| Diffuse | 1 (2.7) | 2 (3.8) | |
| Location of metastasis in LNs | < 0.001 | ||
| Marginal sinus | 17 (45.9) | 1 (1.9) | |
| Non marginal sinus | 20 (54.1) | 52 (98.1) | |
| Gastric resection | 0.423 | ||
| Open subtotal | 27 (73.0) | 40 (75.5) | |
| Open total | 2 (5.4) | 6 (11.3) | |
| LADG | 8 (21.6) | 7 (13.2) | |
| Lymph node dissection | 0.261 | ||
| D1 + β | 15 (40.5) | 15 (28.3) | |
| D2 | 22 (59.5) | 38 (71.7) | |
| Dissected LNs (n) | 36.2 ± 11.1 | 38.6 ± 15.6 | 0.424 |
| Adjuvant chemotherapy | 0.503 | ||
| No | 11 (29.7) | 20 (37.7) | |
| Yes | 26 (70.3) | 33 (62.3) | |
| Recurrence | 0.041 | ||
| Absent | 37 (100) | 47 (88.7) | |
| Present | 0 (0) | 6 (11.3) |
Available data only. Data are expressed as absolute numbers (percentage) or mean ± SD. LN: Lymph node; LADG: Laparoscopically assisted distal gastrectomy.
Biologic features of primary and metastatic nodal tumors
Representative microscopic photos of IHC with cytokeratin, Ki-67 and TUNEL assay are presented in Figure 1. Proliferative and apoptotic activities of MI were significantly decreased than those of MA among the examined tissues (26.7% vs 40.5%, P = 0.004 and 1.0% vs 3.0%, P < 0.001, respectively) (Table 2). The proliferative activity of the primary tumor was not different but the apoptotic activity was different between the two groups. There was a significant correlation between proliferative and apoptotic activities in both the primary tumor and nodal metastasis. Furthermore, both the proliferative and apoptotic activities of nodal metastasis were well correlated to those of the primary tumor (Figure 2).
Figure 1.
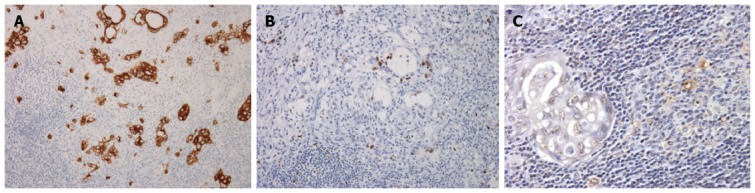
Representative microscopic photos of immunohistochemistry with cytokeratin (A), Ki-67 (B) and terminal deoxynucleotidyl transferase dUTP nick end labeling assay (C).
Table 2.
Biologic features according to the classification of lymph node metastasis (n = 90)
| Micrometastasis | Macrometastasis | P value | |
| Ki-67 (primary tumor) | |||
| Examed tissue | 33 | 51 | |
| Positive cell | 59.3% ± 24.0% | 62.3% ± 20.9% | 0.553 |
| TUNEL (primary tumor) | |||
| Examed tissue | 30 | 51 | |
| Positive cell | 2.4% ± 1.7% | 4.5% ± 4.4% | 0.004 |
| Ki-67 (lymph node) | |||
| Examed tissue | 33 | 50 | |
| Positive cell | 26.7% ± 18.0% | 40.5% ± 24.1% | 0.004 |
| TUNEL (lymph node) | |||
| Examed tissue | 25 | 51 | |
| Positive cell | 1.0% ± 1.0% | 3.0% ± 3.5% | < 0.001 |
Data are expressed as absolute numbers (percentage) or mean ± SD. TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Figure 2.
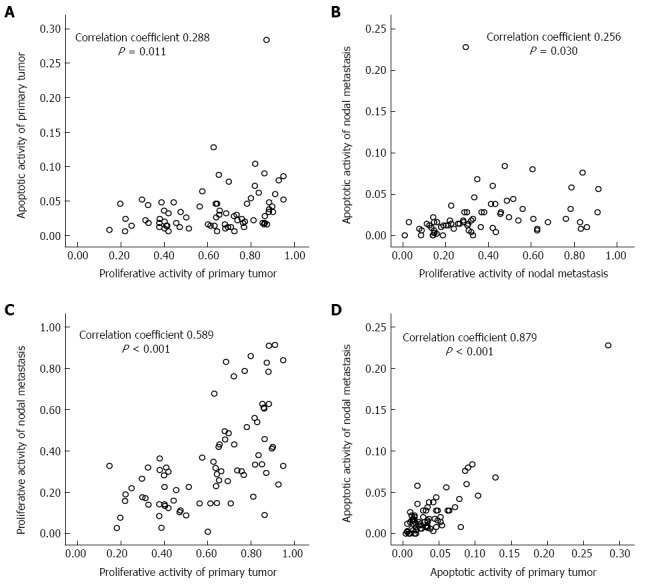
Correlation of proliferative and apoptotic activities in the primary tumor (A), nodal metastasis (B) and with each other (C, D).
