Abstract
Thoracolumbar spine fractures are common injuries that can result in significant disability, deformity and neurological deficit. Controversies exist regarding the appropriate radiological investigations, the indications for surgical management and the timing, approach and type of surgery. This review provides an overview of the epidemiology, biomechanical principles, radiological and clinical evaluation, classification and management principles. Literature review of all relevant articles published in PubMed covering thoracolumbar spine fractures with or without neurologic deficit was performed. The search terms used were thoracolumbar, thoracic, lumbar, fracture, trauma and management. All relevant articles and abstracts covering thoracolumbar spine fractures with and without neurologic deficit were reviewed. Biomechanically the thoracolumbar spine is predisposed to a higher incidence of spinal injuries. Computed tomography provides adequate bony detail for assessing spinal stability while magnetic resonance imaging shows injuries to soft tissues (posterior ligamentous complex [PLC]) and neurological structures. Different classification systems exist and the most recent is the AO spine knowledge forum classification of thoracolumbar trauma. Treatment includes both nonoperative and operative methods and selected based on the degree of bony injury, neurological involvement, presence of associated injuries and the integrity of the PLC. Significant advances in imaging have helped in the better understanding of thoracolumbar fractures, including information on canal morphology and injury to soft tissue structures. The ideal classification that is simple, comprehensive and guides management is still elusive. Involvement of three columns, progressive neurological deficit, significant kyphosis and canal compromise with neurological deficit are accepted indications for surgical stabilization through anterior, posterior or combined approaches.
Keywords: Spinal injuries, thoracolumbar trauma, management
MeSH terms: Spinal injuries, thoracic vertebrae, lumbar vertebrae, treatment protocols
INTRODUCTION
Fractures of the thoracic and lumbar region constitute a spectrum of injuries ranging from the simple undisplaced fractures to complex fracture dislocations.1 Anatomically and functionally, the thoracic and lumbar spine can be divided into three regions – thoracic spine (T1-T10), thoracolumbar junction (T10-L2) and the lumbar spine (L3-L5). The thoracic spine is functionally rigid due to coronally oriented facet joints, thin intervertebral discs and the ribcage. Thus, it requires huge amounts of energy to produce fractures and dislocations. The narrow spinal canal in this region predisposes to spinal cord damage resulting in a high incidence of neurological deficit. The lumbar spine, on the other hand, is relatively flexible due to the thicker intervertebral discs, sagittal orientation of facet joints and the absence of the rib cage. The relatively lesser incidence of neurological injury in lumbar fractures can be attributed to the large size of the neural canal and the greater resilience of the cauda equina nerve roots. The thoracolumbar junction (T10-L2) is uniquely positioned in between the rigid thoracic spine and the mobile lumbar spine. This transition from the less mobile thoracic spine with its associated ribs and sternum to the more dynamic lumbar spine subjects the thoracolumbar region to significant biomechanical stress.1,2 Hence, fractures of the thoracolumbar region are the most common injuries of the vertebral column.
Though fractures of the thoracolumbar spine are common injuries, 50% of these are unstable and can result in significant disability, deformity and neurological deficit. There are standard classification systems that have been described based on fracture morphology, injury mechanism, neurological deficit and injury to posterior ligamentous complex (PLC). Radiographs are the basic investigation while computed tomography (CT) scan provides information on the extent on bony injury and magnetic resonance imaging (MRI) scan shows injury to the spinal cord and soft tissue structures. However, despite extensive studies on this common injury, controversies still exist regarding the appropriate radiological investigations, the type of Nonoperative treatment, the indications for surgical management, the timing of surgery, approach and type of surgery, need for fusion and the role of spinal canal decompression. This review provides an overview of the epidemiology, biomechanical principles, radiological and clinical evaluation, and evolution of classification system and management principles.
EPIDEMIOLOGY
In an epidemiological study by Hu et al. in the Canadian population, the incidence of spinal injuries was 64/100,000 population/year.3 In North America, the incidence of spinal injuries is more than 160,000 every year.2 Among the thoracolumbar injuries, 50-60% affected the transitional zone (T11-L2), 25-40% affected the thoracic spine and 10-14% involved the lower lumbar spine and sacrum.4 Thoracolumbar fractures are more frequent in men, and the peak incidence is observed between 20 and 40 years.4,5 Neurological injury complicates 20-36% of fractures at the thoracolumbar junction in different studies.6,7 The chances and extent of neurological deficit depend on the type of fracture. In a multicenter study, the incidence of neurological deficit ranged from 22% to 51% depending on the fracture type (22% in type A, 28% in type B and 51% in type C fractures, according to the AO classification).8
Injuries to the thoracolumbar spine are usually the result of high-energy blunt trauma. Sixty-five percent of thoracolumbar fractures occur due to motor vehicle injuries and falls from a height, with the remainder contributed by sports injuries and violence. Since these are high-velocity injuries, thoracolumbar fractures are commonly associated with other injuries like rib fractures, pneumo-hemothorax, and rarely great vessel injuries, hemopericardium and diaphragmatic rupture9,10 [Figure 1]. Seat-belt (chance) fractures and flexion distraction injuries are often associated with intraabdominal visceral injuries. Long bone fractures and head injuries are also common and can often lead to missed injuries of the spine.8 Due to such associated “distracting” injuries, the incidence of missed injuries of the thoracolumbar spine has been reported to be as high as 20%, especially in those with high-energy blunt trauma and altered mental status.11 In a review of 508 consecutive spinal injury patients, Saboe et al. identified associated injuries in 47%. Most frequent injuries were head injuries (26%), chest injuries (24%) and long bone injuries (23%).12
Figure 1.
