Abstract
Inflammatory pseudotumor (IPT) is a rare space-occupying lesion of unknown etiology that can mimic malignancy on clinic-radiological and pathological examination. We describe a rare case of ileocecal intussusception from clinically suspected malignancy of the right colon where the patient underwent right hemicolectomy. Histopathology of the resected specimen confirmed IPT of the colon. This patient was observed to have abnormally elevated total leukocyte count and platelets before and after surgery. In an adult with intussusception associated with an abdominal mass, the possibility of IPT of the colon should be considered. Considering the abnormally high total leukocyte and platelet counts and colonic IPT, it is necessary to prevent postoperative adverse effects due to these changes. Although IPT of the colon is usually a benign process, controversy regarding its management still exists. We consider hemicolectomy as a safe treatment approach for colonic IPT and review the existing literature.
Keywords: Inflammatory pseudotumor, Intussusception, Colon
Core tip: Inflammatory pseudotumor (IPT) is a rare space-occupying lesion of unknown etiology that can mimic malignancy on clinic-radiological and pathological examination. We describe a rare case of ileocecal intussusception from clinically suspected malignancy of the right colon where the patient underwent right hemicolectomy. Histopathology of the resected specimen confirmed IPT of the colon. This patient was observed to have abnormally elevated total leukocyte count and platelets before and after surgery. We consider hemicolectomy as a safe treatment approach for colonic IPT and review the existing literature.
INTRODUCTION
Inflammatory pseudotumor (IPT) is a reactive condition which occurs in many organs, including the lung which is the most common site of occurrence. IPT is also found in the central nervous system, major salivary glands, kidney, liver, omentum, ovary, larynx, urinary bladder, breast, pancreas, spleen, lymph nodes, skin, soft tissues, and orbit[1]. IPT is a benign tumor and represents a rare lesion with uncertain etiopathogenesis[2]. According to the location of the lesion, the clinical symptoms of IPT are diverse, and include a mass, fever, weight loss, malaise, pain and site-specific symptoms[3]. However, IPT of the colon is seldom found, and ileocecal intussusception is a rare complication of colonic IPT. We describe a rare case of ileocecal intussusception from clinically suspected malignancy of the right colon where the patient underwent right hemicolectomy. Histopathology of the resected specimen subsequently confirmed IPT of the colon and the outcome was favorable.
CASE REPORT
A 37-year-old Chinese male was referred to our Hospital with a 7-d history of intermittent abdominal pain and fever. He did not present any changes in his bowel habits, nausea or vomiting. His past medical history was unremarkable.
On admission, his axillary temperature was 37.2 °C, heart rate was 82 beats per minute, and blood pressure was 140/90 mmHg. Physical examination showed right lower quadrant tenderness, slight rebound tenderness and localized muscle tension, no mass, shifting dullness negative, bowel sounds slightly active and reduced gurgling sounds.
His hemoglobin (HGB), total leukocyte count, platelet count and neutrophils were 134 g/L (reference range: 130-175 g/L), 13.3 × 109/L (reference range: 3.5-9.5 × 109/L), 533 × 109/L (reference range: 125-350 × 109/L) and 86.4% (reference range: 40%-75%), respectively. His random blood glucose was 7.10 mmol/L and his liver and kidney function tests were within normal limits. The patient had normal coagulation and no hepatitis B, hepatitis C, syphilis or HIV. Ultrasound examination of the abdomen showed an upper abdominal solid mass and possible intussusception (Figure 1). Computerized tomographic (CT) examination of the abdomen revealed intussusception in the right lower quadrant, possible colonic neoplasms and a right renal cyst (Figure 2). Chest X-ray showed no heart or lung abnormalities.
Figure 1.
