Abstract
Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas (UCOGCP) is an unusual pancreatic neoplasm that represents < 1% of all pancreatic malignancies. Moreover, the giant cells of UCOGCP morphologically resemble the benign giant cells of bone tumors. Due to the rarity of this tumor type, the histogenesis and biologic behavior of UCOGCP remain controversial. Here, we report a case of UCOGCP that exhibited an invasive growth pattern involving infiltration of the adjacent bowel loop and portal vein, as well as superior mesenteric vein thrombosis. The patient underwent a distal pancreatectomy with splenectomy and partial colectomy, followed by four cycles of gemcitabine chemotherapy. No evidence of recurrence was detected after ten years. In addition to this case, clinical information on other UCOGCP cases reported in the English literature is summarized.
Keywords: Undifferentiated carcinoma with osteoclast-like giant cells, Pancreas, Locally advanced
Core tip: Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas (UCOGCP) is an unusual pancreatic neoplasm and the histogenesis and biologic behavior of UCOGCP remain controversial. We report a case of locally advanced UCOGCP with infiltration of the adjacent colon and portal vein. Ten years after extended distal pancreatectomy with splenectomy and colectomy, the patient is still alive without any evidence of recurrence.
INTRODUCTION
Extraskeletal tumors containing multinucleated osteoclast-like giant cells (OGCs), which morphologically resemble those found in giant cell tumors of the bone, are uncommon. When they do develop, they are most frequently found in the pancreas and breast. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas (UCOGCP) was first described by Rosai[1], and this rare tumor currently accounts for < 1% of all pancreatic malignancies[2]. UCOGCP was formerly referred to as an OGC tumor, or pleomorphic carcinoma of the pancreas with OGCs. However, UCOGCP is now classified by the World Health Organization as a rare variant of ductal pancreatic adenocarcinoma (PAC), based on the epithelial origin of its OGCs[2]. Over the last decade, the number of reports of UCOGCP has increased. However, the clinical features of UCOGCP remain obscure as many cases are already advanced when detected. For example, at the time of diagnosis, > 80% of UCOGCP tumors are > 5 cm, and 50% are > 10 cm[2]. To date, the largest UCOGCP reported was 24.5 cm[3]. In addition, UCOGCP typically includes various degrees of hemorrhage and necrosis. Herein, we report a case of UCOGCP and review the cases previously published in the English literature in order to summarize the clinicopathologic characteristics which currently describe this rare neoplasm.
CASE REPORT
A previously healthy, 71-year-old female patient was admitted due to a one month history of epigastric pain and anorexia. A 15 cm × 13 cm mass was palpated in the left upper abdomen. Laboratory examination revealed moderate anemia (Hb, 86 g/L). In addition, levels of carbohydrate antigen (CA)19-9 were 42.9 U/mL (normal, < 37.0 U/mL), CA24-2 was 22.8 U/mL (normal, < 20 U/mL), and carcinoembryonic antigen (CEA) was 2.2 ng/mL (normal, < 5.0 ng/mL). Abdominal ultrasonography and contrast-enhanced computed tomography (CECT) revealed an approximately 13 cm × 11 cm irregular cystic and solid lesion in the left upper quadrant of the abdomen. The tumor appeared to extend from the body and tail of the pancreas, and exhibited strong vascularization with peripheral enhancement that was detected by CECT. The tumor had also infiltrated the splenic hilum and adjacent bowel loop, with the splenic vein obstructed by a thrombus or tumor thrombus. Furthermore, regional lymphadenopathy, ascites, and distant metastasis were not detected in preoperative examinations (Figure 1).
Figure 1.
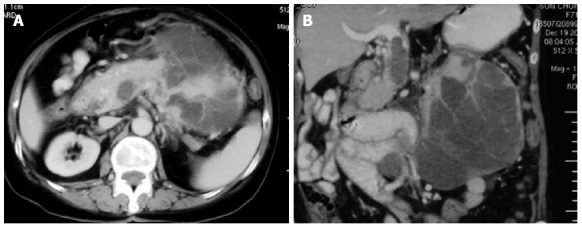
Computed tomography findings. A: Abdominal computed tomography showing a large heterogenous lesion extending from the body and tail of the pancreas with peripheral enhancement; B: Coronal section showing a thrombus or tumor thrombus in the portal vein and superior mesenteric vein.
During laparotomy, a large cystic and solid mass was found extending from the pancreatic body and tail, and the mass was densely adherent to the splenic hilum. The tumor had also invaded the transverse colon and partial jejunum, and extensive vascularization was observed on the surface of the tumor. The distal pancreas, spleen, and adjacent transverse colon and jejunum with their mesentery were resected en bloc. The splenic vein was also simultaneously opened for embolectomy. The operation time was 7 h and blood loss was 2000 mL in total.
