Abstract
Osteonecrosis of femoral head (ONFH) is a disabling condition of young individuals with ill-defined etiology and pathogenesis. Remains untreated, about 70-80% of the patients progress to secondary hip arthritis. Both operative and nonoperative treatments have been described with variable success rate. Early diagnosis and treatment is the key for success in preserving the hip joint. Once femoral head collapses (>2 mm) or if there is secondary degeneration, hip conservation procedures become ineffective and arthroplasty remains the only better option. We reviewed 157 studies that evaluate different treatment modalities of ONFH and then a final consensus on treatment was made.
Keywords: Avascular necrosis, femur head, hip, osteonecrosis, treatment
MeSH terms: Avascular necrosis of bone, femur head, treatment protocols, osteonecrosis
INTRODUCTION
Osteonecrosis of the femoral head (ONFH) is caused by inadequate blood supply leading to death of the osteocytes. Subsequently it progresses to collapse of the femoral head and advanced joint destruction. ONFH thus leads to significant disability in the most productive years of life and is one of the common causes of hip arthroplasty in young individuals. Both traumatic and nontraumatic etiologies have been described for ONFH. The common causes include corticosteroid medications, fractures and dislocations of hip joint and chronic alcohol intake. In about 30% patients, it is idiopathic [Table 1]. Bilateral presentation is frequently seen and males are more commonly affected.1 Contralateral hip may be affected in about 55% of the patients within 2 years.2 About 75% of patients with other sites of involvement will have concurrent ONFH.3
Table 1.
Common etiologies of avascular necrosis of the femoral head

At an early-stage of ONFH, the hip joint is painless. However, it becomes painful and there is limitation of hip range of movement with advancement of disease. Multiple diagnostic and treatment modalities have been described for ONFH but none of them are completely accurate and effective. Earliest X-ray findings of ONFH take at least 2 months to develop, but may take as long as 6 months. Sclerosis and cystic changes are early radiographical changes. With progression of disease, there is asphericity of femoral head (femoral head collapse) and joint space reduction (secondary arthritis). Magnetic resonance imaging (MRI) is the most sensitive diagnostic modality for ONFH. MRI has sensitivity of 90-100% and specificity of 100% in diagnosis of avascular necrosis (AVN).4 It is also useful for early detection of asymptomatic AVN.5 The characteristic appearance of the infarcted area is a hypo-dense on T1 image surrounded by a single hypo-dense line separating normal and osteonecrotic bone. T2 image shows another line within this line representing increased vascularity in granulation tissue. The appearance of the interface is more important in the diagnosis, and the density of the necrotic central part will change with the change in fat content due to death of adipocytes and appearance of reparative tissue. MRI can help in identifying patients at risk of collapse of the femoral head. Presence of bone marrow edema, increased fat content in the proximal femur and joint effusion on MRI are important prognostic factors. Dynamic MRI may be the future investigation for early prediction of vascular insult to femoral head.6,7 Altered hemodynamic changes in the vascular phase of bone scintigraphy may be seen as early as first 24 h of vascular insult. Classical finding is increased uptake in the reparative interface where vascular tissue is invading the dead bone, and new bone is depositing. This surrounds the area of increased uptake by the dead trabeculae. Bone scan will thus detect the lesion before conventional radiograph. It is also helpful to detect disease at multiple sites in the skeleton. Most commonly used isotope is Tc-99m. Studies have shown that bone scintigraphy of the hip has lower resolution and sensitivity in the diagnosis of osteonecrosis compared to MRI.8,9 Disadvantages include radiation exposure, poor morphometric details and expertise needed for interpretation. It is less useful than MRI in ruling out other differential diagnosis for osteonecrosis. It has the advantage in patients where MRI may not be feasible, such as, cardiac pacemakers, intracranial clips and claustrophobia.
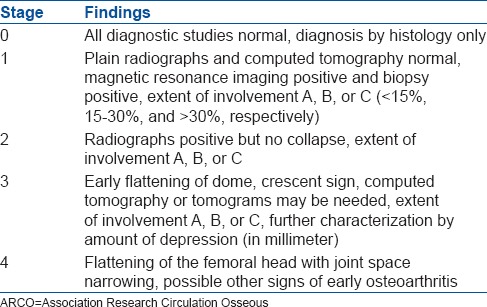
There are many classification systems that describe the clinical and radiological severity/progression of ONFH. The Ficat and Arlet-staging system is still one of the most commonly used systems [Table 2]. It is based on radiological findings, but does not consider the extent of necrosis.10 Quantification of the size of the lesion is helpful in predicting the collapse of the femoral head. Kerboul et al.11 estimated the extent of necrosis radiographically in the early-stages by measuring the sum of arc of area of the femoral head involved on anteroposterior and lateral radiographs. Clinical outcomes were better if this value was <200°. These measurements have also been obtained on mid-sagittal and mid-coronal MRI scans and is useful in predicting outcomes.12 Steinberg et al.13 added quantification of femoral head involvement to the classification system, but could not gain wide popularity as it was difficult to apply. The Association Research Circulation Osseous (ARCO) system of classification incorporated features of both the Ficat and Arlet system and the Steinberg classification [Table 3].5
Table 2.
Ficat and Arlet classification of femoral head osteonecrosis

Table 3.
ARCO classification of femoral head osteonecrosis
The natural history of ONFH is a progression to subchondral fracture leading to femoral head collapse and secondary osteoarthritis.1,2 Accordingly, the treatment can be broadly categorized into two types; treatment in the precollapsed or early collapsed stage <2 mm and treatment after advanced collapse or osteoarthritis of hip joint. Numerous nonoperative treatment modalities and hip preserving surgical procedures have been described for precollapsed and early collapsed stage. Once joint collapse occurs or degeneration starts, the treatment for joint preservation becomes more complex and outcome is poor. This review will discuss the current evidences for treatment of ONFH.
MATERIALS AND METHODS
A Pubmed/Medline search was conducted (between January 1st 1980 and June 30th, 2014) to identify all randomized control trial, meta-analysis, systematic reviews, prospective and retrospective studies published on the treatment of ONFH. The MeSH terms used in different combinations were “AVN, ON, nonoperative treatment, bisphosphonate, extracorporeal shock wave therapy (ESWT), electromagnetic therapy, hyperbaric oxygen, core decompression (CD), stem cell, bone-graft, tantalum rod, osteotomy, arthroplasty.” The searches were limited to English language and human species. The references of these studies and systematic reviews were also searched for inclusion of other relevant studies in this review.
RESULT
Number of articles retrieved with the above search strategy has been mentioned in Table 4. In total 157 studies were reviewed, and a consensus of treatment was planned.
Table 4.
Search strategy of pubmed and extracted articles for review

(A) Nonoperative treatment
Nonoperative management of ONFH includes restricted weight-bearing, pharmacological agents and biophysical modalities of treatment.7 The goal of drug treatment in the precollapsed stage is to improve hip function, provide pain relief, prevent radiographic progression to subchondral fracture and collapse, and allow healing of the necrotic lesions.14,15,16
Nonweight bearing
Restricted weight-bearing using cane, crutches or a walker is effective in early-stages ON hip (Ficat and Arlet Stage-I and II) when the osteonecrotic lesion is <15% and located far from the weight-bearing dome (medial lesions).16 Mont et al.17 reviewed 21 studies (n = 819 patients) based on restricted weight-bearing treatment and observed satisfactory clinical result (no further surgery) in 22% patients after 34 months. Radiological progression was seen in 74% patients. There was no difference in outcomes among patients following full, partial, and nonweight-bearing regimens in the study. In a systematic review (level II evidence), Mont et al.18 again reported that 59% (394 of 664 hips) of asymptomatic hips had onset of symptoms or disease progression to collapse after 7 years (range, 0.2-20 years). The investigators reported increased risk of collapse in sickle cell disease (73%; 29 of 40 hips) and minimal risk of collapse in systemic lupus erythematous (SLE) (17%; 10 of 59 hips). 32% patients with small or medium-sized lesions (<50% of head involvement) progressed to symptoms or collapse, whereas large lesions had 84% of chance of progression. It was stressed that progression to advanced-stage depends largely on location, size of the lesion and etiology. Small size lesion may show spontaneous regression.19 In 21st century, this modality of treatment cannot be accepted as a standard isolated modality of treatment and may be an additive treatment to medical or surgical management.
Bisphosphonates
Bisphosphonate inhibits the osteclastic activity in the osteonecrotic lesion site and thus promotes bone healing. It prevents the onset of subchondral fracture or collapse in early ON hip, and in advanced conditions when already collapse has occurred it delays the need of total hip replacement (THR) surgery.20,21,22,23,24,25,26,27,28,29,30 Agarwala et al. have reported the benefits of alendronate (10 mg/day or 70 mg/week) in ON hip at <1-year, 4 years and 10 years followup.20,21,22 At an average followup of 4 years, Agarwala et al.21 reported radiographic progression to collapse in 12.6% (27 of 215 hips) of hips in Stage-I and 55.8% (72 of 129 hips) of hips in Stage-II (Ficat and Arlet). Over all radiological progression was seen in 46% (99 of 215) of hips in Stage-I, 54% (70 of 129) of Stage-II hips and 20% (10 of 51) of Stage-III hips. The proportions of hips requiring THR were 2%, 8% and 33% for Staged-I, -II and -III ONFH respectively compared with 65%, 69% and 87% respectively as reported by Mont and Hungerford in untreated hips.31 Agarwala and Saha22 in a recent publication of 53 hips (in 40 patients) at 10 year followup reported a 29% collapse rate in the precollapse-stage of ON (10 of 34 hips) following 3 years of continuous alendronate use at 70 mg weekly. The investigators thus concluded that the natural history of untreated ON with more than 70% collapse rate was favorably altered with alendronate use.
In level II, prospective comparative study Nishii et al.23 also found the lower rate of collapse and lesser hip pain after 1-year in ON hip patients receiving alendronate (14 patients with 20 hips, 5 mg daily for 1-year) than patients not receiving alendronate.
Lai et al.24 reported similar efficacy of alendronate in the treatment of nontraumatic ON hip at early-stages (Steinberg Stage-II or III). In a randomized control trial, the authors reported 2 of 29 hip collapse in the alendronate group and 19 of 25 hip collapse in the control group (no treatment or placebo) at 2 years. Radiographic progression was observed in 14% patients in the treatment group compared with 80% in the placebo group. One hip in the alendronate group underwent total hip arthroplasty (THA), whereas 16 hips in the control group needed THA (P < 0.001).
A recent report by Chen et al.25 provided conflicting evidence about bisphosphonate treatment in ONFH. In this prospective, randomized, double-blinded, placebo-controlled trial (level I evidence), there were 65 hips in Stage-IIC and IIIC (University of Pennsylvania classification). They did not notice any significant difference in radiographic disease progression, quality-of-life improvement, and prevention of THA between the alendronate and the placebo groups after 2 years. However, the investigators thought that the study was underpowered to detect statistical significance despite a numerical reduction in the rate of disease progression (61% vs. 66%) and THA conversion (12.5% vs. 15.2%) in the alendronate group.