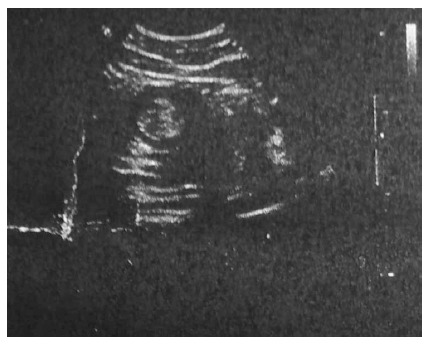
Ultrasound examination of the abdomen showed an upper abdominal solid mass and possible intussusception.
Figure 2.
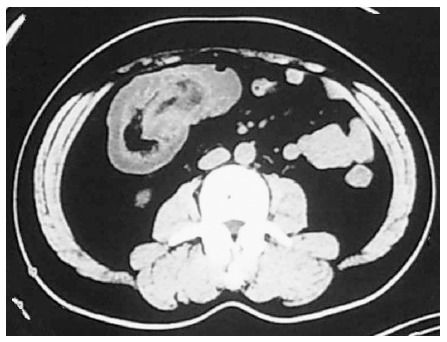
Abdominal computed tomography revealed an intussusception in the right lower quadrant and possible colonic neoplasms.
The patient underwent an emergency exploratory laparotomy with the presumptive diagnosis of intussusception, possible colonic neoplasms and localized peritonitis. Operative findings demonstrated a well-circumscribed, firm mass approximately 6 cm in diameter arising from the central ascending colon, intussusception and edema of the appendix. Intussusception was detected in the right abdomen and involved the terminal ileum and cecum and it could not be reset although no bowel necrosis was present. A few pieces of crisp lymph nodes (maximum diameter approximately 1.5 cm) were detected in the right mesocolon, the roots of the ileocolon and superior colic artery (Figure 3). The patient underwent right hemicolectomy with the intraoperative diagnosis of ileocecal intussusception, colonic neoplasms and localized peritonitis. Histological examination showed that the mass section diameter was about 5 cm, and the incisal surface was gray with a hard texture; microscopic examination revealed a large number of fibroblasts, myofibroblast proliferation, inflammatory changes and no tumor cells (Figure 4A and B). Immunohistochemical staining for smooth muscle actin (SMA) was positive in the colonic mass (Figure 5). The histopathologic diagnosis was colonic IPT. Histopathologic examination also showed enlarged lymph nodes, follicular hyperplasia, and a significantly expanded germinal center; the histopathologic diagnosis was reactive lymphoid hyperplasia.
Figure 3.
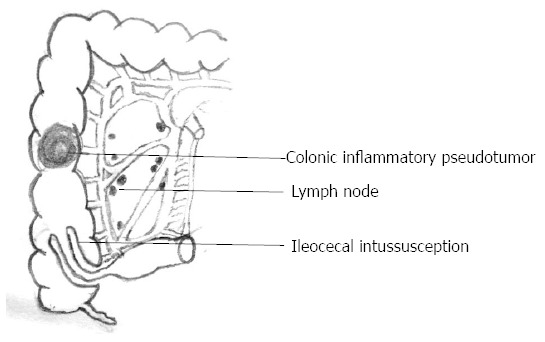
Schematic showing the inflammatory pseudotumor in the central ascending colon, ileocecal intussusception and enlarged lymph nodes.
Figure 4.
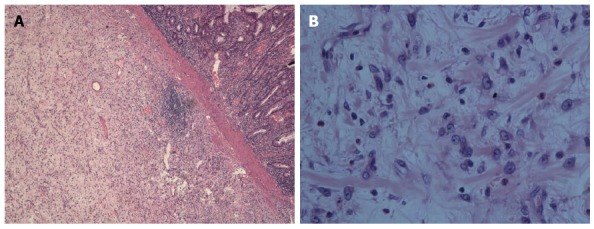
Microscopic examination revealed a large number of fibroblasts, myofibroblast proliferation and inflammatory changes. HE staining, A: magnification × 40; B: magnification × 400.
Figure 5.