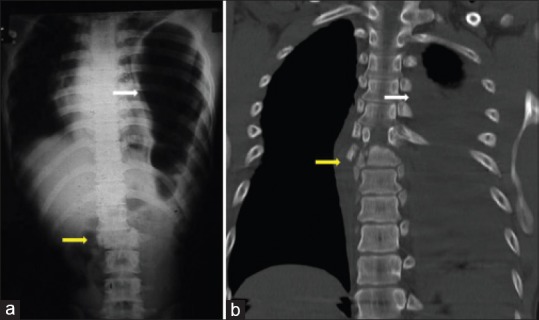
Systemic injuries associated with thoracolumbar fractures. (a) T12-L1 dislocation associated with left diaphragmmatic rupture and herniation of intraabdominal contents. (b) T7-T8 translational injury associated with massive hemothorax on the left side
DIAGNOSIS
Clinical evaluation
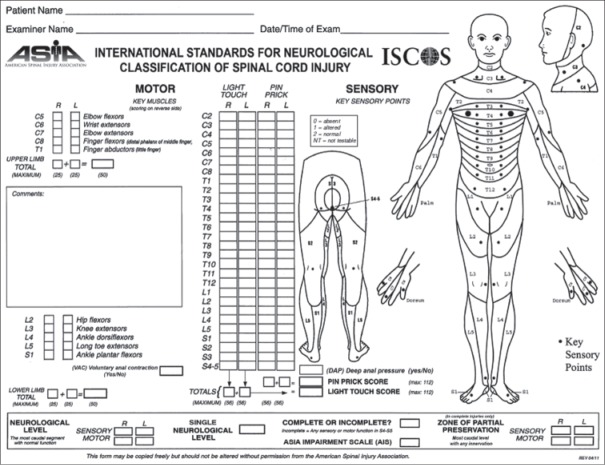
Needless to say, any suspected spinal trauma patient in the emergency room should be evaluated following the basic principles of trauma assessment including primary and secondary survey. Once life-threatening injuries are prioritized, a careful history about the injury mechanism and information pertaining to any back or neck pain and neurological symptoms are acquired. Patients typically present with a history of trauma following a road traffic accident, fall from height, a direct blow to the spine or rarely gunshot injuries. Axial, nonradiating back pain of stabbing or aching quality is the most common symptom. Patients with neurological injury complain of weakness, paresthesia or anesthesia below the injury level and urinary retention. Thorough inspection of the spine should be performed after a careful log roll maneuver to look for abrasions, tenderness, local kyphosis and a palpable gap in between spinous processes. Neurological assessment should follow the standard American Spinal Injury Association (ASIA) guidelines13 [Figure 2]. As the spinal cord ends at the L1-L2 level, and the cauda equina fills the distal canal, varied neurological injury patterns can be observed with thoracolumbar fractures. Neurological injuries above L1 can damage the spinal cord producing a typical upper motor neuron injury. Injuries much below L1-L2 affect only the cauda equina roots involving few or multiple nerve roots resulting in lower motor neuron type injury. Conus medullaris syndrome characterized by exclusive damage to sacral innervations to the bowel and bladder, with intact lumbar nerve roots, is a unique feature of T12-L1 injury [Figure 3].
Figure 2.
American Spinal Injury Association (ASIA) form for standard neurologic classification of spinal cord injury (from ASIA)
Figure 3.
X-ray thoracolumbar spine lateral view (a) and magnetic resonance imaging of conus medullaris syndrome showing fracture of L1 vertebra resulting in injury to the conus medullaris
Radiological evaluation
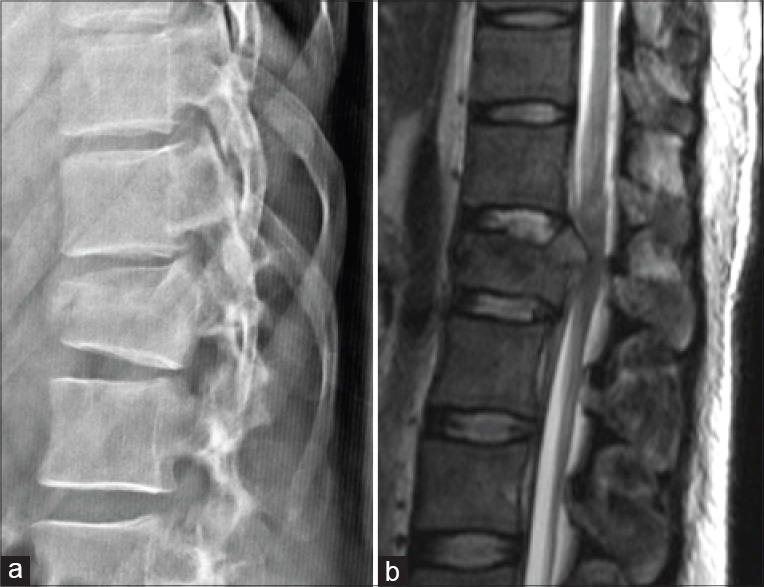
Standard radiographic evaluation includes antero-posterior and lateral radiographs. Radiographic evaluation should include spinal alignment, presence of any rotation or translation, assessment of the kyphosis, loss of vertebral height, and widened inter-pedicular or inter-spinous distance.11,14,15 CT scan of the injured area characterizes the fracture further and provides the degree of canal compromise. Approximately, 25% of burst fractures are misdiagnosed as compression fractures if radiographs alone are evaluated16 [Figure 4]. MRI scan provides information on spinal cord or root injury, presence and extent of cord edema and hemorrhage, and epidural hematoma.17 Other advantages of MRI are its ability to evaluate injury to the intervertebral discs and PLC, and identify the presence of noncontiguous injuries through screening of the whole spine [Figure 5]. The incidence of noncontiguous spinal fractures is 1.6-23.8%.18,19 The incidence of a delayed diagnosis of the second lesion ranges from 23.1% to 83.3%.18,19 Thorough clinical examination of the entire spine to look for bruise and tenderness, radiographs of the other regions of the spine and sagittal MRI screening of the whole spine can potentially avoid missing other injuries.
Figure 4.
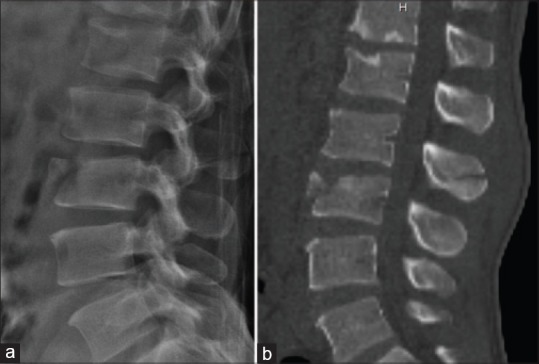
Computed tomography (CT) scan provides excellent delineation of bony injuries. In this patient with A1 injury of the L3 vertbral body seen in the lateral radiograph (a), CT scan showed horizontal split of L2 spinous process indicating a flexion-distraction injury
Figure 5.