Recurrence and associated factors
All 6 recurrent cases had MA and lymphovascular invasion (LVI) of the primary tumor. However, patients without recurrence showed 56.0% MA and 52.4% positive LVI. These factors were statistically significant for recurrence (P = 0.041, P = 0.032, respectively) (Table 3). Biologic features of proliferative and apoptotic activities in the primary tumor and nodal metastasis were not significant. The details of recurrent patients are shown in Table 4. The sites of recurrence were locoregional, hematogenous and peritoneal as well known patterns of gastric cancer. The number of harvested lymph nodes was only 14 even though D1 + β lymphadectomy was done in case 3.
Table 3.
Recurrence and associated factors (n = 90)
| No recurred (n = 84) | Recurred (n = 6) | P value | |
| Age (yr) | 57.8 ± 12.0 | 64.8 ± 7.4 | 0.159 |
| Sex | 1.000 | ||
| Male | 52 (61.9) | 4 (66.7) | |
| Female | 32 (38.1) | 2 (33.3) | |
| Depth of invasion | 1.000 | ||
| Mucosa | 12 (14.3) | 0 (0) | |
| Submucosa | 72 (85.7) | 6 (100) | |
| Tumor size (cm) | 4.7 ± 2.5 | 3.7 ± 1.27 | 0.321 |
| Histology | 0.681 | ||
| Differentiated | 44 (52.4) | 4 (66.7) | |
| Undifferentiated | 40 (47.5) | 2 (33.3) | |
| Lauren | 0.396 | ||
| Intestinal | 49 (58.3) | 5 (83.3) | |
| Diffuse, mixed | 35 (41.7) | 1 (16.7) | |
| Lymphovascular invasion | 0.032 | ||
| Absent | 40 (47.6) | 0 (0) | |
| Present | 44 (52.4) | 6 (100) | |
| Metastatic LNs (n) | 2.0 ± 1.3 | 2.2 ± 1.2 | 0.816 |
| Classification of nodal metastasis | 0.041 | ||
| Micrometastasis | 37 (44.0) | 0 (0) | |
| Macrometastasis | 47 (56.0) | 6 (100) | |
| Japanese N2 station involvement1 | 0.127 | ||
| No | 58 (85.3) | 2 (50.0) | |
| Yes | 10 (14.7) | 2 (50.0) | |
| Main tumor burden in LN (mm) | 3.0 ± 4.1 | 4.0 ± 1.5 | 0.594 |
| Pattern of metastasis in LNs | 0.450 | ||
| Single cluster | 28 (33.3) | 4 (66.7) | |
| Multiple cluster | 54 (64.3) | 1 (16.7) | |
| Diffuse | 2 (2.4) | 1 (16.7) | |
| Location of metastasis in LNs | 0.090 | ||
| Marginal sinus | 31 (36.9) | 0 (0) | |
| Non marginal sinus | 53 (63.1) | 6 (100) | |
| Gastric resection | 1.000 | ||
| Open subtotal | 63 (75.0) | 4 (66.7) | |
| Open total | 7 (8.3) | 1 (16.7) | |
| LADG | 14 (16.7) | 1 (16.7) | |
| Lymph node dissection | 1.000 | ||
| D1 + β | 28 (33.3) | 2 (33.3) | |
| D2 | 56 (66.7) | 4 (66.7) | |
| Dissected LNs (n) | 37.4 ± 13.8 | 41.0 ± 16.4 | 0.538 |
| Adjuvant chemotherapy | 0.660 | ||
| No | 30 (35.7) | 1 (16.7) | |
| Yes | 54 (64.3) | 5 (83.3) | |
| Ki-67 (primary tumor)1 | |||
| Positive cell | 61.3% ± 22.1% | 58.3% ± 24.6% | 0.748 |
| TUNEL (primary tumor)1 | |||
| Positive cell | 3.6% ± 3.9% | 4.6% ± 2.2% | 0.552 |
| Ki-67 (lymph node)1 | |||
| Positive cell | 35.0% ± 23.5% | 35.0% ± 10.8% | 0.996 |
| TUNEL (lymph node)1 | |||
| Positive cell | 2.3% ± 3.2% | 2.8% ± 1.1% | 0.720 |
Available data only. Data are expressed as absolute numbers (percentage) or mean ± SD. LN: Lymph node; LADG: Laparoscopically assisted distal gastrectomy; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Table 4.