Even though the efficacy of alendronate is proven in early-stages ONFH, the doses required and duration of therapy is yet to be clearly established. There are reports of jaw necrosis and subtrochanteric fractures with the longterm use of bisphosphonate.20,21,22,23,24,25,26,27,28,29,30 Most of the studies on the efficacy of this drug in ONFH are underpowered and without the control group. With these limitations and potential side effects, the surgical treatment is still favored.32 With the current evidence, alendronate in ONFH patients can be used in a dose of 70 mg weekly for 3 years in Stage-I, II and III (Steinberg classification).20,21,22,23,24,25,26,27,28,29,30,32
Anticoagulants, statins and other vasodilators
Hypofibrinolysis and thrombophilia leading to venous stasis and reduced arterial flow, thus causing increased intraosseous pressure and hypoxic bone death have been postulated as a major and common etiological factor for ON.33,34 Systemic anticoagulation therapy started before irreversible segmental collapse of the femur head may arrest or, speculatively, sometimes reverse the process of ischemic ON.32,33,34,35 In a prospective study, Glueck et al.35 reported outcome of enoxaparin therapy in Ficat Stage-I or II ON hip after 2 years (mean 3 years, range, 2-4 years) of followup. But they included patients of ON hip with either hypofibinolytic or thrombophilic or combined disorders. They observed 95% of hips (19 of 20 hips) with primary ON and 20% (3 of 15 hips) of patients with secondary ON (secondary to corticosteroid use) with no progression of the disease after enoxaparin treatment (60 mg/day for 3 months). In another recent retrospective study of 36 patients with bilateral idiopathic ON having at least one hip in the precollapsed stage (Ficat and Arlet Stage-I and II), Chotanaphuti et al.36 observed no evidence of radiographic progression (P = 0.042) in 57.7% (15 of 26 hips) of hips in patients receiving enoxaparin therapy (6000 units 3 months) compared to 21.7% (5 of 23 hips) of hips in patients not receiving any treatment at the end 2 years. Only 7 patients (14 hips, 38.9%) had coagulation disorder in the enoxaparin group compared with 5 patients (10 hips, 27.8%) in the control group. Anticoagulant therapy has shown clear benefit in these two small studies35,36 and has prevented the progression of ON from precollapsed stage to advanced-stage in idiopathic ON and/or corticosteroid-induced ON.
Lipid lowering agents are also helpful in ONFH particularly in steroid-induced ON.37 Steroid causes hyperlipidemia which increases the fat content of the femoral head also.37,38 It increases intracortical pressure and lead to sinusoidal collapse and osteonecrosis. Statins are lipid-clearing agents that dramatically reduce lipid levels in blood and tissues. Pritchett39 reported that after mean followup of 7.5 years, only 1% of patients taking high-doses of corticosteroids and statin drugs developed ONFH whereas the prevalence was 3-20% in patients receiving high-dose corticosteroids without statins. But Ajmal et al.40 did not find any significant reduction in ON between patients taking steroid and statin versus steroid without statin (4.4% vs. 7%). Further, large randomized studies are needed to establish its efficacy in ONFH. Another vasodilator named Iloprost (a prostacyclin derivative) has also shown benefit after 1-year treatment in patients of osteonecrosis and bone marrow edema.41 Recently adrenocorticotropic hormone has also shown protection against ONFH induced by steroid. The proposed mechanism of action of this drug is based on enhancement of osteoblastic activity and stimulation of vascular endothelial growth factor that enhance neovascularization in the femoral head.42 These drugs are still under trial and need larger study for regular use as a prophylactic agent.
Extracorporeal shock wave therapy
The exact mechanism how ESWT benefits in ONFH remains unknown. However, researchers believe that it enhances neovascularization by stimulating the expression of angiogenic growth factors.26,28,43,44,45,46,47,48,49 In a randomized clinical trial, Wang et al.44 compared ESWT (one episode of ESWT therapy, 23 patients with 29 hips) to CD with nonvascularized fibular grafting (VFG) (25 patients with 29 hips) in early-stages of ONFH. There was a significant improvement (P < 0.001) in pain, as well as hip function (Harris hip score HHS) and a nonsignificant (P = 0.04), but definite decrease in lesion size in ESWT group compared to CD and fibular graft group at the end of 2 years. 79% of patients in ESWT group improved whereas only 29% patients had improvement in bone-grafting group. The same investigators46 reported the long term outcome (mean, 8.5 years; range, 7.7-8.8 years) of the above two groups of patients. They reported that patients with ESWT had significantly better clinical outcomes (pain score and HHS, 76% vs. 21% good or fair; P < 0.001) and decreased need for THA (24% vs. 64%; P 5.002) compared with the surgery group. MRI also revealed significant decrease in lesion size and bone marrow edema in ESWT group compared to the surgery group (P < 0.05).
In another randomized clinical study, Wang et al. compared ESWT alone (25 patients, 30 hips) to combined ESWT and alendronate therapy (ESWT followed by alendronate 70 mg/week for 1-year, 23 patients, 30 hips). There was significant but statistically similar improvement in pain, function and lesion size in both the groups at the end of 1-year. The authors concluded that the addition of alendronate to ESWT did not provide additional benefit.30
Ludwig et al.47 (n = 22 patients) reported significant improvement in pain (visual analogue score VAS 8.5 to 1.2), function (HHS 43.3-92) and lesion size (size decreased or healed in 10 of 14 successfully treated patients) after 1-year of ESWT in ARCO Stage-I to Stage-III ONFH. Hsu et al.,28 in a prospective randomized study of 98 early ON hips compared the ESWT to a cocktail regimen consisting of ESWT, hyperbaric oxygen, and alendronate. At 2 years followup (range, 1.5-4 years), the overall results (clinical, radiograph and MRI) showed 74% improved, 16% unchanged and 10% worsened in cocktail group; and 79.2% improved, 10.4% unchanged and 10.4% worsened in ESWT group (P = 0.717). THR was performed for 10% of cocktail group and 10.4% of ESWT group (P = 0.946). MRI showed a significant reduction in bone marrow edema and a trend of decrease in the size of the lesions in both groups, however, no difference was noted between the two groups. Wang et al.48 in a randomized trial of 55 hips with ARCO Stage-I to III ON reported no significant difference in pain (P = 5 0.4), hip function (P = 5 0.1), and the need for THA (P = 5 0.8) with ESWT (6000 impulses at 28 kV at each session) in patients with SLE and a non- SLE control group at minimum of 2 years’ followup.
Vulpiani et al.49 evaluated the outcome of ESWT in early-stages ONFH (ARCO I to III). At 1-and 2 years followup, all 10 patients (100%) in Stage-I, 9 of 11 patients (81.8%) in Stage-II and 4 of 15 patients (26.7%) in Stage-III reported excellent or good results on Roles and Maudsley score. Patients from ARCO Stage-I group and Stage-II group achieved significantly better results (pain, HHS and Roles and Maudsley score) than patients from ARCO Stage-III group (P < 0.005). Within 2 years, 10 of the 15-II ON hip needed arthroplasty. ARCO Stages I and II lesions were unchanged on radiographs and on MRI. They concluded that ESWT in ARCO Stages I and II slows down the worsening of the grade of the ONFH and improve clinical features. Short followup and small studies on ESWT are the major limitations for its restricted use.
Pulsed electromagnetic therapy
Pulsed electromagnetic therapy is thought to favorably affect early-stage ON through stimulation of osteogenesis and angiogenesis similar to ESWT.26,50,51,52,53,54,55,56 Massari et al.,55 37 in their retrospective analysis of 76 hips treated with electromagnetic field stimulation in Ficat Stage-I to III, reported that the 94% of hips in Stage-I and II avoided the need for THA with a significantly higher proportion of hips in Stage-III progressing to THA at a mean followup of 2 years. At present, evidence in favor of electromagnetic stimulation is limited and further research is needed to explore its potential role in early-stage ON.
Hyperbaric oxygen
Hyperbaric oxygen improves oxygenation, reduces edema by causing vasoconstriction, and induces angioneogenesis; thus causing a reduction in intra osseous pressure and improvement in microcirculation.28,29,57,58,59 Reis et al.,57 observed normal MRI in 13 hips after hyperbaric oxygen treatment (100% oxygen at 2-2.4 atmospheric pressure for 90 min by mask for 100 days) to 12 patients with 16 ONFH, all with Steinberg stage 1 disease. Camporesi et al.58 also reported clinical improvement at followup of 7 years in the study of 19 patients randomized to receive 30 treatment doses of either hyperbaric oxygen or hyperbaric air for a total period of 6 weeks. None of the hyperbaric oxygen group patients needed THA till the time of final followup. Because of limited data, the use of hyperbaric oxygen in ONFH is controversial.
(B) Operative treatment
Surgical treatment for precollapsed stage ONFH involves hip preserving procedures (CD, nonvascularized bone-graft, vascularized bone-graft) whereas prosthetic hip surgery is reserved for advanced-stage of collapse and arthritic hip.
Core decompression
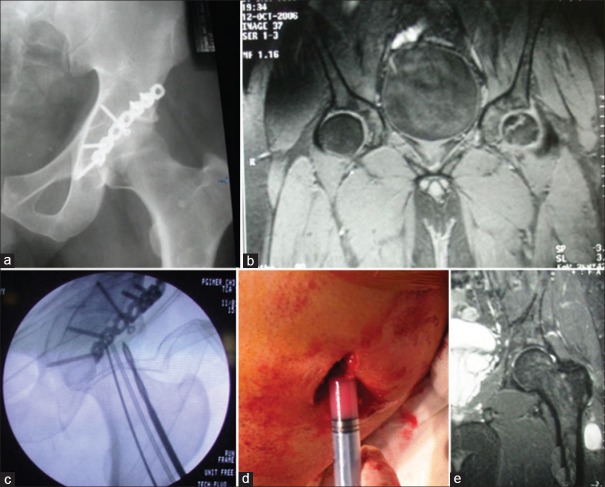
Core decompression is the most commonly performed surgical procedure for treatment of early ONFH. It decreases the intraosseous pressure in the femoral head and increases blood flow to the necrotic area, thus augmenting neobone formation [Figures 1 and 2]. It has been considered as the only cost-effective surgical procedure for ONFH,60,61 but the success of the treatment is largely dependent on the etiology and radiographic parameters such as lesion size, location or collapse of the lesion.62,63 The overall success rate as defined by the need for further surgery has varied between 40% and 80% across multiple studies at 2-7 year followup.60,61,62,63,64,65,66,67,68,69 Conventional core decompression (CD) was performed using 8-10 mm cannula or trephine which had the potential risk of subtrochanteric fracture and hip joint penetration.
Figure 1.
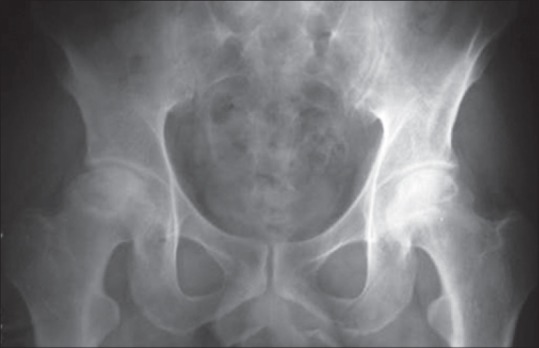
Radiograph of pelvis with both hip joints anteroposterior view showing a bilateral idiopathic osteonecrosis of hip in early stage
Figure 2.