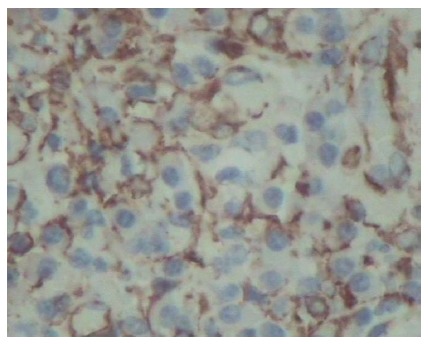
Immunohistochemical staining of smooth muscle actin protein was positive in the colonic mass (magnification × 400).
On postoperative day 2, HGB, total leukocyte count, platelet count, and neutrophils were 146 g/L, 24.1 × 109/L, 666 × 109/L and 90.0%, respectively. Considering the high risk of thrombosis due to the abnormally elevated platelet count, aspirin was administered to inhibit platelet aggregation. The dynamic changes in routine blood samples on postoperative day 6, 10 and 13 are shown in Figure 6A and B. During the course of leukocytosis/thrombocytosis, the patient did not have infectious signs (fever, abscess formation, etc.) and had undetectable C-reactive protein. Serology results showed that serum immunoglobulin G4 (IgG4) was 0.349 g/L (reference range: 0.03-2.01 g/L). Although the patient showed abnormal routine blood samples, the postoperative course was uneventful and he was discharged from hospital on postoperative day 14 and given aspirin to inhibit platelet aggregation. On postoperative day 33, his HGB, total leukocyte count, platelet count and neutrophils were 132 g/L, 11.1 × 109/L, 428 × 109/L and 61.4%, respectively. The dynamic changes in routine blood before and after surgery are shown in Figure 6 A and B. On postoperative day 220, his HGB, total leukocyte count, platelet count and neutrophils were 154 g/L, 7.6 × 109/L, 312 × 109/L and 50.3%, respectively. The patient was free of symptoms 7 mo after surgery with normal laboratory findings.
Figure 6.
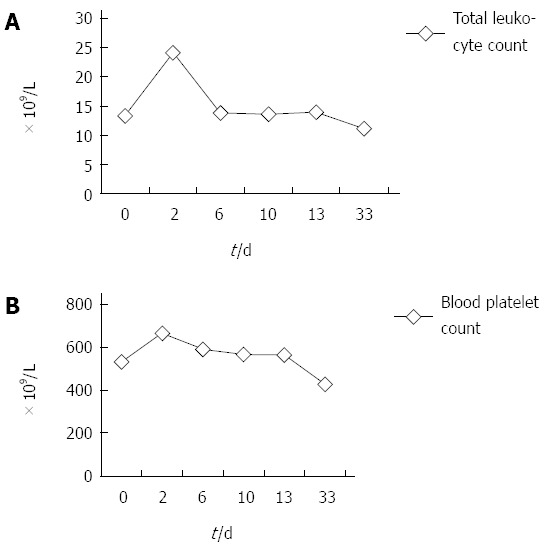
Dynamic changes in routine blood sample. A: Total leukocyte count (× 109/L); B: Blood platelet count (× 109/L) before and after surgery.
DISCUSSION
The etiology and pathogenesis of IPT are unknown[4]. The mechanism of IPT etiology may be due to infections, intraparenchymal hemorrhage or an autoimmune etiology[5]. Microorganisms including Bacteroides caccae, Actinomyces, Klebsiella, Escherichia coli, Gram-positive cocci, B-hemolytic Streptococcus[6] and Mycobacterium tuberculosis[7,8] have been found in many reports of IPT. However, in other reports of IPT, no causative microorganisms were found and an association between IPT and hepatopancreatobiliary autoimmune diseases, such as IgG4 sclerosing cholangitis was indicated[9]. In this case, the past medical history of the patient was unremarkable and serology results for serum IgG4 were within normal limits. Considering the abnormally elevated total leukocyte count, the etiology of colonic IPT in this patient may be associated with infection. It is regrettable that the patient, who underwent emergency surgery, did not also undergo tests for tuberculosis and other infections during the preoperative examination.