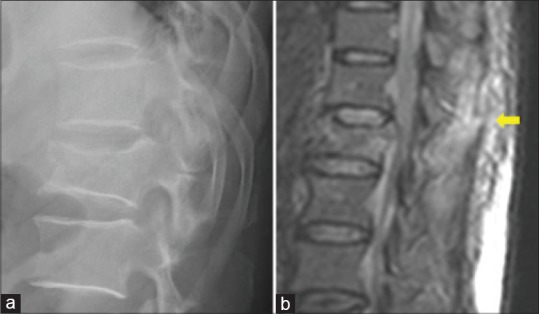
(a) Lateral radiograph of thoracolumbar spine showing a burst fracture of L1 vertebral body. (b) Sagittal magnetic resonance image shows a hyper intense signal of the posterior ligamentous complex (yellow arrow) indicating injury, which is not detected in the radiograph
CLASSIFICATION
Biomechanics of the load bearing and supporting structures of the vertebral column form the basis of understanding the spinal injuries and their classification systems. The classification of thoracolumbar fractures has evolved over the years as the understanding of the spinal biomechanics, mechanism of injury and identification of vertebral stability improved.
Early classification systems described by Boehler (1929) and Jones were descriptive systems, based only on radiographs.20 Holdsworth revolutionized the thoracolumbar injury classification with the introduction of the “two column concept.”21 Denis believed that the middle column is the key to the stability of the fracture and proposed the “three column concept.”22 Based on the three column concept described by Denis and the possible mechanisms of failure of the columns, McAfee described a simplified system of classifying injuries23 [Table 1]. This system is simple and includes most injuries observed in clinical practice. In 1994, Magerl analyzed 1445 cases of thoracolumbar injuries and presented a comprehensive AO classification of thoracolumbar fractures based on the mechanism of injury and morphological pattern of the fracture.6 Despite being a comprehensive classification system, this system was cumbersome with poor inter-observer reliability.
Table 1.
McAfee's classification of thoracolumbar fractures

McCormack et al. introduced a classification (load sharing classification) to predict the risk of implant failure after posterior short segment fixation for thoracolumbar fractures. This classification intends only to identify fractures that would require supplemental anterior fixation following a posterior surgery.24 The Thoracolumbar Injury Classification System (TLICS) created by “The Spine Trauma Study Group,” is based on three major injury characteristics – mechanism of injury, integrity of the PLC and neurological status. Based on the severity scores within these three categories, a total score is calculated that can be used to guide treatment.25
Among these classification systems, the Denis/McAfee classification, AO classification and the TLICS classification are commonly used in clinical practice. Blauth et al. have reported that the inter-observer reliability of the AO classification was low (fair agreement, κ = 0.33), and when the injury was classified into subgroups, the inter-observer reliability decreased further.26 Oner et al.27 and Wood et al.28 also reported that the Denis classification system (κ = 0.60 and 0.606) showed higher inter-observer reliability than the AO classification system (κ = 0.35 and 0.475). Lenarz et al. compared the reliability of Denis, AO, and TLICS systems in 97 thoracolumbar fractures and observed that in all the three systems, variation in reliability was present, with the highest reliability occurring in the senior resident group and attending spine surgeon group.29 The lowest reliabilities were in the nonspine attending orthopedists and junior residents. In each group, the neurologic status had the highest inter observer and intraobserver reliability. They concluded that the TLICS is an acceptably reliable system when compared with the Denis and AO systems.
The AO spine knowledge forum has proposed a recent comprehensive modified AO classification including morphology of the fracture, neurological status, and description of relevant patient-specific modifiers.30
The fracture morphology is assessed based on 3 main injury patterns: Type A (compression-injury to the vertebral body without PLC involvement), type B (tension band disruption - the failure of posterior (PLC) or anterior (anterior longitudinal ligament) constraints), and type C (displacement/translation) injuries. Neurologic status is classified as follows: No neurologic injury (N1), radicular symptoms or deficits (N2), incomplete spinal cord injury (SCI) or any kind of cauda equina injury (N3), complete SCI (N4) and unknown neurologic status (NX). Forty cases with a broad range of injuries were classified independently twice by group members and the reliability in the identification of a morphologic injury type was substantial (κ =0.72). The classification appears much simpler and equally comprehensive when compared with the previous AO classification and includes important information about neurology and posterior ligamentous structures. Based on this classification, type A4, B1, B2, B3 and C injuries will need surgical stabilization. The inter/intraobserver reliability of this classification is yet to be studied, apart from the originators.
MANAGEMENT
Medical management
Throughout resuscitation in the emergency room and subsequent care, all efforts must be taken to immobilize spinal injury patients safely and intermittently log roll to prevent pressure sore formation. Stabilization of unstable injured motion segments plays an important role in preventing further injury. In a patient with SCI, injury to neural structures occurs both at the time of injury (primary – nonmodifiable) and in the subsequent period due to vascular dysfunction, edema, ischemia, electrolyte shifts, free radical production, inflammation and delayed apoptotic cell death (secondary – potentially modifiable).31 Numerous pharmacological agents thought to mitigate the secondary injury have been extensively studied. These include the steroids (antiinflammatory), gangliosides, naloxone (opiate receptor antagonist), calcium channel blockers, free radical scavengers and neurotropic agents.