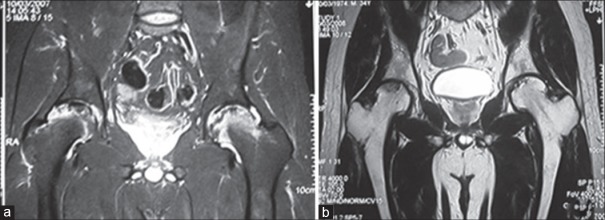
Magnetic resonance imaging of the same patient demonstrates edema inside femoral head as well as effusion in the hip joint (a-preoperative) and, after 1-year of core decompression the lesion has diminished in size, the edema has subsided and hip joint effusion is resolved (b-postoperative 1-year)
But the technique of CD has improved overtime.66,67 The procedure of CD by multiple small drillings was presented in the annual ARCO meeting by Kim et al., in 2004.65 They compared the results of the efficacy of two decompressive methods (multiple drilling MD vs. conventional CD) for the treatment of precollapse ONFH in a consecutive series of 54 patients. They reported that radiographically and clinically, high failure was significantly related to the larger size and laterally located lesion in both groups. The average preoperative and the last HHS was 73.7-86.7 in single CD and 74.6-87.0 in MD. The group who had undergone MD had significantly longer time before the collapse (mean 42.3 months vs. 22.6 months, P = 0.011) and the lower rate of collapse within 3 years after operation (55.0% vs. 85.7%). In a systematic review, Marker et al.67 compared the outcome of recent technique of CD to that of old conventional technique. Recent technique of CD had a better result than old conventional technique. There were 1337 hips treated before 1992 and 1268 hips between 1992 and 2007. The proportion of patients surviving without additional surgery increased (P < 0.001) from 59% (range, 29-85%) in the earlier studies to 70% (range, 39-100%) in the more recent reports. Similarly, the radiographic success also increased (P = 0.027) from 56% (range, 0-94%) for the pre-1992 cohort to 63% (range, 22-90%). Stratification by Ficat stage showed there were fewer (P < 0.001) patients who were Ficat stage III after 1992. CD thus is an effective procedure for early ONFH mainly in Ficat stage I and II. Recent technique of CD involves MD of the necrotic lesion of femur head which is an easy, simple and safe procedure. A recent study by Al Omran68 also reported similar observation as noted by Marker et al. in their review. In his series 61 patients underwent a classical 8 mm drilling and 33 patients underwent 3.2 mm diameter MD. They observed significant improvement in outcome in both groups of patients, but there was no difference in the outcome between the groups at the end of 2 years (100% improvement in pain and HHS of Ficat stage I patients both groups n = 19, 80% in CD vs. 78% in MD group had better pain and HHS in Stage-IIA n = 39, 52% in CD and 52.8% MD had improved pain and HHS in Stage-IIB n = 36). In a retrospective study, Song et al.69 reported the outcome of MD in 163 hips as a treatment for Ficat stage I to III ON. They reported clinical success (defined as HHS >75 and no need of additional surgery) in 79% (31 of 39 hips) of Stage-I hips and 77% (62 of 89 hips) of Stage-II hips. 88% (52 of 59 hips) hips with small to medium-sized lesions required no additional surgical procedure at a mean followup of 7.2 years (range, 5-11.2 years).
Mont et al.66 reported 71% successful outcome (32 of 45 hips) following MD (2-3 perforations) with 3 mm steinmann pin. They observed better outcome in small and medium size lesions of Stage-I compared with large lesions and Stage-II disease. Current recommendation is that CD should be performed with 3.2 mm drill bit with multiple perforation (at least 3). This is an established modality of treatment for early-stage ONFH. This procedure can be safely performed under image intensifier with percutaneous method with minimal risk of subtrochanteric fracture or inadvertent hip joint penetration [Figures 1 and 2].
Mesenchymal stem cells implantation or growth factor based treatment strategies
To augment osseous regeneration in the necrotic lesion site, the applications of osteogenic or angiogenic precursor cells with or without growth factor is an alluring possibility. Adult tissue derived mesenchymal stem cells (MSCs) application represents a highly promising option for treatment of ONFH in the precollpased stage [Figure 3].70 Many researchers have documented decreased quantity of endothelial progenitor cells and colony forming units in patients suffering from ONFH.71,72 Besides that, there is impaired migratory capacity of endothelial progenitor cells and increased cellular senescence resulting in decreased angiogenesis in patients of ONFH.70 All these reasons justify the potential role of stem cells or growth factors in treatment of precollapsed ONFH. MSCs implantation has capability to differentiate into multiple cell lineages including the osteoblast, chondrocytes and adipocytes. This property of stem cells has been observed in an experimental dog model while evaluating its effect in ON.73 However, the efficacy of stem cells in healing ON lesion is because of the osteoblastic differentiation ability or secondary to release of growth factors or cytokines remains unclear. In addition, augmentation of neovascularization or angiogenesis property of stem cells have been described by many researchers which ascribes another reason for its potential role in the treatment of ON.
Figure 3.
(a) Plain x-ray anteroposterior view of left hip in a 32-year old young patient showing posttraumatic AVN in its early stage (b) MRI of both hips showing early avascular changes (c) Intraopartaive photographs demonstrating core decompression and (d) bone marrow concentrate instillation inside the core tract. (e) After 15 months, the necrotic lesion has completely healed
Mesenchymal stem cells can be implanted either as bone marrow concentrate mononuclear cells [Figure 3c and d] or ex vivo culture expanded bone marrow-derived stem cells.70 Application of stem cells in ONFH was pioneered by Hernigou et al.74,75,76 The authors injected the mononuclear cells fractions of the bone marrow aspirate from the iliac crest and injected into the necrotic area. Nine out of 145 patients with early-stage ONFH (Steinberg stage I or II) and 25 out of 45 patients with advanced ONFH (Steinberg stage III or IV) required THR.62 Subsequently Gangji et al.,77,78 Yan et al.79 and Deltro et al.80 also proved the safety and efficacy of MSCs in ONFH.
In a prospective, randomized and double blind trial, Gangji et al.81 reported the outcome of isolated CD and the autologous bone marrow implantation at followup of 5 years. In 24 ON hips at ARCO Stage-I and II, they observed significant improvement in pain and lower rate radiographic progression in bone marrow implantation patients (23%, 3 of 13 hips) compared to CD cohort (73%, 8 of 11 hips). However, they did not notice a significant difference in both the groups in terms of the need of subsequent THA (15% in bone marrow implantation group vs. 27% in CD). A retrospective study by Liu et al.82 reported the outcome of CD and hydroxyapatite/polyamide implantation with or without bone marrow MSCs implantation. At the end of 2 years, they observed better-clinical success (HHS and pain score) and radiographic improvement in stem cells group. 21.4% (6 of 28 hips) of ON hips in bone marrow group collapsed compared to 59.3% (16 of 27 hips) in CD group. In another comparative prospective randomized study, Zhao et al.83 reported significantly lower radiographic progression to collapse in the bone marrow group (23%, 10 of 44 hips) than the CD (4%, 2 of 53 hips) group at 5 years followup in ARCO Stage-I and II ONFH. Within each stage of disease, the author reported significant improvement in HHS and radiographic necrotic volume in the bone marrow group compared to CD (P < 0.05). Sen et al.,84 in a recent randomized control trial of 51 hips with ARCO Stage-I and II ON comparing CD with a bone marrow mononuclear cell instillation, reported significant improvement in pain, deformity, and hip survival in the bone marrow concentrate (P < 0.05) group after 2 years.
The efficacy of ex vivo expanded autologous bone marrow-derived stem cells have been studied in few small studies.85,86 Kawate et al.85 reported the outcome of VFG combined with a synthetic β-TCP ceramic and cultured bone marrow-derived MSCs in 3 patients with advanced-stages of steroid-induced ONFH (Steinberg stage III or IV). There was no progression in any patients within 34 month followup. Noth et al. also observed promising result with similar treatment.87
The growth factors secreted by osteogenic cells, platelets, and inflammatory cells (bone morphogenetic proteins BMPs, insulin-like growth factor-1 and -2, transforming growth factor-β1 (TGF-β1), platelet-derived growth factor, and fibroblast growth factor-2) are functionally involved in bone healing.70 Lieberman et al.88 treated 15 patients (17 hip joints at Ficat stage II or III) with CD and allogenic, antigen-extracted, autolyzed fibula grafts, combined with 50 mg of recombinant human BMP-2 and noncollagenous protein. Radiographic progression of the disease was prevented in 14 of 17 hips at an average of 53 months (range, 26.94 months). Only one of 15 hips, that were classified as Ficat stage IIA developed collapse. The other two hips that progressed already had collapse of the femoral head before the procedure.
Mont et al.89 used a triphasic bone substitute consisting of demineralized bone matrix, processed allograft bone chips, and a thermoplastic carrier plus the addition of BMP-7 for ONFH in 19 patients (21 hips). A successful clinical outcome (HHS >80) was observed in 86% percent at mean followup of 48 months. In another retrospective study, Seyler et al.90 used autologous, nonvascularized bone-grafts loaded with BMP-7. There were 33 patients (39 hips: 22 at Ficat stage II and 17 at Ficat stage III) with ONFH. At the mean time of 36 months, only four of 22 Ficat stage II hips and 11 of 17 Ficat stage III hips needed THR. In several other animal models, the efficacy of BMP-2, BMP-7 and 14 have been evaluated in ONFH. However, the studies on the efficacy of growth factors are still in its preliminary stage. Lack of the control group, small patient numbers and short duration of followup are the major limitations to consider these treatments as a definite modality of management.
The regenerative medicine also widens its application in ONFH in all its stages. Few researchers have used cartilage regenerative technique such as osetochondral graft implantation, mosaicplasty, autologus chondroctes transplantation and acellular matrix application for treatment of ONFH in its advanced-stage (Ficat III and IV, ARCO III and IV).91,92,93,94 Gagala et al.95 recently reported the outcome of autologous osteochondral transfer in ONFH of 20 patients with 21 hips. Seven patients with ARCO IIA and IIB were treated with Osteo Articular Transfer System alone, 13 patients with ARCO IIC, III and IV were treated with OATS and morselized bone allograft. Hip survival in OATS group was 85.71% after 4 years (one conversion to THR) and 61.54% in OATS/allograft group after 3 years (five conversions to THR). Cartilage regenerative techniques include a surgical dislocation of the hip anteriorly to access the osteochondral defect, thus, it is more invasive and surgically demanding. Very few cases have been reported with the above methods, and it has shown variable results; thus, it is very difficult to comment on the efficacy of these techniques.
Nonvascularized bone graft
Nonvascularized bone-grafts (usually tibial autograft and fibular autograft or allograft) are used to support subchondral bone and articular cartilage after removal of the necrotic lesion from the femoral head. The osteoconductive and osteoinductive properties of bone-graft help in healing of the osteonecrotic lesion. This modality of treatment has been reported to be successful in precollapse, and early postcollapse (<2 mm collapse) ONFH when the articular cartilage is relatively undamaged. The surgeons commonly adopt this procedure in Ficat stage I and II ONFH when CD fails.32 Three methods of bone-grafting technique have been described: Phemister technique (grafting through CD track), trap door (grafting through a window created in the femoral head) and light bulb procedure (grafting through a window created in femoral neck or femoral neck-head junction). Although these modalities of treatment is rarely been used now a days as an isolated procedure, many researchers now use these techniques in combination with growth factors and various bone-graft substitutes.32 The position of bone-graft within the necrotic lesion or at the transition zone between necrotic lesion and normal bone has not shown any difference in the outcome, but the type of graft (tibail autograft is better than fibular graft) has a definite impact on the outcome.91,92,93,94,95,96,97 Still though, the findings of finite-element analyses recommend the graft to be placed as close as possible to the subchondral bone, and in the lateral part of head.98
Many studies have reported encouraging results with the use of nonvascularized bone-graft (70-90% excellent result at 2-7 years followup)98,99,100,101,102,103,104 but few studies have shown a high rate of radiological progression (Nelson and Clark,105 Dun and Grow.106). Seyler et al.90 reported 83% survivorship in stage I and II ON and 78% survivorship at a minimum follow up of 2 years in 39 hips using the light bulb procedure. In a retrospective study (80 hips in 65 patients), Keizer et al.97 used tibial autograft and fibular allograft in 18 and 62 patients of ONFH respectively. Of the 78 hips available for evaluation, 42 patients (54%) had clinical failure (secondary surgery or a poor Merle d’Aubigné and Postel score (<8 points) at a mean of 4.5 years. Kaplan–Meier survivorship analysis with clinical and radiological end-point showed a mean survival rate of 55% at 5 years and a mean of 33% at 10 years. Survivorship analysis with revision surgery as an end-point showed a mean survival rate of 66% (95% confidence interval [CI], 55-77) at 5 years and of 52% (95% CI, 39-65) at 10 years. When survivorship analysis was stratified according to Ficat stage, it showed a mean survival rate of 83% for stage 0, 0% for stage I, 80% for stage IIA, 63% for stage IIB, 54% for stage III and 56% for stage IV at 5 years. On comparative evaluation, tibial autograft showed a significantly better survival than fibular graft (P = 0.002). At 6 year followup, the survival rate for tibial graft was 75% (95% CI, 54-96) compared to fibular allograft which showed a mean survival rate of 42% (95% CI, 30-55).