As a quasi-neoplastic lesion, the histological characteristics of IPT include a heterogeneous population of acute and chronic inflammatory cells, particularly plasma cells, macrophages and fibroblasts, accompanied by areas of fibrosis and necrosis[10]. The microscopic appearance varies from case to case. This entity has been called many different names, including plasma cell granuloma, inflammatory myofibroblastic tumor and most commonly inflammatory pseudotumor[11]. In this case, microscopic examination revealed a large number of fibroblasts, myofibroblast proliferation, inflammatory changes and no tumor cells. Immunohistochemical staining of SMA was positive in the colonic mass. The histopathologic diagnosis was colonic IPT.
Early cases of lesions classified as IPTs focused on pulmonary lesions[7,12] which were possibly more common than extrapulmonary lesions. Over the years, IPTs have also been reported at various other sites[3,13]. However, an extensive review of the literature using the PubMed database from 1994 to 2014 found only 18 cases of IPTs originating from the colon[14-29] (Table 1) and only 1 case of colonic IPT causing intussusception[16]. IPT of the colon is an extremely rare process and this unexpected lesion tends to arise from an erroneous impression of malignancy[16].
Table 1.
Studies on colonic inflammatory pseudotumor in PubMed database from 1994 to 2014
| Position of colonic IPT | Patient number | Ref. |
| Indefinite | 2 | Aalbers et al[14], 1999; Velitchkov et al[15], 2000 |
| Descending colon | 1 | Jeong et al[16], 2011 |
| Transverse and descending colon | 1 | Fosi et al[17], 2014 |
| Cecal and sigmoid flexure | 2 | Chetty et al[18], 2011 |
| A mass in the right iliac fossa, IPT infiltrating ileocecal valve | 1 | Salgado-Sánchez et al[19], 2003 |
| Sigmoid | 3 | Rosenbaum et al[20], 2000; De Monti et al[21], 1997; Wendum et al[22], 1994 |
| Terminal ileum, cecum, and ascending colon | 1 | Cviko et al[23], 1999 |
| Urinary bladder and Sigmoid colon | 1 | Saito et al[24], 1999 |
| Colon and rectum | 2 | Sanders et al[25], 2001 |
| Diverticular disease of the sigmoid | 1 | Timofeev et al[26], 2000 |
| Transverse colon | 2 | Díaz Morant et al[27], 1999; Ohno et al[28], 1998 |
| Cecum | 1 | Yoshikawa et al[29], 1994 |
IPT: Inflammatory pseudotumor.
IPT is often incidentally detected on imaging studies without clinical symptoms or during diagnostic evaluation for unexplained fever, weight loss or anemia[16]. The clinical symptoms of IPT are diverse and depend on the location of the lesion. Patients with intra-abdominal tumor most commonly present with abdominal pain, a palpable mass or occasionally, with intestinal obstruction[3,13]. In this case, the patient presented with intermittent abdominal pain resulting from intussusception. In addition, due to its rarity in adults, when intussusception is diagnosed, it strongly suggests the presence of a malignant condition of either primary or metastatic origin[16]. The probability of malignancy, usually adenocarcinoma, is greater for those cases occurring in the colon[30]. In this case, the patient underwent acute exploratory laparotomy with the presumptive diagnosis of intussusception, possible colonic neoplasms and localized peritonitis. Due to cecum and ascending colon of inter-peritoneal viscera and ileum of intra-peritoneal viscera in this patient, the tumor in the central ascending colon caused bowel disorders and resulted in ileo-cecal intussusception instead of colo-colic intussusception.