Steroids were extensively employed in the clinical treatment of SCI beginning in the mid-1960s. In rat SCI models, steroids have been shown to improve neurological recovery.32 After encouraging “positive” results with the use of high dose steroids in North American Spinal Cord Injury Study (NASCIS) 1 and 2 trials, the NASCIS 3 trial studied the effect of methyl prednisolone within 3-8 h of injury and concluded that better neurological recovery is statistically observed if administered over 24-48 h.33
However, a number of articles have strongly criticized the NASCIS trial design, analysis and reporting. Coleman et al. observed that the NASCIS II and III reports have used specific choices of statistical methods that have strongly shaped the reporting of results. The primary outcome analysis of the trials were negative with modest beneficial effects being proven only through post hoc analyses, inappropriately excluding >70% of the patients.34 After a thorough review of the three NASCIS trials, Hurlbert concluded that evidence of the drug's efficacy and impact is weak and may only represent random events, and the use of high dose methyl prednisolone in the treatment of acute SCI is not proven as a standard of care.35 A survey published in 2006 revealed that the majority of respondents continue to administer methylprednisolone, but they are motivated predominantly by fear of litigation.36
Nonoperative management
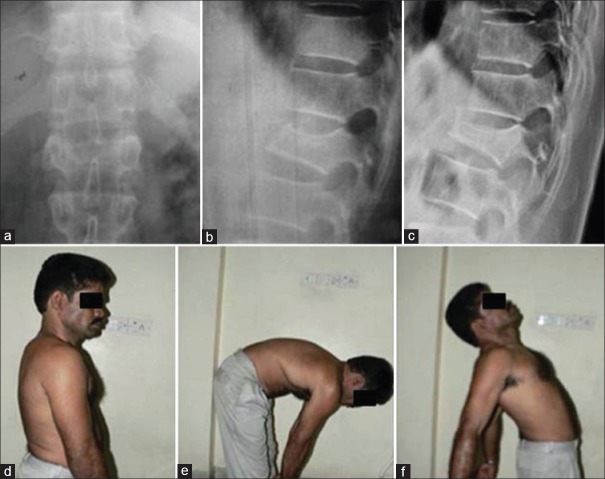
Most thoracolumbar fractures are stable injuries amenable to nonoperative management. Simple compression or stable burst fractures without neurologic complications can typically be treated with commercially available thoracolumbar orthoses, or a hyperextension cast that permit early ambulation37,38 [Figure 6]. There is no consensus on the exact duration of treatment. The advantages of a brace or cast over unprotected ambulation have also not been studied. However, it has been shown that external support has no mechanical stabilizing effect on the lumbar spine.39 In a systematic review of studies, Giele et al. concluded that there is no evidence for the effectiveness of bracing in patients with traumatic thoracolumbar fractures.40 During nonoperative care, it is common to observe a certain degree of increasing fracture kyphosis in most patients, often closer to the pretreatment sagittal alignment. However kyphosis even up to 30° has not been shown to correlate with pain in several studies.37,41,42
Figure 6.
Stable burst fracture of L2 vertebral body treated by conservative care. Lateral radiograph at the end of 1-year shows good fracture healing. Clinical pictures show good functional outcome
Operative management
The advantages of operative treatment of thoracolumbar fractures over the nonoperative approach include avoiding an orthosis in the presence of multiple injuries, skin injuries, and obesity, immediate mobilization and earlier rehabilitation and better restoration of sagittal alignment.4,43 Surgical decompression of compressing bone fragments over the spinal cord also reliably provides a better environment for restoration of neurologic function. On the other hand, the benefits of surgical treatment must be carefully weighed against the potential surgical morbidity. Conventional open surgical techniques can be associated with morbidity because of approach-related muscle injury, increased infection rates and higher blood loss.
Neurological recovery and surgical decompression
In general, operative treatment is indicated mainly for unstable spinal injuries such as flexion distraction injuries, unstable burst fractures and fracture dislocations. Though operative treatment reduces pain and enables early mobilization and rehabilitation, there is no difference between operative and nonoperative treatment regarding neurological recovery and long term functional outcomes.44 Studies in animal models have demonstrated that neurological recovery is enhanced by early decompression.45 However, this has not been proven in human studies on acute SCI. The severity of neurological injury is determined by the extent of neuronal injury incurred at the time of primary injury. But it is still worthwhile considering early surgical decompression in patients with incomplete SCI in the presence of spinal cord compression. Despite the lack of clear level I or II scientific evidence, the general accepted indications for operative treatment are given in Table 2.
Table 2.
Standard indications for surgical treatment in thoracolumbar fractures

Timing of surgery
There is no consensus on how early to operate on a patient with SCI. Vaccaro et al. conducted a prospective randomized controlled trial to determine whether neurologic and functional outcome is improved in traumatic cervical spinal cord injured patients with early surgery (<72 h after SCI) compared with those patients who had late surgery (>5 days). They observed no significant neurologic benefit when spinal cord decompression is performed <72 h after injury.46 However, in a meta-analysis by La Rosa et al., 1687 patients were studied to evaluate the advantages of early decompression in acute SCI.47 The patients were divided into three treatment groups: Early decompression (<24 h), delayed decompression (>24 h), and conservative treatment. Early decompression resulted in better outcomes statistically when compared with delayed decompression and conservative management. In the presence of progressive incomplete neurological deficit and spinal cord compression, it is prudent to perform urgent surgical stabilization and decompression. Patients with normal neurology and those with complete neurological deficit are optimized for surgery which can be performed at the earliest safe situation for the patient. There is no evidence to perform surgery in the midnight.
Surgical approach
Different surgical approaches and techniques have been described for thoracolumbar fractures including posterior, anterior and combined approaches. However, scientific evidence lacks to support the selection of one surgical technique as advantageous over the other. Factors, such as an anesthetic and surgical burden to the patient, morbidity, complication rates, costs, and surgeon's expertise should be taken into account in the choice of surgical approach.
Posterior approach
Posterior short segment fixation including the proximal and distal adjacent normal vertebrae is the most commonly performed surgery for the vast majority of thoracolumbar fractures (unstable burst fractures with intact neurology, flexiondistraction injury, Chance fractures). Fracture reduction can be achieved by a combination of postural reduction, and by distraction through ligamentotaxis. Posterior pedicle screw fixation has been shown to be simple, familiar, efficient, reliable, and safe for the reduction and stabilization of most fractures and remains the most popular technique. Disadvantages include instrumentation failure, pseudarthrosis, infection, risks of SCI, inadequate neurological decompression, insufficient correction of kyphosis and the need for late instrumentation removal.48,49,50 Depending on the extent of vertebral body comminution, additional anterior reconstruction may be needed to prevent implant failure. McCormack et al. retrospectively analyzed 28 patients who had been operated for thoracolumbar injuries with Steffee plates.24 Three important factors in predicting posterior fixation failure were studied which included the amount of vertebral body comminuted as seen in sagittal CT images, the apposition of fracture fragments as seen in axial CT images and the amount of correction of kyphotic deformity as best measured by comparing pre and postoperative films. Each of these factors was subdivided into three grades of severity and was scored on a point system from 1 to 3, with a higher number indicating increased severity. They observed that anterior vertebral reconstruction is essential in patients with a score ≥7 to prevent implant failure. To avoid anterior surgeries, various authors have described other techniques such as transpedicular intracorporeal bone grafting, vertebroplasty and kyphoplasty, intracorporeal filling with hydroxyapatite or calcium phosphate.51,52 Other biomechanical measures to improve the strength of the construct include the use of cross-links, supplemental hook fixation at the levels of the screws and the addition of “intermediate” screw into the fractured vertebra.53,54 Several biomechanical and clinical studies have shown that the addition of intermediate screws significantly increased the strength of a short segment posterior construct thus lowering the rates of loss of kyphosis after correction [Figure 7].55,56,57
Figure 7.