The effect of autologous nonvasculairized fibular graft in combination with BMP-7 was evaluated by many investigators.88,89,107 Use of cancellous chips admixed with BMP-7 during nonvascularized grafting via a trapdoor technique avoided the need for the secondary procedure in 80% of stage II and III ONFH.76,77 Papanagiotou et al.107 in a recent study treated 7 hips which were in precollapsed stage (5 Steinberg stage II and 2 Steinberg stage III). Five hips maintained the sphericity of head and two failed (needed THR) after an average followup of 4 years (2-5.5 years). The author observed a marked improvement of function (mean HHS increase of 49.2) and decrease of pain level (mean VAS decrease of 5) in these five hips.
Porous tantalum implant
Porous tantalum implants provide structural support like that of bone-graft and avoid the risks of infectious complications and donor site morbidity as reported with the use of allograft and autograft respectively. These rods are highly porous (>80% volume) and thus allow secure and rapid bone growth.108,109,110,111,112,113,114 With the addition of bone marrow, growth factors, or bisphosphonates, the efficacy of porous tantalum rod can be further imporved.111,112
Veillette et al.,108 prospectively evaluated 54 patients (60 hips) in whom ONFH was treated with CD and insertion of a porous tantalum rod. At the mean followup of 24 months, 15.5% (n = 9 patients) of patients needed THA. The overall survival rates were 91.8% at 12 months, 81.7% at 24 months, and 68.1% at 48 months. The absence of chronic systemic diseases resulted in a survival rate of 92% at 48 months. Varitimidis et al.115 prospectively evaluated 27 patients who were treated with tantalum rod in nontraumatic ONFH. At the mean followup of 38 months (15-71 months), 13 of 26 hips remained at the same radiographic stage, and 13 deteriorated. Mean HHS improved from 49 to 85, 6 patients needed THA. Survivorship, with conversation to THA, was 70% at 6 years. The authors concluded that tantalum rod implantation is a safe “buy-time” technique, especially when other joint salvage procedures are not an option. But he stressed on careful patient selection (early-stage disease) and careful rod insertion for favorable results.
Tsao et al.113 reported early clinical results of these implants and observed better survival rates (92% at 48 months) than hips treated with CD and VFG. Shuler et al.109 reported a survival rate of 86% after 39 months after insertion of the tantalum implant, and 6 weeks protected weight-bearing. Nadeau et al.116 reported a survival rate of 78% after 12 months and overall success rate of 45%. The reason behind less success rate is because of inclusion of patients with advanced collapse. Tanzer et al.112 reported the results of a retrieval analysis of 15 clinically failed porous tantalum implants that were associated with little bone ingrowth and insufficient mechanical support of subchondral bone. Floerkemeier et al.110 reported 44% survival after implantation of a tantalum rod in ONFH (19 patients with 23 hips) at mean followup of 1.45 years. Thirteen hips needed THA. The authors concluded that treatment of the early-stage of ONFH with CD combined with the implantation of an osteonecrosis intervention rod seems to be no better and no worse than simple CD. Furthermore, the procedure is associated with increased costs and a prolonged operation time. However, the author did not notice any difficulty in removing implant while converting to THR as warned by many surgeons before in their reports. At this stage, porous tantalum rod cannot be considered as a standard mode of treatment in ONFH.
Vascularized bone graft
Vascularized bone-grafting is a recommended modality of treatment for early ONFH (Ficat stage I to III).117,118,119,120,121,122 The graft provides a viable structural support (eg., vascularized iliac crest graft, vascularized fibula graft) and prevents joint collapse Figure 4.117,118,119,120,121,122,123,124,125 As the vascularity is preserved, and the graft has inherent osteogenic potential, it augments bony healing in the necrotic lesion site. However, the outcome is less rewarding in large lesions where the involvement is more than 50% of the femoral head, and the collapse is more than 2 mm. Patients with a history of smoking, alcoholism, peripheral vascular disease or other risk factors should not be considered for the procedure. The major demerit of this technique is its surgical complexity and increased surgical duration.117,118,119,120,121,122,123,124,125
Figure 4.
(a) X-ray (R) hip joint anteroposterior view showing advanced stage osteonecrosis of hip treated with vascularized fibular graft, (b) X-ray pelvis both hip joints anteroposterior view (b) after 11 years showing the lesion has healed and femoral head has maintained the sphericity
Muscle pedicle bone graft
Meyers (1978) 117 first reported the application of muscle pedicle bone-graft for treatment of ONFH. They observed good result in all patients with Stage I and II disease but only in 33% patients in advanced disease (Ficat III and IV disease) at 6 months to 2 years followup. Lee and Rehmatullah118 reported 70% success rate with muscle pedicle bone-graft in idiopathic ONFH. Baksi (1991)119 reported the outcome of 68 hips (61 patients) treated with many types of muscle pedicle bone-graft at 3-12 years (mean, 7 years) followup. Of the several types of muscle pedicle bone-grafts used, the tensor fascia lata anteriorly, and the quadrates femoris posteriorly were preferred. About 83% patients had good or excellent results.
Vascularized Iliac crest graft
The method described is recommended for treatment in the Ficat stage II and early-stage III, when necrosis does not yet involve the complete femoral head. Iwato et al.120 observed 74% success rate (17 of 23 hips) with vascularized iliac crest graft use in ONFH. Most of the patients had no femoral head collapse preoperatively, but more than 50% progressed to radiographic collapse at a mean followup of 3 years. Eisenschenk et al.126 reported stable disease after 5 years followup in 56% of ONFH patients treated with iliac crest graft perfused by the circumflexed ilium profunda artery. Matsusaki et al.,121 used vascularized pedicle iliac bone-graft combined with trans-trochanteric anterior rotational osteotomy in patients with extensive necrosis in whom the necrotic area occupied more than two-thirds of the weight-bearing zone of the femoral head. There was significant clinical improvement and no disease progression in 12 of 17 hips (71%) after mean followup of 50.7 months. They concluded that vascularized pedicle iliac bone-graft combined with trans-trochanteric anterior rotational osteotomy to treat AVN of the femoral head is promising for joint preservation. In a retrospective study, Babhulkar127 reported only one progression to collapse (treated with THR) in 31 patients after treatment with CD and iliac crest with deep circumflex iliac vessels. He included patient with nontraumatic ONFH in ARCO stage IIB and IIIC only and followed them up till 5-8 years.
Vascularized fibular graft
Fang et al.128 reviewed six articles on vascularized fibular graft (VFG) used as a treatment of ONFH published between January 1980 and April 2012. In this meta-analysis of six studies (n = 984 patients), there were 122 conversions to THA (16.5%) from 740 patients who were treated with VFG. In the remaining 244 patients treated with other methods (CD, non-VFG, and vascularized iliac graft), there were 104 conversions to THA (42.6%). VFG can achieve lower conversion rate than the other three methods (odd ratio [OR] 0.19; 95% CI, 0.13-0.28; P = 0.001; I2 = 24%). Among patients evaluated with radiographs for progression to collapse in 3 of these studies (n = 122 patients), a total of 14 of 84 (16.7%) hips treated with VFG collapsed, and a total of 56 of 88 (63.6%) hips treated with non-VFG collapsed. The result favored vascularized grafting more than nonvascularized grafting (OR, 0.09; 95% CI, 0.01-0.57; P < 0.05). In precollapse phase (steinerg I and II), VFG had got a better hip salvage than other three methods (CD, N-VFG, vascularized iliac grafting). Among the 270 hips, a total of 16 of 163 (9.8%) hips treated with VFG failed, and a total of 43 of 107 (40.2%) hips treated with non-VFG failed (OR, 0.17; 95% CI, 0.09-0.33; P = 0.001; I2 = 5%). In precollapse and early postcollapse phase (Steinberg II and III) 116 of 705 (16.5%) hips treated with VFG failed, total of 83 of 194 (42.8%) hips treated with non-VFG failed (OR, 0.17; 95% CI, 0.11-0.26; P < 0.001). A total of 30 complications (23.8%) were reported in 126 VFG treated patients and 13 complications (8.9%) in 146 patients treated with CD, non-VFG, and vascularized iliac graft (n = 272 total patients followed, P = 0.01). However, in the weighted test for overall effect, this difference does not reach significance (OR, 3.44; 95% CI, 0.81-14.62; P = 0.09).
Urbaniak et al.129 in their study of 103 hips treated with vascularized fibula grafting, reported 91% survivorship in stage II and 77% survivorship in stage III at a final followup of 5 years. Yoo et al.128 also reported excellent results, with 89% survivorship in 124 hips in stage II and III at a minimum of 10 years’ followup (mean, 13.9 years; range, 10-23.7 years). Eward et al.130 recently reported the long term followup data (mean, 14.4 years; 10.5-26 years) on 65 hips with precollapse-stage ON treated with vascularized fibula grafting; 75% of the hips survived without the need for THA at a minimum 10 year followup. The investigators noted that demographic and radiographic factors were not associated with changes in graft survivorship.
Proximal femoral osteotomy
The underlying principle behind proximal femoral osteotomies in ONFH is to rotate the necrotic femur head away from the load-bearing area and replace it with the uninvolved healthy portion of the head. It also reduces the intraosseus venous pressure and improves vascularity. There are mainly two types of osteotomy described: Trans-trochanteric rotational osteotomy and intertrochanteric varus or valgus osteotomy (combined with flexion or extension). The success rates of these osteotomies have been reported between 70% and 93%.15,32,131,132,133,134,135
Jacobs et al.136 had 73% success rate at 5.3 years followup after intertrochanteric osteotomy in ONFH. Maistrelli et al.,137 reported satisfactory results in 71% of ON hips after 2 years of intertrochanteric varus or valgus osteotomy and it dropped to 58% at 8.2 years. After 10.2 years followup, Gallinaro and Masse138 observed success rate in 62.5% of cases after flexion osteotomy. Flexion valgus osteotomy with autogenous bone-grafting has shown a survival rate of 87% without replacement arthroplasty 10 years after operation.139
The success rate of rotational osteotomy has been described in 78% of patients after 3-16 years followup.136 Zhao et al.,140 in their study of 73 hips at a mean followup of 12.4 years (range, 5-31 years), reported that 91.8% (67 of 73 hips) of the hips remained intact and did not need conversion to a THA following curved trans-trochanteric varus osteotomy. There was a significant improvement in HHS after surgery, and the mean postoperative intact ratio was 57.2% (range, 27-100%). To prevent progressive collapse they reported that the cutoff for the postoperative intact ratio was 33.6% (sensitivity, 82.9% and specificity, 100%; P =0.001). Moreover, to prevent collapse and joint space narrowing, the cutoff intact ratio was reported to be 41.9% (sensitivity, 88.9%; specificity, 92.1%; P =0.001). Sakano et al.141 similarly reported that, in their series of 20 hips, 90% (18 hips) did not collapse or require conversion to a THA following trans-trochanteric varus osteotomy at a mean followup of 4 years (range, 0.7-4.1 years). The mean postoperative intact ratio was 61%, and the reported mean elevation of the greater trochanteric was 1.2 cm (range, 0.5-2 cm). Ito and colleagues recently reported the longterm results of varus half wedge osteotomy in 34 hips at a mean followup of 18.1 years (range, 10.5-26 years). Overall, 74% (25 hips) had satisfactory results with a mean HHS of more than 80 points despite having a mean limb length discrepancy of 19 mm (range, 8-36 mm). The investigators concluded that the varus osteotomy of the proximal femur provides favorable longterm outcomes in the presence of more than one-third of normal superolateral bone.