Grossly, the resected mass was a multilocular cystic lesion measuring 17 cm along its longest dimension. On the cut surface, there was a cyst filled with necrotic and hemorrhagic content. On the inner aspect of the cyst wall, a firm nodule measuring 8 cm along its longest dimension was found (Figure 2A). Microscopically, the cyst was lined with mucinous epithelium with a few areas of high-grade dysplasia. The mural nodule consisted of variably pleomorphic cancer cells admixed with abundant and diffusely distributed multinucleated OGCs with a bland phenotype (Figure 2B). The pleomorphic cancer cells exhibited atypical, spindle-shaped, giant, and bizarre features, and these cells had densely proliferated in an inflammatory, necrotic stroma that encompassed a hemorrhagic pool. The spindle and giant cells had irregularly shaped nuclei, a thick nuclear membrane, large eosinophilic nucleoli, and abundant cytoplasm. Furthermore, these tumor cells had diffusely infiltrated into adjacent organ tissues, including the spleen, mesentery, and bowel. The OGCs lacked features of atypia, and occasionally were observed to have undergone phagocytosis of the atypical cells.
Figure 2.
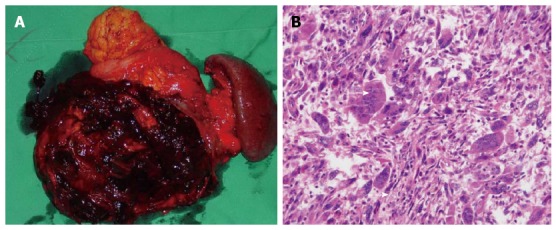
Gross pathology. A: Gross pathologic examination revealed a 17 cm × 12 cm mass in the pancreatic body and tail. The cut surface of the tumor included a cyst filled with necrotic and hemorrhagic content; B: Microscopically, a mixture of pleomorphic cancer cells and osteoclast-like giant cells were observed with hematoxylin and eosin staining (magnification × 20). Arrows indicate an osteoclast-like giant cell.
Immunohistologic examinations showed that the OGCs were strongly positive for the histiocytic marker, CD68, thereby confirming their origin from the mononuclear phagocytic system. In addition, OGCs were negative for p53, while neoplastic cells were positive for p53. No osteoid or lymph node metastasis was detected. Taken together, these results were consistent with a diagnosis of UCOGC, and according to the 6th edition of the TNM classification of the American Joint Committee on Cancer (2010), the tumor was pT3, N0, M0, stage IIB (Figure 3).
Figure 3.
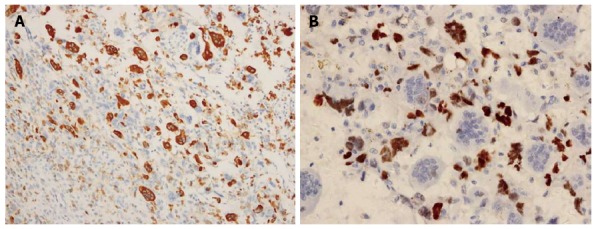
Immunohistochemical findings. Immunohistochemical assays detected CD68 expression in osteoclast-like giant cells (A) (magnification × 20), and p53 expression in epithelial neoplastic cells (B) (magnification × 40).
Overall, the patient remained stable throughout the operation and postoperatively, and had an uneventful recovery. The patient underwent adjuvant chemotherapy, which included four cycles of gemcitabine (1 g/m2 on days 1, 8, and 15). CECT was performed at least annually, and cavernous transformation of the portal vein was detected four months postoperatively. For the past ten years, the patient has done well, and metastasis or any other signs of tumor recurrence have not been detected.
DISCUSSION
Histologically, UCOGCP exhibits pathological features that differ from those of common ductal PAC. In particular, UCOGCP includes highly pleomorphic, frequently spindle-shaped neoplastic cells, and large multinucleated non-neoplastic OGCs. OGCs may develop from bone marrow-derived monocytes that are recruited into the tumor by chemoattractants. Furthermore, the growth pattern and cytologic features of OGCs closely resemble those of giant cell tumors of bone. In the present report, multinucleated, benign-appearing OGCs in the neoplasm were dispersed among infiltrating pleomorphic mononuclear or multinucleated cancer cells. Immunohistologically, the OGCs were positive for the histiomonocytic marker, CD68, and were negative for the epithelial marker, p53. In addition, immunoreactivity to p53 was strong in the cytoplasm of the pleomorphic cancer cells, yet was not detected in the OGCs. These findings confirm an epithelial origin for this UCOGCP, and demonstrate that the giant cell component was not neoplastic.