Details of recurrent cases with pT1N1 in gastric cancer
| Case | Age (yr) | Sex | T depth | T size | Histology | Lauren | LVI | Number of metastatic LNs | Size of metastasis (mm) | Pattern | Location | Tumor burden (mm) | Proportion | Extent of LND | Number of dissected LNs | Site of recurrence |
| 1 | 62 | M | Sm3 | 2.3 | Undiff | Diffuse | Present | 2 | 2.3 | Single cluster | Parenchyme | 2.5 | 40.0% | D1 + β | 31 | N3 LN |
| 2 | 62 | M | Sm3 | 3.0 | Diff | Intestinal | Present | 2 | 3.1 | Single cluster | Parenchyme | 3.88 | 26.0% | D2 | 59 | Liver |
| 3 | 72 | M | Sm3 | 3.0 | Diff | Intestinal | Present | 1 | 3.6 | Diffuse | Diffuse | 9.62 | 65.0% | D1 + β | 14 | Peritoneum |
| 4 | 59 | M | Sm3 | 3.0 | Diff | Intestinal | Present | 4 | 5.5 | Single cluster | Parenchyme | 10.8 | 45.0% | D2 | 45 | Liver, lung |
| 5 | 76 | F | Sm2 | 5.0 | Diff | Intestinal | Present | 1 | 6.0 | Single cluster | Parenchyme | 6.0 | 67.0% | D2 | 54 | Liver |
| 6 | 58 | F | Sm3 | 5.5 | Undiff | Intestinal | Present | 3 | 3.2 | Multiple cluster | Parenchyme | 7.2 | 87.0% | D2 | 43 | PALN |
LVI: Lymphovascular invasion; LN: Lymph node; LND: Lymph node dissection; PALN: Para-aortic lymph node; Diff: Differentiate; Undiff: Undifferentiate.
DISCUSSION
The screening program of gastric cancer for early detection is well established in Asian countries, especially in South Korea and Japan[22,23]. As the proportion of EGC has increased, the biopathological and clinical features of nodal MI in EGC patients have gained attention due to the development of minimally invasive surgery, such as endoscopic submucosal dissection, SNB oriented tailored approach and laparoscopic surgery, in these patients[13,24]. Even though SNB in EGC is not routinely practiced, it is controversial whether lymphadenectomy of D1 + β or more should be performed if MI is detected in SNs[15]. Most nodal MIs in EGC have been studied by comparing its prognostic significance in pN0 patients, and the results are still controversial[15]. However, the present study compared nodal MIs with MAs in pN1 patients to assess the therapeutic strategy. In the present study, we revealed that the nodal MI of EGC has a relatively high proliferative activity and an equivalent apoptotic activity compared to other cancers. Moreover, about 10% of nodal MIs were located at Japanese N2 station. Uncleared lymph nodes at Japanese N2 station in MI, patients may progress to MA and recurrence because most of SNs were located along Japanese N1 station.
The fate of MI in the lymph node is controversial as to whether they will progress to overt metastasis or regress spontaneously by the human immune system. Several animal studies have been reported concerning this issue but direct human evidence is scanty[25,26]. The biology of MI in melanoma and breast cancer was interpreted as a concept of being in a balanced dormant state between proliferation and apoptosis before tumor vascularization[3,4]. Reported proliferative and apoptotic activities were 2.4%-12% and 0.2%-0.7% in melanoma and breast cancer, respectively[5-7]. However, the present study showed inconsistent results with melanoma or breast cancer due to high proliferative activity and equivalent apoptotic activity of nodal MI in EGC. The more aggressive biological nature of nodal MI in gastric cancer is indirectly reflected as the survival difference between various cancer types[27]. Most of recurrence in gastric cancer occur within 2-3 postoperative years representing the difference with hypothesis of dormancy. The correlation of proliferative and apoptotic activities in the primary tumor and nodal metastasis also indirectly represents the different biology of gastric cancer with melanoma and breast cancer. The meaning of apoptosis does not only include cell loss but also represents proliferative activity[28,29].