The major point against limited acceptance for the above osteotomy technique is because of its technical complexity. All these articles are from single surgeon's series and are of level IV evidence. The technique has never been compared to any other method of treatment and hence it is difficult to establish the superiority of this technique to other methods described. Osteotomies are best suited to patients not being treated with longterm steroids, with minimal osteoarthritic changes, with no loss of joint space or acetabular involvement and small combined necrotic angle (Kerboul's angle <200).15,32
Arthroplasty
Patients with ONFH may need THR when all other modalities of treatment have failed, or joint is arthritic secondary to advanced collapse (more than 2 mm). THR is considered as a last resort of treatment because ONFH victims are usually young adults whose functional demands are high and also, there is a high possibility of the need of revision arthroplasty in such patients. Polyethylene wear and osteolysis leading to aseptic loosening are major concerns. Literature has reported 8-37% of aspectic loosening following THR in ONFH.32 With the introduction of third generation ceramic bearings, porous materials and high cross-linked polyethylene, the survivorship of THR has increased.142
Bipolar arthroplasty is no more an acceptable treatment option for ONFH. Young patients, high incidence of protrusion acetabuli, increased rate of loosening and better THR bearings are major reasons for its unacceptability. Revision rate ranging from 13.9% to 27.6% have been reported with bipolar hemiarthroplasty in ONFH after average followup of more than 5 years.142,143,144,145,146
Limited femoral resurfacing arthroplast is also a treatment option in ONFH patients with Ficat and Arlet Stage-III disease, a combined necrotic angle of >200° or >30% involvement, femoral head collapse of >2 mm, and no evidence of damage to the acetabular cartilage.15,32 Although it had shown satisfactory results for up to 10 years, a few recent studies has shown less predictable outcomes of these procedures with overall hip survivorship reaching 75.9% at 3 years.147 In another study by Cucklere et al. 31% failure was noted in a mean followup of 4.5 years (18 failure of 59 hips).148
Better implant designs have improved the outcome of THR in ONFH. In a systematic review of 67 studies (3277 THR in 2593 patients) Johannson et al.149 reported mean survivorship of 97% at 6 years followup in patients operated after 1990. Stratification of the patients as per their associated risk factors demonstrated higher revision rate in sickle cells disease, Gaucher disease and end-stage kidney disease or transplant patients. The revision rate was lower in patients with SLE, idiopathic or after heart transplant. With the poly on poly bearings (n = 172 THR), Min et al.150 reported 100% survivorship at 7.2 years followup. In a study by Kim et al.,151 ceramic head on polyethylene bearing has shown 100% survivorship (excluding infection) at an average 8.5 years followup. Furthermore, when literature was analyzed to search for the component which commonly fails, cup wear or loosening was found to be more common then stem loosening. Kim et al.151 (n = 148 THRs), reported 98% stem survivorship (cemented and cementless) but only 85% cementless cup survivorship after 17.3 years. Issa et al. have expected a still better survivorship with the introduction of tantalum or titanium porous acetabular cup recently.142
The surgeons sometimes expect difficulty in performing a THR in ONFH. Previous surgery with altered hip biomechanics, presence of hardware, bone/fibula grafts, screw track, scar and fibrosis around hip may evoke potential problem. However, many studies have reported that the medium term result of THR is not affected by previous surgery in ONFH.152,153,154,155,156 Helbig et al.157 demonstrated no complications or component loosening, at a mean followup of 54 months in the series of 15 hips, that were converted to THR after they had previous CD. In a study of 15 failed trans-trochanteric rotational osteotomies that were converted to THR, Kawasaki et al.152 reported no significant differences in implant survivorship, stability, or HHS compared with a matching group of 16 hips that had only undergone a primary THR at a mean followup of 5 years. Ball, Le Duff, and Amstutz compared 21 failed hip resurfacings that were converted to a standard THR, with 64 standard THRs (both cohorts included osteonecrosis patients), and reported no differences in aseptic loosening, dislocations, HHS or complications between the two groups.155 Issa et al.158 evaluated 92 hips in 87 patients who had failed prior hip preserving surgery (35 hips that had previous resurfacing, 9 hips that had a hemi-resurfacing, 29 hips that had a nonvascularized bone-grafting, and 19 that had a CD). These patients were compared with 121 hips in 105 osteonecrosis patients who underwent THR and had no prior surgical interventions. At a mean followup of 75 months, they reported no significant differences in survivorship, clinical, and radiological outcomes among the groups.
As described earlier, patients who have sickle cell disease, Gaucher disease, or end-stage kidney failure and/or posttransplantation have been reported to be at an increased risk for revision following THA. However, even in these high-risk groups, outcomes of THR have improved over time.142,159 Issa et al.160 evaluated 42 THRs for osteonecrosis in 32 sickle cell patients who had a mean age of 37 years compared with 102 THRs in 87 nonsickle cell osteonecrosis patients who had a mean age of 43 years. At a mean followup of 7 years (3-10.5), they reported no significant differences in aseptic implant survivorship (95% vs. 97%), HHS (87 vs. 88), and SF-36 physical (43 vs. 44) or mental component scores (59 vs. 58) between the two patient cohorts respectively. Marulanda et al.161 compared the outcomes of two types of peri-operative management (conservative or aggressive protocols) in three patients who had sickle cell anemia and had undergone 31 separate orthopedic surgeries for ONFH. In the conservative protocol, patients received packed red blood cells preoperatively to increase the hemoglobin level to a minimum of 10 g/dL. Fresh frozen plasma or packed red blood cells were given only when excessive bleeding occurred intraoperatively. In the aggressive protocol, patients received preoperative packed red blood cells with the goal of keeping hemoglobin levels between 9 g/dL and 11 g/dL, and of lowering the hemoglobin S levels to <30%. Fresh frozen plasma was given when patients’ Factor VII levels were less than 30%. They reported although both protocols were safe in managing these patients, the most aggressive protocol had resulted in lower rates of postoperative complications, transfusions, and the need to resort to supplementary oxygen.
Chang et al.162 evaluated 74 hips in 52 patients who underwent THR for ONFH after kidney transplantation with cementless THRs. They reported 96.6% cumulative implant survivorship at a mean followup of 10.2 years, which is comparable with survivorship due to other causes of THR. In the light of these findings, the outcomes of THR even in these high-risk patients are improving, potentially due to improved medical and surgical management, as well as the use of modern prosthetic designs, including cementless acetabular and femoral fixation.
CONCLUSION
Symptomatic hip osteonecrosis is a disabling condition with poorly understood etiology and pathogenesis. Numerous treatment options for hip osteonecrosis have been described including nonoperative modalities, joint preserving procedures, and THR. Nonoperative or joint preserving treatment may improve outcomes when an early diagnosis is made before the lesion has become too large, or there is radiographic evidence of femoral head collapse. The presence of a crescent sign, femoral head flattening, and acetabular involvement indicate a more advanced-stage disease in which joint preserving options are less effective than THR. The algorithm of ONFH management as presented is an effective treatment strategy which is practically feasible and based on sound evidence [Figure 5].
Figure 5.
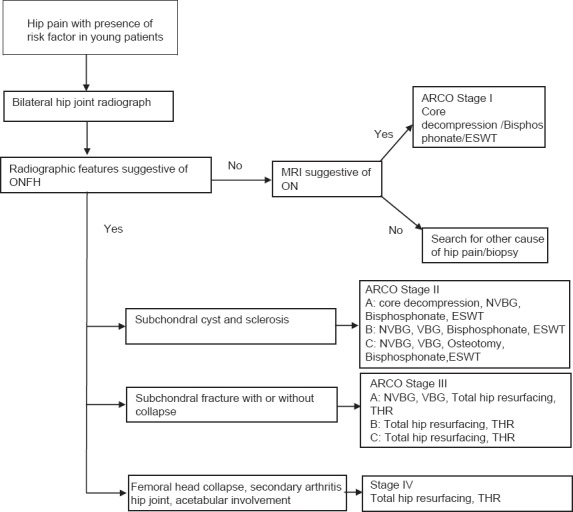
An algorithm of ONFH management
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Powell C, Chang C, Naguwa SM, Cheema G, Gershwin ME. Steroid induced osteonecrosis: An analysis of steroid dosing risk. Autoimmun Rev. 2010;9:721–43. doi: 10.1016/j.autrev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 3.LaPorte DM, Mont MA, Mohan V, Jones LC, Hungerford DS. Multifocal osteonecrosis. J Rheumatol. 1998;25:1968–74. [PubMed] [Google Scholar]
- 4.Lang P, Jergesen HE, Genant HK, Moseley ME, Schulte-Mönting J. Magnetic resonance imaging of the ischemic femoral head in pigs. Dependency of signal intensities and relaxation times on elapsed time. Clin Orthop Relat Res. 1989;244:272–80. [PubMed] [Google Scholar]
- 5.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–5. doi: 10.1007/s007760200108. [DOI] [PubMed] [Google Scholar]
- 6.Fordyce MJ, Solomon L. Early detection of avascular necrosis of the femoral head by MRI. J Bone Joint Surg Br. 1993;75:365–7. doi: 10.1302/0301-620X.75B3.8496201. [DOI] [PubMed] [Google Scholar]
- 7.Konishiike T, Makihata E, Tago H, Sato T, Inoue H. Acute fracture of the neck of the femur. An assessment of perfusion of the head by dynamic MRI. J Bone Joint Surg Br. 1999;81:596–9. doi: 10.1302/0301-620x.81b4.9013. [DOI] [PubMed] [Google Scholar]
- 8.Hirata T, Konishiike T, Kawai A, Sato T, Inoue H. Dynamic magnetic resonance imaging of femoral head perfusion in femoral neck fracture. Clin Orthop Relat Res. 2001;393:294–301. doi: 10.1097/00003086-200112000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell DG, Rao VM, Dalinka MK, Spritzer CE, Alavi A, Steinberg ME, et al. Femoral head avascular necrosis: Correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987;162:709–15. doi: 10.1148/radiology.162.3.3809484. [DOI] [PubMed] [Google Scholar]
- 10.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 11.Kerboul M, Thomine J, Postel M, Merle d’Aubigné R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56:291–6. [PubMed] [Google Scholar]
- 12.Ha YC, Jung WH, Kim JR, Seong NH, Kim SY, Koo KH. Prediction of collapse in femoral head osteonecrosis: A modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am. 2006;88(Suppl 3):35–40. doi: 10.2106/JBJS.F.00535. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 14.Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, et al. Osteonecrosis of the hip: Management in the 21st century. Instr Course Lect. 2003;52:337–55. [PubMed] [Google Scholar]