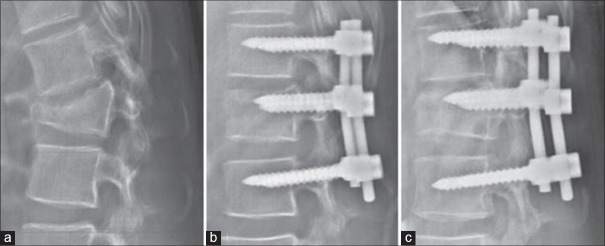
X-ray thoracolumbar spine lateral view showing (a) L1 flexion-distraction injury (b) treatment with posterior short segment fixation and intermediate screws (c) Lateral radiograph performed at the end of 1-year shows good fracture healing
High velocity grossly unstable injuries like fracture dislocations usually requires multilevel spinal stabilization. Fixation of two to three segments above and below the injury is recommended for reducing the dislocation and achieving stable fixation. Similarly, unstable fractures of the thoracic spine are subjected to significant shear stresses and hence are treated with multilevel posterior fixation. For select type A and B fractures, Gotzen et al. have published their technique of posterior mono-segmental reduction and stabilization.58 The injuries are usually confined to the upper-end plate alone, and pedicle screw fixation and fusion involves only the fractured and the proximal normal vertebra. In their 2 year followup of 39 patients, no implant failure was noted.
Anterior approach
About 80% of the axial load of an intact spine is supported by the anterior column. When the anterior column is substantially injured, the anterior column support is reduced leaving majority of the stress to be transmitted by the posterior implant and the bony elements. In such situations, restoration of anterior column through a tricortical bone graft or a cage is advised. The other indication for anterior surgery is the presence of spinal cord compression due to retropulsed bone fragments. Spinal canal compromise in patients presenting with neurological deficits that cannot adequately be resolved by a posterior approach requires anterior decompression. The degree of neurological recovery, rate of spinal fusion, sagittal spine alignment, and return to preinjury activities after anterior decompression appears more favorable compared to techniques that do not decompress the spinal canal.59 Use of anterior vertebral plates and screws, and cages has greatly improved postoperative spinal stability and also reduced donor-site morbidity from major bone graft harvesting techniques. Kaneda et al. have reported a study on 150 consecutive patients who had a burst fracture of the thoracolumbar spine with neurological deficits.60 The patients were managed with a single-stage anterior spinal decompression, strut grafting, and anterior spinal instrumentation. At a mean of 8 years (range: 5-12 years) after the operation, radiographs showed successful fusion of the injured spinal segment in 140 patients (93%). The neurological function improved in 95% of the patients by at least one Frankel grade, while 72% of patients recovered completely.
Relatively few studies compare anterior to posterior approaches for thoracolumbar burst fractures, and most of them show an advantage of the anterior approach. In his series, Gertzbein reported that bladder function significantly improved following anterior compared to posterior procedures.4 Hitchon et al. showed that angular deformity was more successfully corrected and maintained when the anterior approach was used.61 Sasso et al. also showed that although both approaches are associated with a statistically significant initial improvement in sagittal alignment, the posterior approach was associated with increased loss of sagittal correction (8.1°) compared to the anterior approach (1.8°) at followup.62
Combined anterior and posterior approach
Select patients with thoracolumbar burst fractures may benefit from combined surgical approaches. Indications for combined approach would include burst fractures with significant kyphosis (>40°), >50% canal compromise and neurological deficit in the presence of spinal cord compression. The advantages of combined surgical approaches are improved sagittal alignment, thorough spinal canal and neural decompression and stabilization of the disrupted PLC. In a series of 20 consecutive patients with a single-level unstable thoracolumbar burst fracture treated by posterior fixation followed by anterior corpectomy and titanium cage implantation, 12 patients with initial neurological deficits recovered an average of 1.5 grades on the ASIA scale.63 Two years postoperatively, the mean pain score for back pain was 1.6 points and instrumentation failure did not occur. At a mean followup of 6 years, a comparative retrospective study of combined versus posterior-only fixation reported similar clinical outcome and neurological improvement, fusion rate and angle of kyphotic deformity in both groups.64 However, loss of reduction >5° and instrumentation failure were significantly higher in the posterior-only fixation.
Minimally invasive approach
Open surgical approaches for the treatment of thoracolumbar fractures either by anterior or posterior techniques require extensive exposure and often lead to significant postoperative pain and morbidity. Recently, minimally invasive techniques have been described in thoracolumbar fractures. For posterior stabilization, percutaneous minimally invasive pedicle screw fixation and stabilization can be performed to minimize muscle injury and enable early rehabilitation. The application of posterior minimally invasive techniques reduces the approach-related morbidity like iatrogenic muscle denervation, ischemia, pain and functional impairment.65,66,67 It is a useful option in case of poly traumatized patients, obesity, and compromised lung function when conservative treatment is not advisable. Similarly, minimally invasive thoracoscopic vertebral body decompression and cage reconstruction minimizes the morbidity in poly traumatized patients. In an analysis of 65 patients, Kossmann, et al. reported no intra or postoperative complications following thoracoscopic anterior decompression and reconstruction.68 Bühren et al. analyzed 38 patients and concluded that, compared to the open method, minimally invasive surgery had the benefit of reducing postoperative pain, shorter hospitalization, leading to early functional recovery and reducing the morbidity of the operative approach.69
COMPLICATIONS
Posterior pedicle screw fixation has become the mainstay of spinal instrumentation for fracture stabilization. Despite increasing experience, knowledge and technical advancement, pedicle screw insertion is still associated with a certain degree of complications. The most commonly reported complication is screw malpositioning, with an overall incidence of 0-42%.70,71 Most of them are asymptomatic without any major sequelae, and serious screw-related complications, such as neurological, visceral, or vascular are very rare. The overall incidence of nerve root or SCI due to screw malpositioning ranges between 0.6% and 11%.72 A transient self-limiting neurapraxia in the form of numbness is the usual feature and the incidence of permanent neurological deficit is rare. Vascular injuries related to misplacement of screws are potential life- and limb-threatening complications that require early recognition with prompt repair of vascular lesions and screw repositioning.73 Visceral injuries related to pedicle screw insertions are very rare. The proximity of vertebral bodies to structures like lung and pleura can result in pnemothorax, effusion or an esophageal injury inadvertently. Screws can break when there is a deficient anterior column, progressive kyphosis and pseudoarthrosis. This is mainly attributable to metal fatigue due to excessive strain on the implant.