IPT often cannot be differentiated clinically or radiologically from other more aggressive neoplasms, and the accurate diagnosis is based on histopathologic examination. The general appearance of abdominal IPT on ultrasound scan and tomographic scans is a well-circumscribed mass of soft tissue density producing displacement or invasion of the adjacent tissues with a homogenous echo pattern[31]. Because the radiologic findings are variable and nonspecific, it is difficult to diagnose IPT before surgery. Inconsistent radiologic images may be caused by the different degrees of inflammation and various proportions of fibrotic content within the tumor. Jeong et al[16] first reported 18F-FDG Positron Emission Tomography/Computerized tomography (PET/CT) in the detection of intussusception due to IPT in the colon. PET/CT application in the clinic is limited due to its high cost. In this case, CT examination of the abdomen showed intussusception in the right lower quadrant, possible colonic neoplasms and a renal cyst. The diagnosis of IPT of the colon cannot be confirmed by preoperative examination. The patient underwent acute exploratory laparotomy with the presumptive diagnosis of intussusception, possible colonic neoplasms and localized peritonitis. Due to the firm mass arising from the central ascending colon and a few pieces of crisp lymph nodes, the patient underwent right hemicolectomy. The histopathologic diagnosis was IPT of the colon.
Patients who have IgG4-related mass lesions with dysplastic and malignant tumors endoscopically and radiographically, can undergo unnecessary invasive therapy including resection[32]. IgG4-related IPT may respond to conservative treatment with steroids[32]. However, the diagnosis of IPT is difficult due to its rarity, and the clinical history and radiographic findings lead to a high level of suspicion of a true neoplasm[5]. Generally, IPTs have a benign behavior with occasional spontaneous regression, but occasionally they have been reported to recur, metastasize, and undergo sarcomatous transformation[5]. IPT of the spleen may be diagnosed accurately by fine needle aspiration (FNA)[33]. Kawaguchi et al[34] also reported IPTs of the liver and spleen diagnosed by percutaneous needle biopsy. However, careful use of FNA biopsy is required in suspected intestinal IPT, as this invasive examination may cause intestinal perforation, and IPT is often misdiagnosed in pathology due to its heterogeneity and diversity. In order to avoid misdiagnosis, the patient with undiagnosed intestinal IPT should undergo exploratory laparotomy to confirm the diagnosis.
Dynamic changes in routine blood samples were noted in our patient before and after surgery. It was interesting that abnormally elevated total leukocyte count and platelets were observed before and after surgery, and these levels gradually returned to near normal with postoperative recovery of the patient. When a patient with a colon mass is observed to have abnormally elevated total leukocyte count and platelets before surgery, the diagnostic possibility of colonic IPT should be considered. Considering the abnormally high total leukocyte count and platelet count and colonic IPT, it is necessary to prevent postoperative adverse effects due to these changes. The reason for these changes is not clear. Cytokines are possibly involved in the pathogenesis of IPT[35]. Cytokines such as IL-6 and cyclin D1 probably have a paracrine action and sustain myofibroblastic growth[35]. Preoperative leukocytosis and thrombocytosis may be related to the common stimulation by inflammatory cytokines (such as IL-6) in IPT and the hematopoietic system, however, early postoperative leukocytosis and thrombocytosis may be related to surgery and anesthesia-induced trauma. The decline in late postoperative total leukocyte count and platelets is slow, late postoperative near-normal total leukocyte count and platelets may be related to removal of the IPT or the involvement of other factors. There is another possibility, in that the abnormal laboratory data is not related to IPT at all. Specific mechanisms related to the abnormally high total leukocyte count, platelet count and colonic IPT require further study.
IPT of the colon should be considered a diagnostic possibility in an adult who has an intussusception associated with an abdominal mass and has an abnormally elevated total leukocyte count and platelets before and after surgery. IPT is a rare entity that can occur in the colon in association with other inflammatory diseases. The symptoms of IPT are nonspecific, and its diagnosis is intriguing. Surgical resection is necessary and safe in many patients with IPT of the colon. Considering the abnormally high total leukocyte count and platelet changes and colonic IPT, it is necessary to prevent postoperative adverse effects due to these changes. This case report is a significant contribution to the controversy surrounding this medical problem.
ACKNOWLEDGMENTS
We thank the patient and his families, and thank Dr. Yong Zhao for critical reading of the manuscript. The authors declare no conflicts of interest.