- 15.Sen RK. Management of avascular necrosis of femoral head at pre-collapse stage. Indian J Orthop. 2009;43:6–16. doi: 10.4103/0019-5413.45318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–74. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–78. doi: 10.1097/00003086-199603000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: A systematic literature review. J Bone Joint Surg Am. 2010;92:2165–70. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 19.Hernigou P, Poignard A, Nogier A, Manicom O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86-A:2589–93. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Agarwala S, Jain D, Joshi VR, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology (Oxford) 2005;44:352–9. doi: 10.1093/rheumatology/keh481. [DOI] [PubMed] [Google Scholar]
- 21.Agarwala S, Shah S, Joshi VR. The use of alendronate in the treatment of avascular necrosis of the femoral head: Followup to eight years. J Bone Joint Surg Br. 2009;91:1013–8. doi: 10.1302/0301-620X.91B8.21518. [DOI] [PubMed] [Google Scholar]
- 22.Agarwala S, Shah SB. Ten-year followup of avascular necrosis of femoral head treated with alendronate for 3 years. J Arthroplasty. 2011;26:1128–34. doi: 10.1016/j.arth.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Nishii T, Sugano N, Miki H, Hashimoto J, Yoshikawa H. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin Orthop Relat Res. 2006;443:273–9. doi: 10.1097/01.blo.0000194078.32776.31. [DOI] [PubMed] [Google Scholar]
- 24.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–9. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 25.Chen CH, Chang JK, Lai KA, Hou SM, Chang CH, Wang GJ. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: A two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64:1572–8. doi: 10.1002/art.33498. [DOI] [PubMed] [Google Scholar]
- 26.Rajpura A, Wright AC, Board TN. Medical management of osteonecrosis of the hip: A review. Hip Int. 2011;21:385–92. doi: 10.5301/HIP.2011.8538. [DOI] [PubMed] [Google Scholar]
- 27.Kang P, Pei F, Shen B, Zhou Z, Yang J. Are the results of multiple drilling and alendronate for osteonecrosis of the femoral head better than those of multiple drilling? A pilot study. Joint Bone Spine. 2012;79:67–72. doi: 10.1016/j.jbspin.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Hsu SL, Wang CJ, Lee MS, Chan YS, Huang CC, Yang KD. Cocktail therapy for femoral head necrosis of the hip. Arch Orthop Trauma Surg. 2010;130:23–9. doi: 10.1007/s00402-009-0918-5. [DOI] [PubMed] [Google Scholar]
- 29.Wong T, Wang CJ, Hsu SL, Chou WY, Lin PC, Huang CC. Cocktail therapy for hip necrosis in SARS patients. Chang Gung Med J. 2008;31:546–53. [PubMed] [Google Scholar]
- 30.Wang CJ, Wang FS, Yang KD, Huang CC, Lee MS, Chan YS, et al. Treatment of osteonecrosis of the hip: Comparison of extracorporeal shockwave with shockwave and alendronate. Arch Orthop Trauma Surg. 2008;128:901–8. doi: 10.1007/s00402-007-0530-5. [DOI] [PubMed] [Google Scholar]
- 31.Hungerford DS, Mont MA. The natural history of untreated asymptomatic hips in patients who have non-traumatic osteonecrosis. J Bone Joint Surg Am. 1998;80:765–6. [PubMed] [Google Scholar]
- 32.Banerjee S, Issa K, Pivec R, Kapadia BH, Khanuja HS, Mont MA. Osteonecrosis of the hip: Treatment options and outcomes. Orthop Clin North Am. 2013;44:463–76. doi: 10.1016/j.ocl.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Glueck CJ, Freiberg RA, Fontaine RN, Sieve-Smith L, Wang P. Anticoagulant therapy for osteonecrosis associated with heritable hypofibrinolysis and thrombophilia. Expert Opin Investig Drugs. 2001;10:1309–16. doi: 10.1517/13543784.10.7.1309. [DOI] [PubMed] [Google Scholar]
- 34.Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001;386:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Glueck CJ, Freiberg RA, Sieve L, Wang P. Enoxaparin prevents progression of stages I and II osteonecrosis of the hip. Clin Orthop Relat Res. 2005;435:164–70. doi: 10.1097/01.blo.0000157539.67567.03. [DOI] [PubMed] [Google Scholar]
- 36.Chotanaphuti T, Thongprasert S, Laoruengthana A. Low molecular weight heparin prevents the progression of precollapse osteonecrosis of the hip. J Med Assoc Thai. 2013;96:1326–30. [PubMed] [Google Scholar]
- 37.Oinuma K, Harada Y, Nawata Y, Takabayashi K, Abe I, Kamikawa K, et al. Sustained hemostatic abnormality in patients with steroid-induced osteonecrosis in the early period after high-dose corticosteroid therapy. J Orthop Sci. 2000;5:374–9. doi: 10.1007/s007760070046. [DOI] [PubMed] [Google Scholar]
- 38.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 39.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;386:173–8. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Ajmal M, Matas AJ, Kuskowski M, Cheng EY. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population? Orthop Clin North Am. 2009;40:235–9. doi: 10.1016/j.ocl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Disch AC, Matziolis G, Perka C. The management of necrosis-associated and idiopathic bone-marrow oedema of the proximal femur by intravenous iloprost. J Bone Joint Surg Br. 2005;87:560–4. doi: 10.1302/0301-620X.87B4.15658. [DOI] [PubMed] [Google Scholar]
- 42.Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 2010;107:8782–7. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma HZ, Zeng BF, Li XL. Upregulation of VEGF in subchondral bone of necrotic femoral heads in rabbits with use of extracorporeal shock waves. Calcif Tissue Int. 2007;81:124–31. doi: 10.1007/s00223-007-9046-9. [DOI] [PubMed] [Google Scholar]
- 44.Wang CJ, Wang FS, Huang CC, Yang KD, Weng LH, Huang HY. Treatment for osteonecrosis of the femoral head: Comparison of extracorporeal shock waves with core decompression and bone-grafting. J Bone Joint Surg Am. 2005;87:2380–7. doi: 10.2106/JBJS.E.00174. [DOI] [PubMed] [Google Scholar]
- 45.Alves EM, Angrisani AT, Santiago MB. The use of extracorporeal shock waves in the treatment of osteonecrosis of the femoral head: A systematic review. Clin Rheumatol. 2009;28:1247–51. doi: 10.1007/s10067-009-1231-y. [DOI] [PubMed] [Google Scholar]
- 46.Wang CJ, Huang CC, Wang JW, Wong T, Yang YJ. Long term results of extracorporeal shockwave therapy and core decompression in osteonecrosis of the femoral head with eight- to nine-year followup. Biomed J. 2012;35:481–5. doi: 10.4103/2319-4170.104413. [DOI] [PubMed] [Google Scholar]
- 47.Ludwig J, Lauber S, Lauber HJ, Dreisilker U, Raedel R, Hotzinger H. High-energy shock wave treatment of femoral head necrosis in adults. Clin Orthop Relat Res. 2013;87:119–26. doi: 10.1097/00003086-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Wang CJ, Ko JY, Chan YS, Lee MS, Chen JM, Wang FS, et al. Extracorporeal shockwave for hip necrosis in systemic lupus erythematosus. Lupus. 2009;18:1082–6. doi: 10.1177/0961203309103151. [DOI] [PubMed] [Google Scholar]
- 49.Vulpiani MC, Vetrano M, Trischitta D, Scarcello L, Chizzi F, Argento G, et al. Extracorporeal shock wave therapy in early osteonecrosis of the femoral head: Prospective clinical study with long term followup. Arch Orthop Trauma Surg. 2012;132:499–508. doi: 10.1007/s00402-011-1444-9. [DOI] [PubMed] [Google Scholar]
- 50.Eftekhar NS, Schink-Ascani MM, Mitchell SN, Bassett CA. Osteonecrosis of the femoral head treated by pulsed electromagnetic fields (PEMFs): A preliminary report. Hip. 1983:306–30. [PubMed] [Google Scholar]
- 51.Aaron RK, Lennox D, Bunce GE, Ebert T. The conservative treatment of osteonecrosis of the femoral head. A comparison of core decompression and pulsing electromagnetic fields. Clin Orthop Relat Res. 1989;249:209–18. [PubMed] [Google Scholar]
- 52.Bassett CA, Schink-Ascani M, Lewis SM. Effects of pulsed electromagnetic fields on Steinberg ratings of femoral head osteonecrosis. Clin Orthop Relat Res. 1989;246:172–85. [PubMed] [Google Scholar]
- 53.Aaron RK, Steinberg ME. Electrical stimulation of osteonecrosis of the femoral head. Semin Arthroplasty. 1991;2:214–21. [PubMed] [Google Scholar]
- 54.Aaron RK. Treatment of osteonecrosis of the femoral head with electrical stimulation. Instr Course Lect. 1994;43:495–8. [PubMed] [Google Scholar]
- 55.Massari L, Fini M, Cadossi R, Setti S, Traina GC. Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):56–60. doi: 10.2106/JBJS.F.00536. [DOI] [PubMed] [Google Scholar]
- 56.Mont MA, Jones LC, Seyler TM, Marulanda GA, Saleh KJ, Delanois RE. New treatment approaches for osteonecrosis of the femoral head: An overview. Instr Course Lect. 2007;56:197–212. [PubMed] [Google Scholar]
- 57.Reis ND, Schwartz O, Militianu D, Ramon Y, Levin D, Norman D, et al. Hyperbaric oxygen therapy as a treatment for stage-I avascular necrosis of the femoral head. J Bone Joint Surg Br. 2003;85:371–5. doi: 10.1302/0301-620x.85b3.13237. [DOI] [PubMed] [Google Scholar]
- 58.Camporesi EM, Vezzani G, Bosco G, Mangar D, Bernasek TL. Hyperbaric oxygen therapy in femoral head necrosis. J Arthroplasty. 2010;25:118–23. doi: 10.1016/j.arth.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Kot J, Mathieu D. Controversial issues in hyperbaric oxygen therapy: A European Committee for Hyperbaric Medicine Workshop. Diving Hyperb Med. 2011;41:101–4. [PubMed] [Google Scholar]
- 60.Soohoo NF, Vyas S, Manunga J, Sharifi H, Kominski G, Lieberman JR. Cost-effectiveness analysis of core decompression. J Arthroplasty. 2006;21:670–81. doi: 10.1016/j.arth.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Wang GJ, Dughman SS, Reger SI, Stamp WG. The effect of core decompression on femoral head blood flow in steroid-induced avascular necrosis of the femoral head. J Bone Joint Surg Am. 1985;67:121–4. [PubMed] [Google Scholar]
- 62.Ficat RP, Arlet J. Ischemia and Necroses of Bone. In: Hungerford DS, editor. Baltimore: Williams and Wilkins; 1980. [Google Scholar]
- 63.Hungerford DS. Treatment of ischemic necrosis of the femoral head. In: Evarts CD, editor. Sugery of the Musculoskeletal System. Vol. 3. New York: Churchill Livingstone; 1983. pp. 5029–43. [Google Scholar]
- 64.Bozic KJ, Zurakowski D, Thornhill TS. Survivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1999;81:200–9. doi: 10.2106/00004623-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Kim SY, Kim DH, Park IH. Multiple drilling compared with core decompression for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Br. 2004;86:149. [Google Scholar]
- 66.Mont MA, Ragland PS, Etienne G. Core decompression of the femoral head for osteonecrosis using percutaneous multiple small-diameter drilling. Clin Orthop Relat Res. 2004;429:131–8. doi: 10.1097/01.blo.0000150128.57777.8e. [DOI] [PubMed] [Google Scholar]