The abdomen contains many vascular structures including the aorta, inferior vena cava, segmental vessels, and numerous veins, which are exposed during anterior surgeries and hence at risk for injury. Venous laceration is the most common vascular injury and usually occurs during manipulation and retraction of the great vessels.74 Vascular injury can also occur while performing the corpectomy, placing the graft, and inserting the screws. Manual compression or primary repair of the tear is generally effective at treating this complication. Visceral injuries and postoperative lymphocele, or chyloretroperitoneum are uncommon events. This is usually evident intraoperatively and requires the expertise of the gastrointestinal surgeon to repair. Injuries to the peritoneum are very common but are easily repaired and do not lead to significant problems.
CONCLUSION
Despite tremendous improvements in spinal imaging and management techniques in the last two decades, there is still lack of consensus in several areas in the management of thoracolumbar fractures. Principally treatment decisions in these patients require a complete evaluation of the neurological status and identification of the presence of spinal instability. CT scan provides the best information regarding the extent of bony injury and MRI scan shows the extent and severity of cord compression and injury to PLC. The ideal classification that is simple, comprehensive and guides management are still elusive. The most recently described is the AO KF thoracolumbar classification, which appears simple and includes most information regarding the extent of vertebral body injury, neurological injury and patient modifiers. Involvement of all the three columns, progressive neurological deficit, significant kyphosis >30° and canal compromise in the presence of neurological deficit are accepted indications for surgical stabilization. Compression fractures and stable burst fractures can be treated by nonoperative methods. Posterior surgery remains the most preferred technique, and anterior approach is the access of choice when decompression of the spinal cord is the priority. Minimally invasive surgeries are increasingly used to reduce surgical morbidity in the acutely traumatized patient.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Wood KB, Li W, Lebl DS, Ploumis A. Management of thoracolumbar spine fractures. Spine J. 2014;14:145–64. doi: 10.1016/j.spinee.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 2.el-Khoury GY, Whitten CG. Trauma to the upper thoracic spine: Anatomy, biomechanics, and unique imaging features. AJR Am J Roentgenol. 1993;160:95–102. doi: 10.2214/ajr.160.1.8416656. [DOI] [PubMed] [Google Scholar]
- 3.Hu R, Mustard CA, Burns C. Epidemiology of incident spinal fracture in a complete population. Spine (Phila Pa 1976) 1996;21:492–9. doi: 10.1097/00007632-199602150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Gertzbein SD. Scoliosis Research Society. Multicenter spine fracture study. Spine (Phila Pa 1976) 1992;17:528–40. doi: 10.1097/00007632-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Gertzbein SD, Khoury D, Bullington A, St John TA, Larson AI. Thoracic and lumbar fractures associated with skiing and snowboarding injuries according to the AO Comprehensive Classification. Am J Sports Med. 2012;40:1750–4. doi: 10.1177/0363546512449814. [DOI] [PubMed] [Google Scholar]
- 6.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 7.Kraemer WJ, Schemitsch EH, Lever J, McBroom RJ, McKee MD, Waddell JP. Functional outcome of thoracolumbar burst fractures without neurological deficit. J Orthop Trauma. 1996;10:541–4. doi: 10.1097/00005131-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Knop C, Blauth M, Bühren V, Hax PM, Kinzl L, Mutschler W, et al. Surgical treatment of injuries of the thoracolumbar transition 1: Epidemiology. Unfallchirurg. 1999;102:924–35. doi: 10.1007/s001130050507. [DOI] [PubMed] [Google Scholar]
- 9.McLain RF, Sparling E, Benson DR. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures. A preliminary report. J Bone Joint Surg Am. 1993;75:162–7. doi: 10.2106/00004623-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Benson DR, Burkus JK, Montesano PX, Sutherland TB, McLain RF. Unstable thoracolumbar and lumbar burst fractures treated with the AO fixateur interne. J Spinal Disord. 1992;5:335–43. doi: 10.1097/00002517-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Keene JS. Radiographic evaluation of thoracolumbar fractures. Clin Orthop Relat Res. 1984;189:58–64. [PubMed] [Google Scholar]
- 12.Saboe LA, Reid DC, Davis LA, Warren SA, Grace MG. Spine trauma and associated injuries. J Trauma. 1991;31:43–8. doi: 10.1097/00005373-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–74. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- 14.Dalinka MK, Kessler H, Weiss M. The radiographic evaluation of spinal trauma. Emerg Med Clin North Am. 1985;3:475–90. [PubMed] [Google Scholar]
- 15.Harris JH., Jr Radiographic evaluation of spinal trauma. Orthop Clin North Am. 1986;17:75–86. [PubMed] [Google Scholar]
- 16.Ballock RT, Mackersie R, Abitbol JJ, Cervilla V, Resnick D, Garfin SR. Can burst fractures be predicted from plain radiographs? J Bone Joint Surg Br. 1992;74:147–50. doi: 10.1302/0301-620X.74B1.1732246. [DOI] [PubMed] [Google Scholar]
- 17.Tarr RW, Drolshagen LF, Kerner TC, Allen JH, Partain CL, James AE., sJr MR imaging of recent spinal trauma. J Comput Assist Tomogr. 1987;11:412–7. doi: 10.1097/00004728-198705000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop Relat Res. 2003:95–102. doi: 10.1097/01.blo.0000068362.47147.a2. [DOI] [PubMed] [Google Scholar]
- 19.Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine (Phila Pa 1976) 1991;16:128–31. [PubMed] [Google Scholar]
- 20.Jones RW. The results of postural reduction of fractures of the spine. J Bone Joint Surg. 1938;20:567–86. [Google Scholar]
- 21.Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am. 1970;52:1534–51. [PubMed] [Google Scholar]