Based on a review of the English medical literature, UCOGCP usually presents in the sixth or seventh decade of life, although a wide range of patient ages has been reported (e.g., 32-82 years). Moreover, no gender predilection is associated with UCOGCP. The main signs and symptoms of UCOGCP include nonspecific upper abdominal pain, abdominal distension, a palpable mass, weight loss, fatigue, and anorexia. Patients with PAC also frequently present with jaundice. UCOGCP can arise from any portion of the pancreas, although it commonly develops from the body and tail of the pancreas. In contrast, ductal PAC usually involves the head of the pancreas. Similar to giant cell tumors of bone, UCOGCP is also an aggressive tumor that commonly invades adjacent organs. However, lymph node involvement and distant metastasis are rarely observed. UCOGCP also tends to be more extensively vascularized than other cystic neoplasms of the pancreas. The sensitivity of abdominal ultrasonography, CECT, magnetic resonance imaging (MRI), and endoscopic ultrasonography (EUS) for the detection of UCOGCP are similar to those for the detection of PAC. However, while ductal PAC appears hypovascular on contrast computed tomography (CT) scans, UCOGCP appears hypervascular, which is possibly related to the rapid growth of UCOGCP, or the associated inflammatory reaction. On CT scanning, an irregular solid and cystic mass with strong enhancement are typically observed for UCOGCP. With regard to tumor markers, particularly CEA and CA19-9, elevated levels are less common, or are not distinct, in cases of UCOGCP compared with PAC. For OGCs, although they rarely express epithelial markers, they do typically stain for histomonocytic markers, especially CD68, as shown in the present report. Levels of inflammatory markers, such as white blood cell count, C-reactive protein level, and levels of interleukins, have also been found to be elevated in > 50% of patients with UCOGCP.
An accurate pretreatment diagnosis is crucial to determine the most appropriate therapy and to obtain an optimal prognosis. However, the differential diagnosis of UCOGCP from other unusual pancreatic tumors is difficult, particularly from pancreatic serous and mucinous cystic tumors, pancreatic pseudocysts, ductal pancreatic carcinomas, and neuroendocrine tumors. The presence of non-neoplastic OGCs is a histological hallmark of UCOGCP, and the diagnosis can be straightforward when examining tissue sections. Moreover, although there are limited data to support the differentiation of pancreatic lesions by CT or MRI alone, an accurate analysis of cross-sectional imaging in conjunction with clinical data may provide valuable insight into a correct diagnosis. For example, a cytologic/pathologic diagnosis is often necessary. In some cases, EUS-guided fine needle aspiration cytology (FNAC) was found to be an effective and accurate means of achieving a cytological diagnosis. For example, in a series of five patients reported by Moore et al[4], EUS-guided FNAC was performed, and the EUS appearance differed from that of typical ductal PAC and neuroendocrine tumors. Cytological features observed with FNAC can also distinguish primary giant cell-containing neoplasms from non-neoplastic giant cell-containing lesions of the pancreas, or giant cell-containing neoplasms that do not arise from the pancreas.
For patients with unresectable UCOGCP, the overall median survival period is 6.5 mo[5]. Therefore, the primary treatment for UCOGCP includes en bloc surgical resection when it is an option. However, it remains difficult to determine the best treatment modality for this neoplasm due to its rarity. An additional consideration is that more than half of giant cell tumors are locally advanced at the time of presentation, and are detected as a result of their large size or local invasion. Consequently, a partial or total pancreatectomy combined with the resection of adjacent organs is often necessary. In a previous report, a patient underwent a total pancreatectomy, and survived an additional 15 years[6]. However, an additional consideration is that the extensive vascularization that can characterize UCOGCP, as demonstrated in the present report, can lead to significant blood loss during pancreatectomy. Sporadic case reports have also demonstrated a reduction in tumor mass and prolonged survival can be achieved following treatment with 5-fluorouracil. In the present case, a chemotherapy regimen including gemcitabine was administered according to the treatment protocol of ductal PAC. Thus, it may be reasonable to consider agents such as gemcitabine for palliation. Furthermore, based on the radiosensitivity exhibited by giant cell tumors of bone, we hypothesize that radiation may also have benefits in a neo-adjuvant setting for UCOGCP. However, this remains to be evaluated in a larger cohort.
UCOGC may represent a distinct clinicopathologic entity with a more favorable prognosis than an undifferentiated carcinoma without OGCs, possibly because it is slower to metastasize and rarely metastasizes to the lymph nodes. In the literature, the outcome of UCOGCP is extremely variable, with the interval to death ranging from 4 mo to 10 years[5]. However, in a study of 35 patients, 29/35 patients did not survive, and the average survival period was 5.2 mo[7]. In contrast, for three of the patients still alive at the last follow-up, two had been disease-free for 14.6 and 7.2 years, respectively, while tumor recurrence was detected in the third patient after 14.7 years. Other studies have also reported UCOGCP patients with 10-year survival periods[7]. For the patient in the present report, she remains disease-free ten years after undergoing surgery for locally advanced UCOGCP. Correspondingly, a favorable prognosis is predicted for her long-term follow-up.