- 67.Marker DR, Seyler TM, Ulrich SD, Srivastava S, Mont MA. Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clin Orthop Relat Res. 2008;466:1093–103. doi: 10.1007/s11999-008-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al Omran A. Multiple drilling compared with standard core decompression for avascular necrosis of the femoral head in sickle cell disease patients. Arch Orthop Trauma Surg. 2013;133:609–13. doi: 10.1007/s00402-013-1714-9. [DOI] [PubMed] [Google Scholar]
- 69.Song WS, Yoo JJ, Kim YM, Kim HJ. Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res. 2007;454:139–46. doi: 10.1097/01.blo.0000229342.96103.73. [DOI] [PubMed] [Google Scholar]
- 70.Rackwitz L, Eden L, Reppenhagen S, Reichert JC, Jakob F, Walles H, et al. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res Ther. 2012;3:7. doi: 10.1186/scrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81:349–55. doi: 10.1302/0301-620x.81b2.8818. [DOI] [PubMed] [Google Scholar]
- 72.Feng Y, Yang SH, Xiao BJ, Xu WH, Ye SN, Xia T, et al. Decreased in the number and function of circulation endothelial progenitor cells in patients with avascular necrosis of the femoral head. Bone. 2010;46:32–40. doi: 10.1016/j.bone.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res. 2009;27:442–6. doi: 10.1002/jor.20759. [DOI] [PubMed] [Google Scholar]
- 74.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Hernigou P, Manicom O, Poignard A, Nogier A, Filippini P, De Abreu L. Core decompression with marrow stem cells. Oper Tech Orthop. 2004;14:68–74. [Google Scholar]
- 76.Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87:896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 77.Gangji V, Toungouz M, Hauzeur JP. Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther. 2005;5:437–42. doi: 10.1517/14712598.5.4.437. [DOI] [PubMed] [Google Scholar]
- 78.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2005;87(Suppl 1):106–12. doi: 10.2106/JBJS.D.02662. [DOI] [PubMed] [Google Scholar]
- 79.Yan ZQ, Chen YS, Li WJ, Yang Y, Huo JZ, Chen ZR, et al. Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol. 2006;9:3–7. [PubMed] [Google Scholar]
- 80.Daltro GC, Fortuna VA, de Araújo SA, FerrazLessa PI, Sobrinho UA, Borojevic R. Femoral head necrosis treatment with autologous stem cells in sickle cell disease. Acta Orthop Bras. 2008;16:44–8. [Google Scholar]
- 81.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year followup of a prospective controlled study. Bone. 2011;49:1005–9. doi: 10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Liu S, Su X. Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2013;133:125–33. doi: 10.1007/s00402-012-1623-3. [DOI] [PubMed] [Google Scholar]
- 83.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–30. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: A randomized control study. J Arthroplasty. 2012;27:679–86. doi: 10.1016/j.arth.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 85.Müller I, Vaegler M, Holzwarth C, Tzaribatchev N, Pfister SM, Schütt B, et al. Secretion of angiogenic proteins by human multipotent mesenchymal stromal cells and their clinical potential in the treatment of avascular osteonecrosis. Leukemia. 2008;22:2054–61. doi: 10.1038/leu.2008.217. [DOI] [PubMed] [Google Scholar]
- 86.Kawate K, Yajima H, Ohgushi H, Kotobuki N, Sugimoto K, Ohmura T, et al. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: Transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs. 2006;30:960–2. doi: 10.1111/j.1525-1594.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 87.Nöth U, Reichert J, Reppenhagen S, Steinert A, Rackwitz L, Eulert J, et al. Cell based therapy for the treatment of femoral head necrosis. Orthopade. 2007;36:466–71. doi: 10.1007/s00132-007-1087-2. [DOI] [PubMed] [Google Scholar]
- 88.Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139–45. doi: 10.1097/01.blo.0000150312.53937.6f. [DOI] [PubMed] [Google Scholar]
- 89.Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003;417:84–92. doi: 10.1097/01.blo.0000096826.67494.38. [DOI] [PubMed] [Google Scholar]
- 90.Seyler TM, Marker DR, Ulrich SD, Fatscher T, Mont MA. Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res. 2008;466:1125–32. doi: 10.1007/s11999-008-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meyers MH, Jones RE, Bucholz RW, Wenger DR. Fresh autogenous grafts and osteochondral allografts for the treatment of segmental collapse in osteonecrosis of the hip. Clin Orthop Relat Res. 1983;174:107–12. [PubMed] [Google Scholar]
- 92.Meyers MH. Resurfacing of the femoral head with fresh osteochondral allografts. Long term results. Clin Orthop Relat Res. 1985;197:111–4. [PubMed] [Google Scholar]
- 93.Sotereanos NG, DeMeo PJ, Hughes TB, Bargiotas K, Wohlrab D. Autogenous osteochondral transfer in the femoral head after osteonecrosis. Orthopedics. 2008;31:177. doi: 10.3928/01477447-20080201-33. [DOI] [PubMed] [Google Scholar]
- 94.Rittmeister M, Hochmuth K, Kriener S, Richolt J. Five-year results following autogenous osteochondral transplantation to the femoral head. Orthopade. 2005;34:320, 322–6. doi: 10.1007/s00132-005-0776-y. [DOI] [PubMed] [Google Scholar]
- 95.Gagala J, Tarczynska M, Gaweda K, et al. Response to comment on Gagala Clinical and radiological outcomes of treatment of avascular necrosis of the femoral head using autologous osteochondral transfer (mosaicplasty). Preliminary report. Int Orthop. 2013;37:1641–2. doi: 10.1007/s00264-013-1975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckley PD, Gearen PF, Petty RW. Structural bone-grafting for early atraumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1991;73:1357–64. [PubMed] [Google Scholar]
- 97.Keizer SB, Kock NB, Dijkstra PD, Taminiau AH, Nelissen RG. Treatment of avascular necrosis of the hip by a non-vascularised cortical graft. J Bone Joint Surg Br. 2006;88:460–6. doi: 10.1302/0301-620X.88B4.16950. [DOI] [PubMed] [Google Scholar]
- 98.Penix AR, Cook SD, Skinner HB, Weinstein AM, Haddad RJ., Jr Femoral head stresses following cortical bone grafting for aseptic necrosis. A finite element study. Clin Orthop Relat Res. 1983;173:159–65. [PubMed] [Google Scholar]
- 99.Israelite C, Nelson CL, Ziarani CF, Abboud JA, Landa J, Steinberg ME. Bilateral core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2005;441:285–90. doi: 10.1097/01.blo.0000192365.58958.84. [DOI] [PubMed] [Google Scholar]
- 100.Plakseychuk AY, Kim SY, Park BC, Varitimidis SE, Rubash HE, Sotereanos DG. Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85-A:589–96. doi: 10.2106/00004623-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Ko JY, Meyers MH, Wenger DR. “Trapdoor” procedure for osteonecrosis with segmental collapse of the femoral head in teenagers. J Pediatr Orthop. 1995;15:7–15. doi: 10.1097/01241398-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 102.Mont MA, Einhorn TA, Sponseller PD, Hungerford DS. The trapdoor procedure using autogenous cortical and cancellous bone grafts for osteonecrosis of the femoral head. J Bone Joint Surg Br. 1998;80:56–62. doi: 10.1302/0301-620x.80b1.7989. [DOI] [PubMed] [Google Scholar]
- 103.Rosenwasser MP, Garino JP, Kiernan HA, Michelsen CB. Long term followup of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthop Relat Res. 1994;306:17–27. [PubMed] [Google Scholar]
- 104.Smith KR, Bonfiglio M, Montgomery WJ. Non-traumatic necrosis of the femoral head treated with tibial bone-grafting. A followup note. J Bone Joint Surg Am. 1980;62:845–7. [PubMed] [Google Scholar]
- 105.Nelson LM, Clark CR. Efficacy of phemister bone grafting in nontraumatic aseptic necrosis of the femoral head. J Arthroplasty. 1993;8:253–8. doi: 10.1016/s0883-5403(06)80086-0. [DOI] [PubMed] [Google Scholar]
- 106.Dunn AW, Grow T. Aseptic necrosis of the femoral head. Treatment with bone grafts of doubtful value. Clin Orthop Relat Res. 1977;122:249–54. [PubMed] [Google Scholar]
- 107.Papanagiotou M, Malizos KN, Vlychou M, Dailiana ZH. Autologous (non-vascularised) fibular grafting with recombinant bone morphogenetic protein-7 for the treatment of femoral head osteonecrosis: Preliminary report. Bone Joint J. 2014;96-B:31–5. doi: 10.1302/0301-620X.96B1.32773. [DOI] [PubMed] [Google Scholar]
- 108.Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):48–55. doi: 10.2106/JBJS.F.00538. [DOI] [PubMed] [Google Scholar]
- 109.Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007;22:26–31. doi: 10.1016/j.arth.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 110.Floerkemeier T, Thorey F, Daentzer D, Lerch M, Klages P, Windhagen H, et al. Clinical and radiological outcome of the treatment of osteonecrosis of the femoral head using the osteonecrosis intervention implant. Int Orthop. 2011;35:489–95. doi: 10.1007/s00264-009-0940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Babis GC, Sakellariou V, Parvizi J, Soucacos P. Osteonecrosis of the femoral head. Orthopedics. 2011;34:39. doi: 10.3928/01477447-20101123-19. [DOI] [PubMed] [Google Scholar]
- 112.Tanzer M, Karabasz D, Krygier JJ, Cohen R, Bobyn JD. The Otto Aufranc Award: Bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res. 2005;441:30–9. doi: 10.1097/01.blo.0000194728.62996.2d. [DOI] [PubMed] [Google Scholar]
- 113.Tsao AK, Roberson JR, Christie MJ, Dore DD, Heck DA, Robertson DD, et al. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005;87(Suppl 2):22–7. doi: 10.2106/JBJS.E.00490. [DOI] [PubMed] [Google Scholar]
- 114.Tanzer M, Bobyn JD, Krygier JJ, Karabasz D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008;90:1282–9. doi: 10.2106/JBJS.F.00847. [DOI] [PubMed] [Google Scholar]
- 115.Varitimidis SE, Dimitroulias AP, Karachalios TS, Dailiana ZH, Malizos KN. Outcome after tantalum rod implantation for treatment of femoral head osteonecrosis: 26 hips followed for an average of 3 years. Acta Orthop. 2009;80:20–5. doi: 10.1080/17453670902804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nadeau M, Séguin C, Theodoropoulos JS, Harvey EJ. Short term clinical outcome of a porous tantalum implant for the treatment of advanced osteonecrosis of the femoral head. Mcgill J Med. 2007;10:4–10. [PMC free article] [PubMed] [Google Scholar]
- 117.Meyers MH. The treatment of osteonecrosis of the hip with fresh osteochondral allografts and with the muscle pedicle graft technique. Clin Orthop Relat Res. 1978;130:202–9. [PubMed] [Google Scholar]
- 118.Lee CK, Rehmatullah N. Muscle-pedicle bone graft and cancellous bone graft for the “silent hip” of idiopathic ischemic necrosis of the femoral head in adults. Clin Orthop Relat Res. 1981;158:185–94. [PubMed] [Google Scholar]
- 119.Baksi DP. Treatment of osteonecrosis of the femoral head by drilling and muscle-pedicle bone grafting. J Bone Joint Surg Br. 1991;73:241–5. doi: 10.1302/0301-620X.73B2.2005147. [DOI] [PubMed] [Google Scholar]