- 22.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817–31. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 23.McAfee PC, Yuan HA, Fredrickson BE, Lubicky JP. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983;65:461–73. [PubMed] [Google Scholar]
- 24.McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine (Phila Pa 1976) 1994;19:1741–4. doi: 10.1097/00007632-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Vaccaro AR, Lehman RA, Jr, Hurlbert RJ, Anderson PA, Harris M, Hedlund R, et al. A new classification of thoracolumbar injuries: The importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976) 2005;30:2325–33. doi: 10.1097/01.brs.0000182986.43345.cb. [DOI] [PubMed] [Google Scholar]
- 26.Blauth M, Bastian L, Knop C, Lange U, Tusch G. Inter-observer reliability in the classification of thoracolumbar spinal injuries. Orthopade. 1999;28:662–81. doi: 10.1007/s001320050397. [DOI] [PubMed] [Google Scholar]
- 27.Oner FC, Ramos LM, Simmermacher RK, Kingma PT, Diekerhof CH, Dhert WJ, et al. Classification of thoracic and lumbar spine fractures: Problems of reproducibility. A study of 53 patients using CT and MRI. Eur Spine J. 2002;11:235–45. doi: 10.1007/s00586-001-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood KB, Khanna G, Vaccaro AR, Arnold PM, Harris MB, Mehbod AA. Assessment of two thoracolumbar fracture classification systems as used by multiple surgeons. J Bone Joint Surg Am. 2005;87:1423–9. doi: 10.2106/JBJS.C.01530. [DOI] [PubMed] [Google Scholar]
- 29.Lenarz CJ, Place HM, Lenke LG, Alander DH, Oliver D. Comparative reliability of 3 thoracolumbar fracture classification systems. J Spinal Disord Tech. 2009;22:422–7. doi: 10.1097/BSD.0b013e31818a38cd. [DOI] [PubMed] [Google Scholar]
- 30.Vaccaro AR, Oner C, Kepler CK, Dvorak M, Schnake K, Bellabarba C, et al. AOSpine thoracolumbar spine injury classification system: Fracture description, neurological status, and key modifiers. Spine (Phila Pa 1976) 2013;38:2028–37. doi: 10.1097/BRS.0b013e3182a8a381. [DOI] [PubMed] [Google Scholar]
- 31.Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 32.Taoka Y, Okajima K, Uchiba M, Johno M. Methylprednisolone reduces spinal cord injury in rats without affecting tumor necrosis factor-alpha production. J Neurotrauma. 2001;18:533–43. doi: 10.1089/089771501300227332. [DOI] [PubMed] [Google Scholar]
- 33.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 h or tirilazad mesylate for 48 h in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial ational acute spinal cord injury study. J Am Med Assoc. 1997;277:1597–604. [PubMed] [Google Scholar]
- 34.Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green B, et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13:185–99. doi: 10.1097/00002517-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Hurlbert RJ. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 36.Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Questionnaire survey of spine surgeons on the use of methylprednisolone for acute spinal cord injury. Spine (Phila Pa 1976) 2006;31:E250–3. doi: 10.1097/01.brs.0000214886.21265.8c. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein JN, Collalto P, Lehmann TR. Thoracolumbar “burst” fractures treated conservatively: A long term followup. Spine (Phila Pa 1976) 1988;13:33–8. doi: 10.1097/00007632-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Mumford J, Weinstein JN, Spratt KF, Goel VK. Thoracolumbar burst fractures. The clinical efficacy and outcome of nonoperative management. Spine (Phila Pa 1976) 1993;18:955–70. [PubMed] [Google Scholar]
- 39.Shen WJ, Shen YS. Nonsurgical treatment of three-column thoracolumbar junction burst fractures without neurologic deficit. Spine (Phila Pa 1976) 1999;24:412–5. doi: 10.1097/00007632-199902150-00024. [DOI] [PubMed] [Google Scholar]
- 40.Giele BM, Wiertsema SH, Beelen A, van der Schaaf M, Lucas C, Been HD, et al. No evidence for the effectiveness of bracing in patients with thoracolumbar fractures. Acta Orthop. 2009;80:226–32. doi: 10.3109/17453670902875245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood K, Buttermann G, Mehbod A, Garvey T, Jhanjee R, Sechriest V. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85-A:773–81. doi: 10.2106/00004623-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Cantor JB, Lebwohl NH, Garvey T, Eismont FJ. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine (Phila Pa 1976) 1993;18:971–6. doi: 10.1097/00007632-199306150-00004. [DOI] [PubMed] [Google Scholar]
- 43.Denis F, Armstrong GW, Searls K, Matta L. Acute thoracolumbar burst fractures in the absence of neurologic deficit. A comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984:142–9. [PubMed] [Google Scholar]
- 44.Vaccaro AR, Kim DH, Brodke DS, Harris M, Chapman JR, Schildhauer T, et al. Diagnosis and management of thoracolumbar spine fractures. Instr Course Lect. 2004;53:359–73. [PubMed] [Google Scholar]
- 45.Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: Update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13–26. doi: 10.1016/j.injury.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Vaccaro AR, Daugherty RJ, Sheehan TP, Dante SJ, Cotler JM, Balderston RA, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976) 1997;22:2609–13. doi: 10.1097/00007632-199711150-00006. [DOI] [PubMed] [Google Scholar]
- 47.La Rosa G, Conti A, Cardali S, Cacciola F, Tomasello F. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503–12. doi: 10.1038/sj.sc.3101627. [DOI] [PubMed] [Google Scholar]
- 48.Alanay A, Acaroglu E, Yazici M, Oznur A, Surat A. Short-segment pedicle instrumentation of thoracolumbar burst fractures: Does transpedicular intracorporeal grafting prevent early failure? Spine (Phila Pa 1976) 2001;26:213–7. doi: 10.1097/00007632-200101150-00017. [DOI] [PubMed] [Google Scholar]