Most studies concerning proliferative and apoptotic activities in gastric cancer were performed in primary tumors rather than in nodal metastasis. Data regarding the biopathologic findings of nodal metastasis are very few in gastric cancer. Yonemura et al[8] reported that the proliferative activity was 46.6% in ITC of EGC and concluded that ITC has a poor prognosis. Yanagita et al[9] reported that the proliferative activity was 29% in ITC, 92% in MI, and 96% with MA and concluded that ITC and MI should be removed during SNB. They used IHC with anti Ki-67 antibody, as was the case this study, but they did not measure apoptotic activity in nodal metastasis. Apoptotic activity should be measured to estimate the fate of nodal MI combined with the proliferative activity. Variability of proliferative activity measured by Ki-67 in nodal metastasis between these studies and our study might be from several issues such as different handling techniques of tissue samples, subjective nature of IHC and technical diversity[30]. However, the common finding of all of these studies is that a significant proliferative activity is present in nodal MI, even in ITC.
Previous studies with melanoma reported that the tumor burden in SNs is well correlated with the involvement of non-SN and survival[31-33]. The Rotterdam criteria simply measures SN tumor burden by the maximum diameter (in any direction) of the largest lesion. In this study, the main tumor burden in EGC had no clinical significance in terms of recurrence unlike melanoma. However, recurrence was observed only in the MA group. Another important factor for recurrence was determined as LVI in this study. LVI is a well known prognostic factor in gastric cancer[34,35]. Recurrence was not observed in MI group probably because the enrolled patients already received lymphadenectomy of D1 + β or more and this finding offer the indirect suggestion about the surgical strategy when we identified the MI in SN.
Other important factor predicting non SN involvement is the location of metastasis in SNs[20,21]. The location of MI in the parenchyma of SN is significantly related with non SN involvement in melanoma and EGC studies. In this study, a similar finding of non SN involvement could not be proven but the fact that MA had less marginal sinus location than MI indirectly implies disease progression in the lymph node from MI to MA.
For the evaluation of biopathologic and clinical significance of nodal MI and assessment of surgical strategy during SNB, we should have used tissues and information of patients who experienced SNB with EGC at our institution[36]. However, obtaining available tissues from patients for SNB was very limited in our study. Thus, as a second choice we used tissue of pT1N1 patients that simulated the positive SNB results. Therefore, the interpretation of the results of this study has some limitations.
In conclusion, nodal MI in EGC patients has a relatively high proliferative activity and an equivalent apoptotic activity compared to other cancers. Also, not a few patients had Japanese N2 station MI involvement. Therefore, if MI is identified during SNB in EGC, lymphadenectomy of D1 + β or more may be necessary to prevent recurrence by clearing MI involving Japanese N2 station.
COMMENTS
Background
Nodal metastasis is the one of the important prognostic factors along with the depth of invasion of the primary tumor and distant metastasis in solid cancers. However, there are still controversies regarding the clinical significance of micrometastasis (MI) in a variety of tumors including gastric cancer. Surgical strategy in early gastric cancer (EGC) with nodal MI is controversial during the sentinel node biopsy due to the lack of biopathologic and clinical data.
Research frontiers
The current study aimed to evaluate the biopathologic features and clinical significance of nodal MI in EGC patients and assess the surgical strategy in these patients during application of sentinel node biopsy.
Innovations and breakthroughs
Nodal micrometastasis in EGC has a relatively high proliferative and an equivalent apoptotic activities compared to other cancers such as breast cancer or melanoma. The incidence of Japanese N2 station micrometastasis involvement is about 10%.
Applications
Lymphadenectomy of D1 + β or more might be necessary if micrometastasis is identified during sentinel node biopsy in EGC.
Terminology
The main tumor burden with a diameter of no greater than 0.2 mm was defined as isolated tumor cell, greater than 0.2 mm but no greater than 2 mm as MI, and finally, greater than 2 mm as macrometastasis of the representative metastatic node according to the 6th International Union Against Cancer/American Joint Committee on Cancer staging system.
Peer review
In this retrospective study, the authors assessed quite a large number of gastrectomy cases to identify factors associated with nodal metastasis for early gastric cancer. They classified nodal metastasis into 2 groups; micrometastasis and macrometastasis based on the main tumor burden. They assessed proliferative and apoptotic activities of the primary tumor by immunohistochemical staining. From their results, nodal micrometastasis showed a relatively high proliferative activity and an equivalent apoptotic activity. They concluded that extensive lymphadenectomy might be necessary in patients with micrometastasis to prevent recurrence. This is a carefully done study and the findings are of considerable interest.