COMMENTS
Case characteristics
A 37-year-old Chinese male presented with a 7-d history of intermittent abdominal pain and fever.
Clinical diagnosis
Physical examination showed right lower quadrant tenderness, slight rebound tenderness and localized muscle tension, no mass, shifting dullness negative, bowel sounds slightly active and reduced gurgling sounds.
Differential diagnosis
The differential diagnosis included acute appendicitis, colonic neoplasms and right iliac fossa neoplasms.
Laboratory diagnosis
HGB, total leukocyte count, platelet count and neutrophils were 134 g/L, 13.3 × 109/L, 533 × 109/L and 86.4%, respectively: and liver and kidney function tests were within normal limits.
Imaging diagnosis
Ultrasound examination of the abdomen showed an upper abdominal solid mass and possible intussusception, and computed tomography examination of the abdomen revealed intussusception in the right lower quadrant, possible colonic neoplasms and a right renal cyst.
Pathological diagnosis
The histopathologic diagnosis was colonic inflammatory pseudotumor (IPT), which was smooth muscle actin positive.
Treatment
The patient underwent acute right hemicolectomy with the intraoperative diagnosis of ileocecal intussusception, colonic neoplasms and localized peritonitis.
Related reports
IPT is a rare space-occupying lesion of unknown etiology that can mimic malignancy on clinic-radiological and pathological examination, and its diagnosis is intriguing. Surgical resection is necessary and safe in many patients with IPT of the colon.
Term explanation
Fine needle aspiration is a method that is used for the diagnosis of solid tumors.
Experiences and lessons
This case report not only represents a rare case of ileocecal intussusception induced by colonic IPT where the patient underwent right hemicolectomy, but also revealed abnormally elevated total leukocyte count and platelets before and after surgery.
Peer review
This article presents a rare case of ileocecal intussusception induced by colonic IPT.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 24, 2014
First decision: May 29, 2014
Article in press: July 30, 2014
P- Reviewer: Ashurst J, Akbulut S, Galvan-Montano A S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Fukuya T, Honda H, Matsumata T, Kawanami T, Shimoda Y, Muranaka T, Hayashi T, Maeda T, Sakai H, Masuda K. Diagnosis of inflammatory pseudotumor of the liver: value of CT. AJR Am J Roentgenol. 1994;163:1087–1091. doi: 10.2214/ajr.163.5.7976880. [DOI] [PubMed] [Google Scholar]
- 2.Dominis M, Dzebro S, Kusić B, Antica M. Inflammatory pseudotumor of the spleen. Acta Cytol. 1998;42:1053–1056. [PubMed] [Google Scholar]
- 3.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith PJ, Loganathan A, Jacob M, Ahmad N, Toogood GJ, Lodge JP, Prasad KR. Inflammatory pseudotumours of the liver: a spectrum of presentation and management options. Eur J Surg Oncol. 2009;35:1295–1298. doi: 10.1016/j.ejso.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Hosler GA, Steinberg DM, Sheth S, Hamper UM, Erozan YS, Ali SZ. Inflammatory pseudotumor: a diagnostic dilemma in cytopathology. Diagn Cytopathol. 2004;31:267–270. doi: 10.1002/dc.20113. [DOI] [PubMed] [Google Scholar]