- 120.Iwata H, Torii S, Hasegawa Y, Itoh H, Mizuno M, Genda E, et al. Indications and results of vascularized pedicle iliac bone graft in avascular necrosis of the femoral head. Clin Orthop Relat Res. 1993;295:281–8. [PubMed] [Google Scholar]
- 121.Matsusaki H, Noguchi M, Kawakami T, Tani T. Use of vascularized pedicle iliac bone graft combined with transtrochanteric rotational osteotomy in the treatment of avascular necrosis of the femoral head. Arch Orthop Trauma Surg. 2005;125:95–101. doi: 10.1007/s00402-004-0777-z. [DOI] [PubMed] [Google Scholar]
- 122.Zhang C, Zeng B, Xu Z, Song W, Shao L, Jing D, et al. Treatment of femoral head necrosis with free vascularized fibula grafting: A preliminary report. Microsurgery. 2005;25:305–9. doi: 10.1002/micr.20118. [DOI] [PubMed] [Google Scholar]
- 123.Marciniak D, Furey C, Shaffer JW. Osteonecrosis of the femoral head. A study of 101 hips treated with vascularized fibular grafting. J Bone Joint Surg Am. 2005;87:742–7. doi: 10.2106/JBJS.D.02004. [DOI] [PubMed] [Google Scholar]
- 124.Yoo MC, Kim KI, Hahn CS, Parvizi J. Long term followup of vascularized fibular grafting for femoral head necrosis. Clin Orthop Relat Res. 2008;466:1133–40. doi: 10.1007/s11999-008-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aldridge JM, 3rd, Urbaniak JR. Vascularized fibular grafting for osteonecrosis of the femoral head with unusual indications. Clin Orthop Relat Res. 2008;466:1117–24. doi: 10.1007/s11999-008-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eisenschenk A, Lautenbach M, Schwetlick G, Weber U. Treatment of femoral head necrosis with vascularized iliac crest transplants. Clin Orthop Relat Res. 2001;386:100–5. doi: 10.1097/00003086-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 127.Babhulkar S. Osteonecrosis of femoral head: Treatment by core decompression and vascular pedicle grafting. Indian J Orthop. 2009;43:27–35. doi: 10.4103/0019-5413.45320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fang T, Zhang EW, Sailes FC, McGuire RA, Lineaweaver WC, Zhang F. Vascularized fibular grafts in patients with avascular necrosis of femoral head: A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2013;133:1–10. doi: 10.1007/s00402-012-1627-z. [DOI] [PubMed] [Google Scholar]
- 129.Urbaniak JR, Coogan PG, Gunneson EB, Nunley JA. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting. A long term followup study of one hundred and three hips. J Bone Joint Surg Am. 1995;77:681–94. doi: 10.2106/00004623-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 130.Eward WC, Rineer CA, Urbaniak JR, Richard MJ, Ruch DS. The vascularized fibular graft in precollapse osteonecrosis: Is long term hip preservation possible? Clin Orthop Relat Res. 2012;470:2819–26. doi: 10.1007/s11999-012-2429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sugioka Y, Hotokebuchi T, Tsutsui H. Transtrochanteric anterior rotational osteotomy for idiopathic and steroid-induced necrosis of the femoral head. Indications and long term results. Clin Orthop Relat Res. 1992;277:111–20. [PubMed] [Google Scholar]
- 132.Ito H, Tanino H, Yamanaka Y, Nakamura T, Takahashi D, Minami A, et al. Long term results of conventional varus half-wedge proximal femoral osteotomy for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Br. 2012;94:308–14. doi: 10.1302/0301-620X.94B3.27814. [DOI] [PubMed] [Google Scholar]
- 133.Sugioka Y, Katsuki I, Hotokebuchi T. Transtrochanteric rotational osteotomy of the femoral head for the treatment of osteonecrosis. Followup statistics. Clin Orthop Relat Res. 1982;169:115–26. [PubMed] [Google Scholar]
- 134.Sugioka Y. Transtrochanteric anterior rotational osteotomy of the femoral head in the treatment of osteonecrosis affecting the hip: A new osteotomy operation. Clin Orthop Relat Res. 1978;130:191–201. [PubMed] [Google Scholar]
- 135.Hasegawa Y, Sakano S, Iwase T, Iwasada S, Torii S, Iwata H. Pedicle bone grafting versus transtrochanteric rotational osteotomy for avascular necrosis of the femoral head. J Bone Joint Surg Br. 2003;85:191–8. doi: 10.1302/0301-620x.85b2.13190. [DOI] [PubMed] [Google Scholar]
- 136.Jacobs MA, Hungerford DS, Krackow KA. Intertrochanteric osteotomy for avascular necrosis of the femoral head. J Bone Joint Surg Br. 1989;71:200–4. doi: 10.1302/0301-620X.71B2.2925735. [DOI] [PubMed] [Google Scholar]
- 137.Maistrelli G, Fusco U, Avai A, Bombelli R. Osteonecrosis of the hip treated by intertrochanteric osteotomy. A four- to 15-year followup. J Bone Joint Surg Br. 1988;70:761–6. doi: 10.1302/0301-620X.70B5.3192575. [DOI] [PubMed] [Google Scholar]
- 138.Gallinaro P, Massè A. Flexion osteotomy in the treatment of avascular necrosis of the hip. Clin Orthop Relat Res. 2001;386:79–84. doi: 10.1097/00003086-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 139.Scher MA, Jakim I. Intertrochanteric osteotomy and autogenous bone-grafting for avascular necrosis of the femoral head. J Bone Joint Surg Am. 1993;75:1119–33. doi: 10.2106/00004623-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 140.Zhao G, Yamamoto T, Ikemura S, Motomura G, Mawatari T, Nakashima Y, et al. Radiological outcome analysis of transtrochanteric curved varus osteotomy for osteonecrosis of the femoral head at a mean followup of 12.4 years. J Bone Joint Surg Br. 2010;92:781–6. doi: 10.1302/0301-620X.92B6.23621. [DOI] [PubMed] [Google Scholar]
- 141.Sakano S, Hasegawa Y, Torii Y, Kawasaki M, Ishiguro N. Curved intertrochanteric varus osteotomy for osteonecrosis of the femoral head. J Bone Joint Surg Br. 2002;84:817–24. doi: 10.1302/0301-620x.86b3.14383. [DOI] [PubMed] [Google Scholar]
- 142.Issa K, Pivec R, Kapadia BH, Banerjee S, Mont MA. Osteonecrosis of the femoral head: The total hip replacement solution. Bone Joint J. 2013;95-B:46–50. doi: 10.1302/0301-620X.95B11.32644. [DOI] [PubMed] [Google Scholar]
- 143.Chan YS, Shih CH. Bipolar versus total hip arthroplasty for hip osteonecrosis in the same patient. Clin Orthop Relat Res. 2000;379:169–77. doi: 10.1097/00003086-200010000-00020. [DOI] [PubMed] [Google Scholar]
- 144.Ito H, Matsuno T, Kaneda K. Bipolar hemiarthroplasty for osteonecrosis of the femoral head. A 7- to 18-year followup. Clin Orthop Relat Res. 2000;374:201–11. doi: 10.1097/00003086-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 145.Sanjay BK, Moreau PG. Bipolar hip replacement in sickle cell disease. Int Orthop. 1996;20:222–6. doi: 10.1007/s002640050068. [DOI] [PubMed] [Google Scholar]
- 146.Takaoka K, Nishina T, Ohzono K, Saito M, Matsui M, Sugano N, et al. Bipolar prosthetic replacement for the treatment of avascular necrosis of the femoral head. Clin Orthop Relat Res. 1992;277:121–7. [PubMed] [Google Scholar]
- 147.Adili A, Trousdale RT. Femoral head resurfacing for the treatment of osteonecrosis in the young patient. Clin Orthop Relat Res. 2003;417:93–101. doi: 10.1097/01.blo.0000096815.78689.3e. [DOI] [PubMed] [Google Scholar]
- 148.Cuckler JM, Moore KD, Estrada L. Outcome of hemiresurfacing in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2004;429:146–50. doi: 10.1097/01.blo.0000150121.88033.50. [DOI] [PubMed] [Google Scholar]
- 149.Johannson HR, Zywiel MG, Marker DR, Jones LC, McGrath MS, Mont MA. Osteonecrosis is not a predictor of poor outcomes in primary total hip arthroplasty: A systematic literature review. Int Orthop. 2011;35:465–73. doi: 10.1007/s00264-010-0979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Min BW, Lee KJ, Song KS, Bae KC, Cho CH. Highly cross-linked polyethylene in total hip arthroplasty for osteonecrosis of the femoral head: A minimum 5-year followup study. J Arthroplasty. 2013;28:526–30. doi: 10.1016/j.arth.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 151.Kim YH, Kim JS, Park JW, Joo JH. Contemporary total hip arthroplasty with and without cement in patients with osteonecrosis of the femoral head: A concise followup, at an average of seventeen years, of a previous report. J Bone Joint Surg Am. 2011;93:1806–10. doi: 10.2106/JBJS.J.01312. [DOI] [PubMed] [Google Scholar]
- 152.Kawasaki M, Hasegawa Y, Sakano S, Masui T, Ishiguro N. Total hip arthroplasty after failed transtrochanteric rotational osteotomy for avascular necrosis of the femoral head. J Arthroplasty. 2005;20:574–9. doi: 10.1016/j.arth.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 153.McGrath MS, Marker DR, Seyler TM, Ulrich SD, Mont MA. Surface replacement is comparable to primary total hip arthroplasty. Clin Orthop Relat Res. 2009;467:94–100. doi: 10.1007/s11999-008-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gilbert RE, Cheung G, CarrBall ST, Le Duff MJ, Amstutz HC. Early results of conversion of a failed femoral component in hip resurfacing arthroplasty. J Bone Joint Surg Am. 2007;89:735–41. doi: 10.2106/JBJS.F.00708. [DOI] [PubMed] [Google Scholar]
- 155.Ball ST, Le Duff MJ, Amstutz HC. Early results of conversion of a failed femoral component in hip resurfacing arthroplasty. J Bone Joint Surg Am. 2007;89:735–41. doi: 10.2106/JBJS.F.00708. [DOI] [PubMed] [Google Scholar]
- 156.Beaulé PE, Schmalzried TP, Campbell P, Dorey F, Amstutz HC. Duration of symptoms and outcome of hemiresurfacing for hip osteonecrosis. Clin Orthop Relat Res. 2001;385:104–17. doi: 10.1097/00003086-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 157.Helbig L, Simank HG, Kroeber M, Schmidmaier G, Grützner PA, Guehring T. Core decompression combined with implantation of a demineralised bone matrix for non-traumatic osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2012;132:1095–103. doi: 10.1007/s00402-012-1526-3. [DOI] [PubMed] [Google Scholar]
- 158.Issa K, Johnson AJ, Naziri Q, Khanuja HS, Delanois RE, Mont MA. Hip osteonecrosis: Does prior hip surgery alter outcomes compared to an initial primary total hip arthroplasty? J Arthroplasty. 2014;29:162–6. doi: 10.1016/j.arth.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 159.Ilyas I, Moreau P. Simultaneous bilateral total hip arthroplasty in sickle cell disease. J Arthroplasty. 2002;17:441–5. doi: 10.1054/arth.2002.31084. [DOI] [PubMed] [Google Scholar]
- 160.Issa K, Naziri Q, Maheshwari AV, Rasquinha VJ, Delanois RE, Mont MA. Excellent results and minimal complications of total hip arthroplasty in sickle cell hemoglobinopathy at mid-term followup using cementless prosthetic components. J Arthroplasty. 2013;28:1693–8. doi: 10.1016/j.arth.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 161.Marulanda GA, Minniti CP, Ulrich SD, Seyler TM, Mont MA. Perioperative management for orthopaedic patients with sickle cell anaemia. J Orthop Surg (Hong Kong) 2009;17:346–50. doi: 10.1177/230949900901700321. [DOI] [PubMed] [Google Scholar]
- 162.Chang JS, Han DJ, Park SK, Sung JH, Ha YC. Cementless total hip arthroplasty in patients with osteonecrosis after kidney transplantation. J Arthroplasty. 2013;28:824–7. doi: 10.1016/j.arth.2013.01.020. [DOI] [PubMed] [Google Scholar]