Based on the present case and limited previous data, UCOGCP is a rare malignant lesion of the pancreas that has a more favorable prognosis than ductal PAC. UCOGCP also often presents as a large cystic neoplasm accompanied by invasion into adjacent organs, and levels of tumor markers (e.g., CEA and CA19-9) are usually normal or mildly elevated. For cases with advanced UCOGCP, en bloc resection of the pancreas and other invaded organs may be an effective treatment. It remains to be demonstrated whether the response of UCOGCP to chemotherapy or radiotherapy will be efficacious. Therefore, additional studies, preferably with larger cohorts, are needed to further improve our understanding of this rare and interesting tumor.
COMMENTS
Case characteristics
A previously healthy, 71-year-old female patient was admitted due to a one month history of epigastric pain and anorexia.
Clinical diagnosis
A 15 cm × 13 cm mass was palpated in the left upper abdomen.
Differential diagnosis
There are limited data to support the differentiation of pancreatic lesions by computed tomography (CT) or magnetic resonance imaging alone. Endoscopic ultrasonography-guided fine needle aspiration cytology was an effective and accurate means of achieving a cytological diagnosis.
Laboratory diagnosis
Elevated levels of carcinoembryonic antigen and carbohydrate antigen 19-9 are less common, or are not distinct compared with pancreatic adenocarcinoma (PAC).
Imaging diagnosis
On CT scanning, an irregular solid and cystic mass with strong enhancement are typically observed for undifferentiated carcinoma with osteoclast-like giant cells of the pancreas (UCOGCP).
Pathological diagnosis
UCOGCP includes highly pleomorphic, frequently spindle-shaped neoplastic cells, and large multinucleated non-neoplastic osteoclast-like giant cells.
Treatment
The primary treatment for UCOGCP includes en bloc surgical resection when it is an option.
Term explanation
The patient described in this report remains disease-free ten years after undergoing surgery for locally advanced UCOGCP.
Experiences and lessons
For cases with advanced UCOGCP, en bloc resection of the pancreas and other invaded organs may be an effective treatment.
Peer review
The case presentation is interesting. For advanced cases of UCOGCP, en bloc resection of the pancreas may be an effective treatment and has a more favorable prognosis than ductal PAC. However, since this report presented as just one case, additional studies are needed to further improve our understanding of this rare and interesting tumor.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 29, 2014
First decision: May 29, 2014
Article in press: July 25, 2014
P- Reviewer: Alsolaiman M, Lakatos PL, Yokoyama Y S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Rosai J. Carcinoma of pancreas simulating giant cell tumor of bone. Electron-microscopic evidence of its acinar cell origin. Cancer. 1968;22:333–344. doi: 10.1002/1097-0142(196808)22:2<333::aid-cncr2820220210>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010. [Google Scholar]
- 3.Jotsuka T, Hirota M, Tomioka T, Ohshima H, Katsumori T, Miyanari N, Nakano S, Okabe A, Izaki T, Tomiyasu S, et al. Giant cell carcinoma of the pancreas: a case report and review of the literature. Pancreas. 1999;18:415–417. doi: 10.1097/00006676-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Moore JC, Hilden K, Bentz JS, Pearson RK, Adler DG. Osteoclastic and pleomorphic giant cell tumors of the pancreas diagnosed via EUS-guided FNA: unique clinical, endoscopic, and pathologic findings in a series of 5 patients. Gastrointest Endosc. 2009;69:162–166. doi: 10.1016/j.gie.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Singhal A, Shrago SS, Li SF, Huang Y, Kohli V. Giant cell tumor of the pancreas: a pathological diagnosis with poor prognosis. Hepatobiliary Pancreat Dis Int. 2010;9:433–437. [PubMed] [Google Scholar]
- 6.Shiozawa M, Imada T, Ishiwa N, Rino Y, Hasuo K, Takanashi Y, Nakatani Y, Inayama Y. Osteoclast-like giant cell tumor of the pancreas. Int J Clin Oncol. 2002;7:376–380. doi: 10.1007/s101470200059. [DOI] [PubMed] [Google Scholar]
- 7.Paal E, Thompson LD, Frommelt RA, Przygodzki RM, Heffess CS. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129–140. doi: 10.1053/adpa.2001.25404. [DOI] [PubMed] [Google Scholar]