- 49.Shin TS, Kim HW, Park KS, Kim JM, Jung CK. Short-segment pedicle instrumentation of thoracolumbar burst-compression fractures; Short term followup results. J Korean Neurosurg Soc. 2007;42:265–70. doi: 10.3340/jkns.2007.42.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu BS, Tang TS, Yang HL. Long term results of thoracolumbar and lumbar burst fractures after short-segment pedicle instrumentation, with special reference to implant failure and correction loss. Orthop Surg. 2009;1:85–93. doi: 10.1111/j.1757-7861.2009.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho DY, Lee WY, Sheu PC. Treatment of thoracolumbar burst fractures with polymethyl methacrylate vertebroplasty and short-segment pedicle screw fixation. Neurosurgery. 2003;53:1354–60. doi: 10.1227/01.neu.0000093200.74828.2f. [DOI] [PubMed] [Google Scholar]
- 52.Marco RA, Kushwaha VP. Thoracolumbar burst fractures treated with posterior decompression and pedicle screw instrumentation supplemented with balloon-assisted vertebroplasty and calcium phosphate reconstruction. J Bone Joint Surg Am. 2009;91:20–8. doi: 10.2106/JBJS.G.01668. [DOI] [PubMed] [Google Scholar]
- 53.Chiba M, McLain RF, Yerby SA, Moseley TA, Smith TS, Benson DR. Short-segment pedicle instrumentation. Biomechanical analysis of supplemental hook fixation. Spine (Phila Pa 1976) 1996;21:288–94. doi: 10.1097/00007632-199602010-00006. [DOI] [PubMed] [Google Scholar]
- 54.Dick JC, Jones MP, Zdeblick TA, Kunz DN, Horton WC. A biomechanical comparison evaluating the use of intermediate screws and cross-linkage in lumbar pedicle fixation. J Spinal Disord. 1994;7:402–7. [PubMed] [Google Scholar]
- 55.Guven O, Kocaoglu B, Bezer M, Aydin N, Nalbantoglu U. The use of screw at the fracture level in the treatment of thoracolumbar burst fractures. J Spinal Disord Tech. 2009;22:417–21. doi: 10.1097/BSD.0b013e3181870385. [DOI] [PubMed] [Google Scholar]
- 56.Mahar A, Kim C, Wedemeyer M, Mitsunaga L, Odell T, Johnson B, et al. Short-segment fixation of lumbar burst fractures using pedicle fixation at the level of the fracture. Spine (Phila Pa 1976) 2007;32:1503–7. doi: 10.1097/BRS.0b013e318067dd24. [DOI] [PubMed] [Google Scholar]
- 57.Farrokhi MR, Razmkon A, Maghami Z, Nikoo Z. Inclusion of the fracture level in short segment fixation of thoracolumbar fractures. Eur Spine J. 2010;19:1651–6. doi: 10.1007/s00586-010-1449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gotzen L, Puplat D, Junge A. Indications, technique and results of monosegmental dorsal spondylodesis in wedge compression fractures (grade II) of the thoracolumbar spine. Unfallchirurg. 1992;95:445–54. [PubMed] [Google Scholar]
- 59.Haas N, Blauth M, Tscherne H. Anterior plating in thoracolumbar spine injuries. Indication, technique, and results. Spine (Phila Pa 1976) 1991;16:S100–11. doi: 10.1097/00007632-199103001-00015. [DOI] [PubMed] [Google Scholar]
- 60.Kaneda K, Taneichi H, Abumi K, Hashimoto T, Satoh S, Fujiya M. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg Am. 1997;79:69–83. doi: 10.2106/00004623-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Hitchon PW, Torner J, Eichholz KM, Beeler SN. Comparison of anterolateral and posterior approaches in the management of thoracolumbar burst fractures. J Neurosurg Spine. 2006;5:117–25. doi: 10.3171/spi.2006.5.2.117. [DOI] [PubMed] [Google Scholar]
- 62.Sasso RC, Renkens K, Hanson D, Reilly T, McGuire RA, Jr, Best NM. Unstable thoracolumbar burst fractures: Anterior-only versus short-segment posterior fixation. J Spinal Disord Tech. 2006;19:242–8. doi: 10.1097/01.bsd.0000211298.59884.24. [DOI] [PubMed] [Google Scholar]
- 63.Payer M. Unstable burst fractures of the thoracolumbar junction: Treatment by posterior bisegmental correction/fixation and staged anterior corpectomy and titanium cage implantation. Acta Neurochir (Wien) 2006;148:299–306. doi: 10.1007/s00701-005-0681-5. [DOI] [PubMed] [Google Scholar]
- 64.Been HD, Bouma GJ. Comparison of two types of surgery for thoracolumbar burst fractures: Combined anterior and posterior stabilisation vs. Posterior instrumentation only. Acta Neurochir (Wien) 1999;141:349–57. doi: 10.1007/s007010050310. [DOI] [PubMed] [Google Scholar]
- 65.Beisse R, Mückley T, Schmidt MH, Hauschild M, Bühren V. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128–36. doi: 10.3171/spi.2005.2.2.0128. [DOI] [PubMed] [Google Scholar]
- 66.Beisse R. Endoscopic surgery on the thoracolumbar junction of the spine. Eur Spine J. 2010;19(Suppl 1):S52–65. doi: 10.1007/s00586-009-1124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khoo LT, Beisse R, Potulski M. Thoracoscopic-assisted treatment of thoracic and lumbar fractures: A series of 371 consecutive cases. Neurosurgery. 2002;51:S104–17. [PubMed] [Google Scholar]
- 68.Kossmann T, Jacobi D, Trentz O. The use of a retractor system (SynFrame) for open, minimal invasive reconstruction of the anterior column of the thoracic and lumbar spine. Eur Spine J. 2001;10:396–402. doi: 10.1007/s005860100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bühren V, Beisse R, Potulski M. Minimally invasive ventral spondylodesis in injuries to the thoracic and lumbar spine. Chirurg. 1997;68:1076–84. doi: 10.1007/s001040050326. [DOI] [PubMed] [Google Scholar]
- 70.Hicks JM, Singla A, Shen FH, Arlet V. Complications of pedicle screw fixation in scoliosis surgery: A systematic review. Spine (Phila Pa 1976) 2010;35:E465–70. doi: 10.1097/BRS.0b013e3181d1021a. [DOI] [PubMed] [Google Scholar]
- 71.Lonstein JE, Denis F, Perra JH, Pinto MR, Smith MD, Winter RB. Complications associated with pedicle screws. J Bone Joint Surg Am. 1999;81:1519–28. doi: 10.2106/00004623-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Matsuzaki H, Tokuhashi Y, Matsumoto F, Hoshino M, Kiuchi T, Toriyama S. Problems and solutions of pedicle screw plate fixation of lumbar spine. Spine (Phila Pa 1976) 1990;15:1159–65. doi: 10.1097/00007632-199011010-00014. [DOI] [PubMed] [Google Scholar]
- 73.Foxx KC, Kwak RC, Latzman JM, Samadani U. A retrospective analysis of pedicle screws in contact with the great vessels. J Neurosurg Spine. 2010;13:403–6. doi: 10.3171/2010.3.SPINE09657. [DOI] [PubMed] [Google Scholar]
- 74.Inamasu J, Guiot BH. Vascular injury and complication in neurosurgical spine surgery. Acta Neurochir (Wien) 2006;148:375–87. doi: 10.1007/s00701-005-0669-1. [DOI] [PubMed] [Google Scholar]