Footnotes
Supported by Grants from the National Cancer Center, Republic of Korea, Grant No. 0910560-1 and No. 1010490-1.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 13, 2014
First decision: July 9, 2014
Article in press: July 25, 2014
P- Reviewer: Espinel J, Muguruma N S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Greene FL. AJCC cancer staging manual. New York: Springer; 2002. [Google Scholar]
- 2.Kell MR, Winter DC, O’Sullivan GC, Shanahan F, Redmond HP. Biological behaviour and clinical implications of micrometastases. Br J Surg. 2000;87:1629–1639. doi: 10.1046/j.1365-2168.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 3.Crowley NJ, Seigler HF. Relationship between disease-free interval and survival in patients with recurrent melanoma. Arch Surg. 1992;127:1303–1308. doi: 10.1001/archsurg.1992.01420110045011. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer A. Dormancy and breast cancer. J Surg Oncol. 1990;43:181–188. doi: 10.1002/jso.2930430312. [DOI] [PubMed] [Google Scholar]
- 5.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 6.Barnhill RL, Piepkorn MW, Cochran AJ, Flynn E, Karaoli T, Folkman J. Tumor vascularity, proliferation, and apoptosis in human melanoma micrometastases and macrometastases. Arch Dermatol. 1998;134:991–994. doi: 10.1001/archderm.134.8.991. [DOI] [PubMed] [Google Scholar]
- 7.Klauber-DeMore N, Van Zee KJ, Linkov I, Borgen PI, Gerald WL. Biological behavior of human breast cancer micrometastases. Clin Cancer Res. 2001;7:2434–2439. [PubMed] [Google Scholar]
- 8.Yonemura Y, Endo Y, Hayashi I, Kawamura T, Yun HY, Bandou E. Proliferative activity of micrometastases in the lymph nodes of patients with gastric cancer. Br J Surg. 2007;94:731–736. doi: 10.1002/bjs.5604. [DOI] [PubMed] [Google Scholar]
- 9.Yanagita S, Natsugoe S, Uenosono Y, Kozono T, Ehi K, Arigami T, Arima H, Ishigami S, Aikou T. Sentinel node micrometastases have high proliferative potential in gastric cancer. J Surg Res. 2008;145:238–243. doi: 10.1016/j.jss.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Ryu KW, Eom BW, Nam BH, Lee JH, Kook MC, Choi IJ, Kim YW. Is the sentinel node biopsy clinically applicable for limited lymphadenectomy and modified gastric resection in gastric cancer? A meta-analysis of feasibility studies. J Surg Oncol. 2011;104:578–584. doi: 10.1002/jso.21995. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–3710. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 12.Ichikura T, Sugasawa H, Sakamoto N, Yaguchi Y, Tsujimoto H, Ono S. Limited gastrectomy with dissection of sentinel node stations for early gastric cancer with negative sentinel node biopsy. Ann Surg. 2009;249:942–947. doi: 10.1097/SLA.0b013e3181a77e7e. [DOI] [PubMed] [Google Scholar]
- 13.Ryu KW. The future of sentinel node oriented tailored approach in patients with early gastric cancer. J Gastric Cancer. 2012;12:1–2. doi: 10.5230/jgc.2012.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01544413.