- 6.Ntinas A, Kardassis D, Miliaras D, Tsinoglou K, Dimitriades A, Vrochides D. Inflammatory pseudotumor of the liver: a case report and review of the literature. J Med Case Rep. 2011;5:196. doi: 10.1186/1752-1947-5-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekosan M, Cleto M, Senseng C, Farolan M, Sekosan J. Spindle cell pseudotumors in the lungs due to Mycobacterium tuberculosis in a transplant patient. Am J Surg Pathol. 1994;18:1065–1068. doi: 10.1097/00000478-199410000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Srinivas R, Aggarwal AN. Parenchymal pseudotumoral tuberculosis: case series and systematic review of literature. Respir Med. 2008;102:382–389. doi: 10.1016/j.rmed.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14:3948–3955. doi: 10.3748/wjg.14.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda J, Yoshida H, Taniai N, Onda M, Hayashi H, Tajiri T. Inflammatory pseudotumor in the liver associated with intrahepatic bile duct stones mimicking malignancy. J Nippon Med Sch. 2009;76:154–159. doi: 10.1272/jnms.76.154. [DOI] [PubMed] [Google Scholar]
- 11.Dehner LP. The enigmatic inflammatory pseudotumours: the current state of our understanding, or misunderstanding. J Pathol. 2000;192:277–279. doi: 10.1002/1096-9896(200011)192:3<277::AID-PATH749>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Demir HA, Yalcin B, Ciftci AO, Orhan D, Varan A, Akyuz C, Kutluk T, Buyukpamukcu M. Primary pleuropulmonary neoplasms in childhood: fourteen cases from a single center. Asian Pac J Cancer Prev. 2011;12:543–547. [PubMed] [Google Scholar]
- 13.Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23:719–729. doi: 10.1148/rg.233025073. [DOI] [PubMed] [Google Scholar]
- 14.Aalbers AG, De Wilt JH, Zondervan PE, Ijzermans JN. A colon-derived inflammatory pseudotumor. Dig Dis Sci. 1999;44:578–581. doi: 10.1023/a:1026665609461. [DOI] [PubMed] [Google Scholar]
- 15.Velitchkov N, Losanoff J, Kjossev K, Michaylova V. Inflammatory pseudotumor of the colon. Dig Dis Sci. 2000;45:515–516. doi: 10.1023/a:1005441106719. [DOI] [PubMed] [Google Scholar]
- 16.Jeong JH, Cho IH, Kong EJ, Chun KA, Kim YJ, Kim JH. (18)F-FDG PET/CT in inflammatory pseudotumor of the colon causing intussusception. Ann Nucl Med. 2011;25:447–450. doi: 10.1007/s12149-011-0481-3. [DOI] [PubMed] [Google Scholar]
- 17.Fosi S, Altobelli S, Bindi A, Villa M, De Sanctis F, Montuori M, Ricciardi E, Rossi P, Petrella G, Simonetti G. Gradual colonic impaction of a chicken bone associated with inflammatory pseudotumor formation and nonocclusive colon ischemia. Case Rep Radiol. 2014;2014:215465. doi: 10.1155/2014/215465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chetty R, Serra S, Gauchotte G, Märkl B, Agaimy A. Sclerosing nodular lesions of the gastrointestinal tract containing large numbers of IgG4 plasma cells. Pathology. 2011;43:31–35. doi: 10.1097/PAT.0b013e328340e450. [DOI] [PubMed] [Google Scholar]
- 19.Salgado-Sánchez E, Flores-Flores J, Pérez-Toriz MU, Pérez-Cruz R, Salgado-Sánchez J. [Myofibroblast tumor] Rev Gastroenterol Mex. 2003;68:219–221. [PubMed] [Google Scholar]
- 20.Rosenbaum A, Arnold JC, Rebel M, Riemann JF. Pseudotumor of the sigmoid mimicking carcinoma. Endoscopy. 2000;32:546–548. doi: 10.1055/s-2000-3811. [DOI] [PubMed] [Google Scholar]
- 21.De Monti M, Ghilardi G, Cavenati S, Reale D, Pezzica E, Scorza R. [Plasma-cell granuloma of the sigmoid colon concomitant with adenocarcinoma of the cecum. Viewpoint for debate, literature review on pseudotumors, idiopathic colitis and cancer] Ann Ital Chir. 1997;68:245–251. [PubMed] [Google Scholar]