- 15.Arigami T, Uenosono Y, Yanagita S, Nakajo A, Ishigami S, Okumura H, Kijima Y, Ueno S, Natsugoe S. Clinical significance of lymph node micrometastasis in gastric cancer. Ann Surg Oncol. 2013;20:515–521. doi: 10.1245/s10434-012-2355-x. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432; discussion 432-433. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 19.Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 20.Dewar DJ, Newell B, Green MA, Topping AP, Powell BW, Cook MG. The microanatomic location of metastatic melanoma in sentinel lymph nodes predicts nonsentinel lymph node involvement. J Clin Oncol. 2004;22:3345–3349. doi: 10.1200/JCO.2004.12.177. [DOI] [PubMed] [Google Scholar]
- 21.Yanagita S, Natsugoe S, Uenosono Y, Arima H, Kozono T, Ehi K, Arigami T, Higashi H, Aikou T. Morphological distribution of metastatic foci in sentinel lymph nodes with gastric cancer. Ann Surg Oncol. 2008;15:770–776. doi: 10.1245/s10434-007-9713-0. [DOI] [PubMed] [Google Scholar]
- 22.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 23.Lee KS, Oh DK, Han MA, Lee HY, Jun JK, Choi KS, Park EC. Gastric cancer screening in Korea: report on the national cancer screening program in 2008. Cancer Res Treat. 2011;43:83–88. doi: 10.4143/crt.2011.43.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyashiro I. What is the problem in clinical application of sentinel node concept to gastric cancer surgery? J Gastric Cancer. 2012;12:7–12. doi: 10.5230/jgc.2012.12.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata H, Arai T, Soejima Y, Suzuki H, Ishii H, Hibi T. Limited capability of regional lymph nodes to eradicate metastatic cancer cells. Cancer Res. 2004;64:8239–8248. doi: 10.1158/0008-5472.CAN-04-1182. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama H, Nakanishi H, Kodera Y, Ikehara Y, Ohashi N, Ito Y, Koike M, Fujiwara M, Tatematsu M, Nakao A. Biological significance of isolated tumor cells and micrometastasis in lymph nodes evaluated using a green fluorescent protein-tagged human gastric cancer cell line. Clin Cancer Res. 2006;12:361–368. doi: 10.1158/1078-0432.CCR-05-1963. [DOI] [PubMed] [Google Scholar]
- 27.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 28.Tsamandas AC, Kardamakis D, Tsiamalos P, Liava A, Tzelepi V, Vassiliou V, Petsas T, Vagenas K, Zolota V, Scopa CD. The potential role of Bcl-2 expression, apoptosis and cell proliferation (Ki-67 expression) in cases of gastric carcinoma and correlation with classic prognostic factors and patient outcome. Anticancer Res. 2009;29:703–709. [PubMed] [Google Scholar]
- 29.Aizawa K, Ueki K, Suzuki S, Yabusaki H, Kanda T, Nishimaki T, Suzuki T, Hatakeyama K. Apoptosis and Bbcl-2 expression in gastric carcinomas: correlation withclinicopathological variables, p53 expression, cell proliferation and prognosis. Int J Oncol. 1999;14:85–176. doi: 10.3892/ijo.14.1.85. [DOI] [PubMed] [Google Scholar]
- 30.Arciero CA. Ki-67 proliferation index and gastric cancer: answers or more questions. J Surg Oncol. 2010;102:199–200. doi: 10.1002/jso.21626. [DOI] [PubMed] [Google Scholar]
- 31.van Akkooi AC, de Wilt JH, Verhoef C, Schmitz PI, van Geel AN, Eggermont AM, Kliffen M. Clinical relevance of melanoma micrometastases (< 0.1 mm) in sentinel nodes: are these nodes to be considered negative? Ann Oncol. 2006;17:1578–1585. doi: 10.1093/annonc/mdl176. [DOI] [PubMed] [Google Scholar]
- 32.van Akkooi AC, Nowecki ZI, Voit C, Schäfer-Hesterberg G, Michej W, de Wilt JH, Rutkowski P, Verhoef C, Eggermont AM. Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248:949–955. doi: 10.1097/SLA.0b013e31818fefe0. [DOI] [PubMed] [Google Scholar]
- 33.Gershenwald JE, Andtbacka RH, Prieto VG, Johnson MM, Diwan AH, Lee JE, Mansfield PF, Cormier JN, Schacherer CW, Ross MI. Microscopic tumor burden in sentinel lymph nodes predicts synchronous nonsentinel lymph node involvement in patients with melanoma. J Clin Oncol. 2008;26:4296–4303. doi: 10.1200/JCO.2007.15.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, Jhangri GS, Saunders LD, Damaraju S, Cass C. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243:64–73. doi: 10.1097/01.sla.0000194087.96582.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunisaki C, Makino H, Kimura J, Takagawa R, Kosaka T, Ono HA, Akiyama H, Fukushima T, Nagahori Y, Takahashi M. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery. 2010;147:204–211. doi: 10.1016/j.surg.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Ryu KW, Nam BH, Kook MC, Cho SJ, Lee JY, Kim CG, Choi IJ, Park SR, Kim YW. Factors associated with detection failure and false-negative sentinel node biopsy findings in gastric cancer: results of prospective single center trials. J Surg Oncol. 2009;99:137–142. doi: 10.1002/jso.21222. [DOI] [PubMed] [Google Scholar]