- 22.Wendum D, Vissuzaine C, Bellanger J, Le Goff JY, Benhamou G, Potet F. [A case of polypoid solitary colonic plasmocytoma] Ann Pathol. 1994;14:248–250. [PubMed] [Google Scholar]
- 23.Cviko A, Milic Z, Cizmic A, Seiwerth S, Kruslin B. Inflammatory myofibroblastic tumor with extensive involvement of the bowel in a 7-year-Old child. Croat Med J. 1999;40:550–553. [PubMed] [Google Scholar]
- 24.Saito M, Watanabe N, Abe B, Matsui K. Inflammatory pseudotumor of the urinary bladder and sigmoid colon. Urol Int. 1999;62:119–121. doi: 10.1159/000030372. [DOI] [PubMed] [Google Scholar]
- 25.Sanders BM, West KW, Gingalewski C, Engum S, Davis M, Grosfeld JL. Inflammatory pseudotumor of the alimentary tract: clinical and surgical experience. J Pediatr Surg. 2001;36:169–173. doi: 10.1053/jpsu.2001.20045. [DOI] [PubMed] [Google Scholar]
- 26.Timofeev IuM, Perevoshchikov AG. [A pseudotumorous form of diverticular disease of the sigmoid] Vopr Onkol. 2000;46:344–346. [PubMed] [Google Scholar]
- 27.Díaz Morant V, Fúnez Liébana R, Manteca González R, García González E, Morales Jiménez J, Pradas Caravaca M. [An actinomycotic inflammatory pseudotumor of the transverse colon] Gastroenterol Hepatol. 1999;22:206–207. [PubMed] [Google Scholar]
- 28.Ohno M, Nakamura T, Ohbayashi C, Tabuchi Y, Nogi Y, Saitoh Y. Colonic obstruction induced by plasma cell granuloma of the transverse colon: report of a case. Surg Today. 1998;28:416–419. doi: 10.1007/s005950050153. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa I, Murata I, Abe S, Tabaru A, Endo M, Otsuki M. Plasma cell granuloma of the colon: a report of a case removed by endoscopic polypectomy. Am J Gastroenterol. 1994;89:1249–1252. [PubMed] [Google Scholar]
- 30.Martín-Lorenzo JG, Torralba-Martinez A, Lirón-Ruiz R, Flores-Pastor B, Miguel-Perelló J, Aguilar-Jimenez J, Aguayo-Albasini JL. Intestinal invagination in adults: preoperative diagnosis and management. Int J Colorectal Dis. 2004;19:68–72. doi: 10.1007/s00384-003-0514-z. [DOI] [PubMed] [Google Scholar]
- 31.Brown G, Shaw DG. Inflammatory pseudotumours in children: CT and ultrasound appearances with histopathological correlation. Clin Radiol. 1995;50:782–786. doi: 10.1016/s0009-9260(05)83220-9. [DOI] [PubMed] [Google Scholar]
- 32.Kim do H, Kim J, Park do H, Lee JH, Choi KD, Lee GH, Jung HY, Kim JH. Immunoglobulin G4-related inflammatory pseudotumor of the stomach. Gastrointest Endosc. 2012;76:451–452. doi: 10.1016/j.gie.2011.07.061. [DOI] [PubMed] [Google Scholar]
- 33.Mundi I, Singhal N, Punia RP, Dalal U, Mohan H. Inflammatory pseudotumor of the spleen: a rare case diagnosed on FNAC. Diagn Cytopathol. 2012;40:1104–1106. doi: 10.1002/dc.21719. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi T, Mochizuki K, Kizu T, Miyazaki M, Yakushijin T, Tsutsui S, Morii E, Takehara T. Inflammatory pseudotumor of the liver and spleen diagnosed by percutaneous needle biopsy. World J Gastroenterol. 2012;18:90–95. doi: 10.3748/wjg.v18.i1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gómez-Román JJ, Ocejo-Vinyals G, Sánchez-Velasco P, Nieto EH, Leyva-Cobián F, Val-Bernal JF. Presence of human herpesvirus-8 DNA sequences and overexpression of human IL-6 and cyclin D1 in inflammatory myofibroblastic tumor (inflammatory pseudotumor) Lab Invest. 2000;80:1121–1126. doi: 10.1038/labinvest.3780118. [DOI] [PubMed] [Google Scholar]


